Abstract
Traumatic brain injury (TBI), due to its high mortality and morbidity, is an important research topic. Apoptosis plays a pathogenic role in a series of neurological disorders, from neurodegenerative diseases to acute neurological lesions.
In this study, we analyzed the association between apoptosis and the Glasgow Outcome Scale (GOS), to examine the potential of apoptosis as a biomarker for a TBI outcome. Patients with severe TBI were recruited at the Department of Neurosurgery, Wujin Hospital Affiliated with Jiangsu University, between January 2018 and December 2019. As a control group, healthy subjects were recruited. The concentrations of caspase-3, cytochrome c, sFas, and caspase-9 in the cerebrospinal fluid (CSF) were analyzed by enzyme-linked immunosorbent assay (ELISA). The association between the GOS and the clinical variables age, sex, initial Glasgow Coma Scale (GCS) score, intracranial pressure (ICP), cerebral perfusion pressure (CPP), initial computed tomography (CT) findings, and apoptotic factors was determined using logistic regression. The area under the receiver operator characteristic (ROC) curve (AUC), and thus the sensitivity and specificity of each risk factor, were obtained.
The levels of caspase-3, cytochrome c, sFas, and caspase-9 in the TBI group were significantly higher than those in the control group (P < .05). The logistic regression results showed that ICP and caspase-3 were significant predictors of outcome at 6 months post-TBI (P < .05). The AUC was 0.925 and 0.888 for ICP and caspase-3, respectively. However, the AUC for their combined prediction was 0.978, with a specificity and sensitivity of 96.0% and 95.2%, respectively, showing that the combined prediction was more reliable than that of the 2 separate factors.
We demonstrated that caspase-3, cytochrome C, sFas, and caspase-9 were significantly increased in the CSF of patients following severe TBI. Furthermore, we found that ICP and caspase-3 were more reliable for outcome prediction in combination, rather than separately.
Keywords: apoptosis, cerebrospinal fluid, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is a hot topic among researchers because of its high mortality and morbidity. Apoptosis has been recently recognized as a relevant factor in this context, since it may be important for secondary brain injury. Indeed, apoptosis plays a pathogenic role in a series of neurological disorders, from neurodegenerative diseases to acute neurological lesions.[1–3] Apoptosis requires energy and protein synthesis; moreover, it is morphologically characterized by condensation and fragmentation of heterochromatin, and by formation of apoptotic bodies. Several studies showed neuronal apoptosis in patients with TBI, as well as in post-mortem specimens and in vivo analyses.[2,4–6]
Apoptotic cell death after trauma is achieved through two distinct caspase-dependent pathways, an intrinsic and an extrinsic one. The intrinsic pathway is initiated by the release of cytochrome c, which leads to the activation of a cascade of caspases. In contrast, the extrinsic pathway is activated when Fas ligand (FasL) binds to a death receptor, which also leads to activation of caspases.[2,7,8] Although overexpression of cytochrome c, FasL, and caspase-3 in brain tissue has been demonstrated both experimentally and clinically, little is known about the presence of such factors in the CSF of patients after TBI.[6,9,10]
In this study, we analyzed demographic and clinical injury variables, and their impact on the presence of apoptotic elements in the CSF of TBI patients, compared to a control group. The association between the Glasgow Outcome Scale (GOS) and several clinical variables, including apoptotic factors, was determined using a bivariate logistic regression model. Furthermore, we used receiver operator characteristic (ROC) curves to examine the potential of apoptosis as a biomarker for TBI outcome.
2. Methods
2.1. Subjects
Patients with severe TBI were recruited at the Department of Neurosurgery, Wujin Hospital Affiliated with Jiangsu University, between January 2018 and December 2019. A diagnosis of severe TBI required a Glasgow Coma Scale (GCS) score ≤8 and a positive cranial CT scan. The patients received medical treatment according to the Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition (Neurosurgery. September 20, 2016). For all TBI subjects, the inclusion criteria were:
-
(1)
patient age between 16 and 70 years,
-
(2)
an external ventricular drain placed for intracranial pressure monitoring, and
-
(3)
signed consent from next of kin.
As a control group, healthy subjects were recruited. For control subjects, the inclusion criteria were:
-
(1)
patient age between 16 and 70 years,
-
(2)
no current or pre-existing brain injuries, neurological diseases, or bleeding disorders, and
-
(3)
signed consent from the subject or next of kin.
Ultimately, 45 patients with severe TBI and 25 controls were included. The study was approved by the ethical committee of the Wujin Hospital Affiliated with Jiangsu University. Written informed consent was obtained from each participant.
2.2. Demographic and clinical injury variables
The independent variables included sex, age, initial GCS, intracranial pressure (ICP), cerebral perfusion pressure (CPP), and initial computed tomography (CT) findings. The GCS was taken within 8 hours of injury, to limit the influence of alcohol, sedatives, or paralytics. The level of brain injury was determined according to the Marshall classification of traumatic brain injury based on initial CT findings.
2.3. Outcome variables
The GOS scores were assigned to the TBI subjects 6 months after injury. Accordingly, the patients were rated with “good recovery” (5), “moderate disability” (4), “severe disability” (3), “persistent vegetative state” (2), or “death” (1). In this study, the GOS categories were then collapsed into 1/2/3 (unfavorable outcome) vs 4/5 (favorable outcome) for analysis. The GOS is frequently dichotomized this way to discriminate outcomes.
2.4. Samples
For the TBI group, CSF was collected under sterile conditions, from the extra-ventricular drain apparatus, within 24 hours after trauma. The control subjects’ CSF was obtained from patients who had no intracranial or spinal pathology, and required spinal anesthesia for other reasons. The samples were centrifuged at 3,000 rpm for 10 minutes to remove cellular materials, and then stored at −80°C until analysis.
2.5. Measurement of apoptotic factors
The concentrations of caspase-3, cytochrome c, sFas, and caspase-9 were analyzed by enzyme-linked immunosorbent assays (ELISAs) using commercial kits. The optimal dilutions were determined and ELISAs were performed according to the manufacturer's instructions. The following kits were used: human caspase-3 ELISA kit (ab181418; Abcam, Cambridge, MA, USA; detection threshold of 58 pg/ml); human cytochrome c ELISA kit (ab221832; Abcam, Cambridge, MA; detection threshold of 1.1 ng/ml); human sFas ELISA kit (ab119571; Abcam, Cambridge, MA; detection threshold of 3 pg/ml); and human caspase-9 ELISA kit (ab119508; Abcam, Cambridge, MA; detection threshold of 0.4 ng/ml).
2.6. Statistical analyses
The measurement data are expressed as mean ± SD, and the enumeration data as percentages. The differences between the groups were statistically analyzed using the t test or the χ2 test, depending on the variables. The associations between the GOS and the clinical variables age, sex, initial GCS score, ICP, CPP, initial CT findings, and apoptotic factors were determined using a bivariate logistic regression model. The analyses were performed after having removed the outliers, and the area under the ROC curve and 95% confidence interval (CI) were determined. The sensitivity and specificity of each independent risk factor were obtained for a range of different cut-off points. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analyses. P < .05 was regarded as statistically significant.
3. Results
3.1. Clinical characteristics of TBI patients
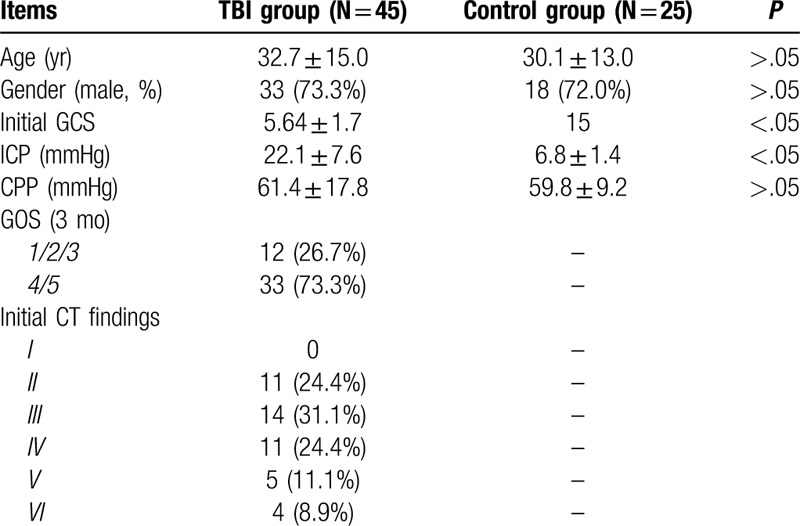
Based on our inclusion criteria, 45 patients with severe TBI and 25 controls were analyzed. For the TBI group, there were 33(73.3%) males with mean age 31.3±14.9 year and 12(26.7%) females with mean age 33.6 ± 13.1 years. For the control group, there were 18(72.0%) males with mean age 29.8 ± 16.7 year and 7(28.0%) females with mean age 31.2 ± 11.6 years. There were no significant age or sex differences between the 2 groups (P > .05). The initial GCS score of the TBI group was 5.64 ± 1.7, which was significantly lower than that of the control group. The ICP of the TBI group was 22.1 ± 7.6 mmHg, which was significantly higher than that of the control group (6.8 ± 1.4 mmHg) (P < .05). On the other hand, there was no significant difference in the CPP between the TBI (61.4 ± 17.8 mmHg) and the control group (59.8 ± 9.2 mmHg) (P > .05). In the TBI group, there were 12 (26.7%) patients with an unfavorable outcome and 33 (73.3%) with a favorable outcome. The initial CT findings of the TBI group were II (11, 24.4%), III (14, 31.3%), IV (11, 24.4%), V (5, 11.1%), and VI (4, 8.9%) (Table 1).
Table 1.
Clinical summary of patients’ data.
3.2. Levels of apoptotic factors in the CSF
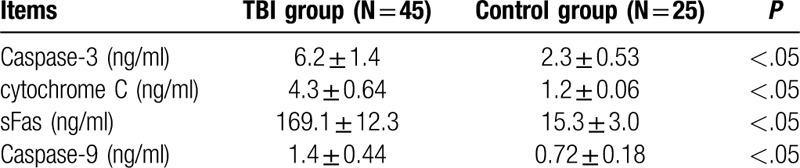
The CSF levels of caspase-3, cytochrome c, sFas, and caspase-9 in the TBI group were 6.2 ± 1.4 ng/ml, 4.3 ± 0.64 ng/ml, 169.1 ± 12.3 ng/ml, and 1.4 ± 0.44 ng/ml, respectively. The CSF levels of caspase-3, cytochrome c, sFas, and caspase-9 in the control group were 2.3 ± 0.53 ng/ml, 1.2 ± 0.06 ng/ml, 15.3±3.0 ng/ml, and 0.72 ± 0.18 ng/ml, respectively. Hence, the levels of all the above apoptotic factors were significantly higher in the TBI than in the control group (P < .05) (Table 2).
Table 2.
The caspase-3 and cytochrome C levels in control and TBI groups.
3.3. Multivariate logistic regression
The above results showed that there was a significant difference between the two groups in the initial GCS, ICP, initial CT findings, and apoptotic factors. Consequently, we analyzed the association between the GOS and the initial GCS score, ICP, initial CT findings, and apoptosis, using a bivariate logistic regression model. The results showed that ICP and caspase-3 were significant predictors of outcome at 6 months post-TBI (P < .05) (Table 3). The initial GCS score, initial CT findings, and other apoptotic factors did not significantly predict the GOS in the multivariate model.
Table 3.
Multivariate logistic regression result for GOS.

3.4. ICP and caspase-3 are promising TBI outcome biomarkers
Next, we determined whether ICP and caspase-3 are reliable biomarkers for TBI outcome. The area under the ROC curve (AUC) for ICP was 0.925, whereas that for caspase-3 was 0.888, which indicated that both ICP and caspase-3 are reliable biomarkers for TBI outcome. We also sought to understand whether ICP and caspase-3 in combination was better at predicting TBI outcome than the two factors taken separately. The AUC for the combined prediction was 0.978, with a specificity and sensitivity of 96.0% and 95.2%, respectively, showing that the combined prediction was even more reliable than that of ICP or caspase-3 alone (Table 4, Fig. 1).
Table 4.
ROC analysis results.
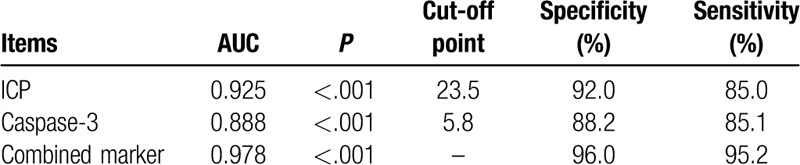
Figure 1.
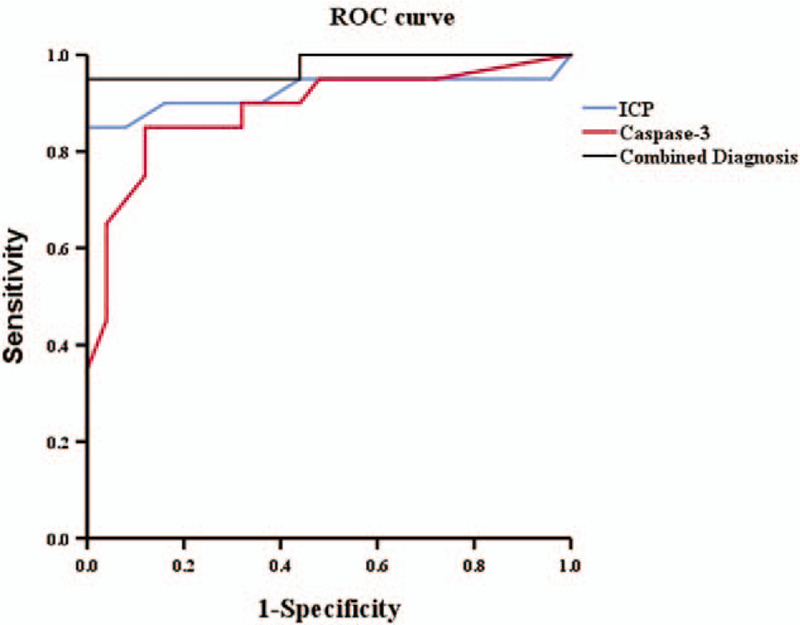
The ROC curve of ICP, caspase-3. The area under the curve (AUC) for ICP was 0.925, and caspase-3 was 0.888. The AUC for the combined marker was 0.978, which showed that the combined marker was more reliable than that separately.
4. Discussion
We demonstrated that the levels of 4 key apoptotic molecules, namely caspase-3, cytochrome c, sFas, and caspase-9, were significantly increased in the CSF of patients following severe TBI. The logistic regression results showed that ICP and caspase-3 were significant predictors of outcome 6 months after TBI. Furthermore, we found that the combination of ICP and caspase-3 represented a better biomarker for TBI outcome than the two separately.
There have only been a few reports investigating the intrathecal release of sFas, caspase-9, or caspase-3 in patients with TBI through serial CSF measurements.[6,9,11,12] Ertel et al and Lenzlinger et al demonstrated a prolonged presence of sFas in the CSF, for up to two weeks after head trauma.[13,14] Previous studies had shown a significant correlation between sFas, cytochrome c, and caspase-9 with ICP or CPP.[15–17] In our work, the increased concentrations of caspase-3, cytochrome c, sFas, and caspase-9 in the CSF of patients shortly after trauma led us to speculate that the increasing ICP may correlate with the levels of these apoptotic factors.
In this study, we found that caspase-3 was a significant predictor of outcome in TBI patients except ICP. Hence, caspase-3 was the only GOS predictor among the analyzed apoptotic factors. Previous studies had shown that the CSF concentration of caspase-3 significantly correlated with the ICP, indicating that the CSF caspase-3 levels closely correlated with the extent of brain damage. In addition, in some studies, western blot analysis revealed that active caspase-3 expression was significantly increased starting 48 hours post-injury, and persisted until the end of day 5.[16,18] Both intrinsic and extrinsic apoptotic pathways activate caspase-3 and lead to cell death. Caspase-3 cleaves DNA fragmentation factor subunit alpha (DFFA), also known as Inhibitor of caspase-activated DNase (ICAD), thus activating it and triggering DNA fragmentation during apoptosis. In rat models, the administration of caspase-3 inhibitors reduced caspase-3 activity and apoptosis in brain tissue. Thus, from a therapeutic perspective, the use of modulators of apoptotic activity by caspase-3 inhibition could represent a new treatment option for TBI.[19,20]
CSF sampling has been frequently used as a minimally invasive method, and a readily available source, to draw conclusions on the state of brain injuries and to obtain markers of predictive or diagnostic value. Besides various cytokines indicating ongoing inflammatory processes, increased concentrations of many apoptotic factors have also been detected.[21–24] In our study, we found that both ICP and caspase-3 are reliable biomarkers of TBI outcome. Furthermore, the combination of the two is even more reliable.
5. Conclusion
We demonstrated that the levels of caspase-3, cytochrome c, sFas, and caspase-9 were significantly increased in the CSF of patients following severe TBI. Furthermore, ICP and caspase-3 were significant predictors of TBI outcome. Finally, the combination of ICP and caspase-3 was more reliable, as a TBI outcome predictor, than the two factors alone.
Author contributions
Wenqing Jiang conducted the study and drafted the manuscript. Peng Jin, Wenfeng Wei, and Wei Jiang participated in the design of the study and performed statistical analyses. All of the authors read and approved the final manuscript.
Footnotes
Abbreviations: AUC = area under the receiver operator characteristic curve, CPP = cerebral perfusion pressure, CSF = cerebrospinal fluid, GOS = Glasgow outcome scale, ICP = intracranial pressure, ROC = receiver operator characteristic, TBI = traumatic brain injury.
How to cite this article: Jiang W, Jin P, Wei W, Jiang W. Apoptosis in cerebrospinal fluid as outcome predictors in severe traumatic brain injury: an observational study. Medicine. 2020;99:26(e20922).
This work was supported by the Changzhou Key R & D Program (CJ20180006), Wujin Science and Technology Support Program (WS201815), Clinical Science and Technology Foundation of Jiangsu University (JLY20180085).
The authors declare that they have no conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Zhang P, Ye Y, Qian Y, et al. The effect of pyrroloquinoline quinone on apoptosis and autophagy in traumatic brain injury. CNS Neurol Disord Drug Targets 2017;16:724–36. [DOI] [PubMed] [Google Scholar]
- [2].Wong J, Hoe NW, Zhiwei F, et al. Apoptosis and traumatic brain injury. Neurocritical care 2005;3:177–82. [DOI] [PubMed] [Google Scholar]
- [3].Glushakova OY, Glushakov AO, Borlongan CV, et al. Role of caspase-3-mediated apoptosis in chronic caspase-3-cleaved tau accumulation and blood-brain barrier damage in the corpus callosum after traumatic brain injury in rats. J Neurotrauma 2018;35:157–73. [DOI] [PubMed] [Google Scholar]
- [4].Eroglu O, Deniz T, Kisa U, et al. Effect of hypothermia on apoptosis in traumatic brain injury and hemorrhagic shock model. Injury 2017;48:2675–82. [DOI] [PubMed] [Google Scholar]
- [5].Ding K, Xu J, Wang H, et al. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 2015;91:46–54. [DOI] [PubMed] [Google Scholar]
- [6].Kacira T, Kemerdere R, Atukeren P, et al. Detection of caspase-3, neuron specific enolase, and high-sensitivity C-reactive protein levels in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. Neurosurgery 2007;60:674–9. discussion 9-80. [DOI] [PubMed] [Google Scholar]
- [7].Qiu J, Whalen MJ, Lowenstein P, et al. Upregulation of the Fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J Neurosci 2002;22:3504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Satchell MA, Lai Y, Kochanek PM, et al. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J Cereb Blood Flow Metab 2005;25:919–27. [DOI] [PubMed] [Google Scholar]
- [9].Felderhoff-Mueser U, Buhrer C, Groneck P, et al. Soluble Fas (CD95/Apo-1), soluble Fas ligand, and activated caspase 3 in the cerebrospinal fluid of infants with posthemorrhagic and nonhemorrhagic hydrocephalus. Pediatr Res 2003;54:659–64. [DOI] [PubMed] [Google Scholar]
- [10].Wagner AK, Amin KB, Niyonkuru C, et al. CSF Bcl-2 and cytochrome C temporal profiles in outcome prediction for adults with severe TBI. J Cereb Blood Flow Metab 2011;31:1886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harter L, Keel M, Hentze H, et al. Caspase-3 activity is present in cerebrospinal fluid from patients with traumatic brain injury. J Neuroimmunol 2001;121:76–8. [DOI] [PubMed] [Google Scholar]
- [12].Au AK, Aneja RK, Bell MJ, et al. Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. J Neurotrauma 2012;29:2013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ertel W, Keel M, Stocker R, et al. Detectable concentrations of Fas ligand in cerebrospinal fluid after severe head injury. J Neuroimmunol 1997;80:93–6. [DOI] [PubMed] [Google Scholar]
- [14].Lenzlinger PM, Marx A, Trentz O, et al. Prolonged intrathecal release of soluble Fas following severe traumatic brain injury in humans. J Neuroimmunol 2002;122:167–74. [DOI] [PubMed] [Google Scholar]
- [15].Darwish RS, Amiridze NS. Detectable levels of cytochrome C and activated caspase-9 in cerebrospinal fluid after human traumatic brain injury. Neurocritical care 2010;12:337–41. [DOI] [PubMed] [Google Scholar]
- [16].Uzan M, Erman H, Tanriverdi T, et al. Evaluation of apoptosis in cerebrospinal fluid of patients with severe head injury. Acta neurochirurgica 2006;148:1157–64. discussion. [DOI] [PubMed] [Google Scholar]
- [17].Paul R, Angele B, Sporer B, et al. Inflammatory response during bacterial meningitis is unchanged in Fas- and Fas ligand-deficient mice. J Neuroimmunol 2004;152:78–82. [DOI] [PubMed] [Google Scholar]
- [18].Rebolledo RA, Hoeksma D, Hottenrott CM, et al. Slow induction of brain death leads to decreased renal function and increased hepatic apoptosis in rats. J Transl Med 2016;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sun Y, Xu Y, Geng L. Caspase-3 inhibitor prevents the apoptosis of brain tissue in rats with acute cerebral infarction. Exp Therap Med 2015;10:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Clark RS, Kochanek PM, Watkins SC, et al. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem 2000;74:740–53. [DOI] [PubMed] [Google Scholar]
- [21].Zhu M, Feng Y, Dangelmajer S, et al. Human cerebrospinal fluid regulates proliferation and migration of stem cells through insulin-like growth factor-1. Stem Cells Dev 2015;24:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wallisch JS, Simon DW, Bayir H, et al. Cerebrospinal fluid NLRP3 is increased after severe traumatic brain injury in infants and children. Neurocrit care 2017;27:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ding YW, Pan SY, Xie W, et al. Elevated soluble fas and FasL in cerebrospinal fluid and serum of patients with Anti-N-methyl-D-aspartate receptor encephalitis. Front Neurol 2018;9:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen X, Yu X, Wang Y, et al. Soluble Fas/FasLare elevated in the serum and cerebrospinal fluid of patients with neurocysticercosis. Parasitol Res 2017;116:3027–36. [DOI] [PubMed] [Google Scholar]


