Supplemental Digital Content is available in the text
Keywords: leiomyosarcoma, regorafenib, soft tissue sarcoma, synovial sarcoma, vascular sarcoma
Abstract
Background:
Regorafenib, a multitargeted tyrosine kinase inhibitor, proved to be active in patients with soft tissue sarcomas (STS).
Methods:
We conducted an open-label, non-randomized, single-center phase II study in advanced pretreated STS patients. Patients received regorafenib 160 mg daily on days 1 enrule 21 of a 28-day cycle. The primary endpoint was the progression-free survival (PFS) at 8 weeks. Toxicity was registered.
Results:
Between April 2015 and November 2016, 21 patients were enrolled in the trial. A total of 13 out of 21 evaluable patients (61.9%) were progression-free at 8 weeks. Median PFS was 3.8 months (95% CI: 2.1–9.4). Median overall survival was 14.8 months (95% CI: 7.7–27.8). In the intention-to-treat population, we reported a PFS of 66.7% at 3 months (95% CI: 40.4–83.4) and 16.7% at 12 months (95% CI: 4.1–36.5). As per the RECIST criteria, the response rate was 4.7% (1 partial response out of 21 evaluable patients) with a clinical benefit rate of 61.9%; no complete response was observed. Treatment was well tolerated.
Conclusion:
Regorafenib shows signs of clinical activity in patients with advanced STS.
Clinical Trial Registration:
ClinicalTrials.gov NCT02307500.
1. Introduction
Despite the recent approval of new drugs, the prognosis of patients with metastatic soft tissue sarcomas (STS) remains poor, with a median overall survival (OS) of 18–24 months.[1]
Single-agent doxorubicin still represents the standard of care in the first-line metastatic setting. In 2014, the randomized European Organisation for Research and Treatment of Cancer (EORTC) 62012 trial confirmed a lack of benefit with doxorubicin + ifosfamide compared with doxorubicin alone with regards to OS.[2] However, the higher response rate (RR) in the combined arm (26% vs 14%) supports a combined regimen in tumor shrinkage for symptomatic patients. The advantage of doxorubicin has been also confirmed in selected subtypes where an advantage of tailored chemotherapy was hypothesized.[3,4]
In the further-line setting, other drugs proved to be active in STS with specific indications by histotype.[5,6] Besides the standard chemotherapeutic agents, the role of antiangiogenetics has also been investigated. Sunitinib and sorafenib were associated with a benefit in progression-free survival (PFS) in pretreated patients in phase II studies.[7–9] Pazozanib was approved based on the results of the phase III, EORTC-sponsored trial, showing a 3-month improvement in PFS compared with placebo, without a benefit in OS.[10]
Regorafenib (STIVARGA, Bayer, Leverkusen, Germany), an oral multikinase inhibitor, has been also approved by the FDA and the European Medicines Agency (EMA) for the treatment of pretreated metastatic colorectal cancer patients, gastrointestinal stromal tumors and, more recently, hepatocellular carcinoma.[11–13] In STS, the activity of regorafenib has been investigated in a large multicenter randomized double-blind, placebo-controlled phase II study (REGOSARC) of adult patients, showing a benefit in terms of PFS in subtypes other than adipocytic STS; it confirmed the expected toxicity profile of kinase inhibitors.[14]
Based on the activity of regorafenib in different histotypes, we conducted a prospective phase II basket trial in advanced, pretreated, solid tumors, including STS, pancreatic cancer, thymomas, and ovarian cancer (RESOUND trial; NCT02307500).[15] In this paper, we report the results of the STS cohort, which did not include liposarcoma patients as per the results of the REGOSARC trial.
2. Patients and methods
2.1. Study design
In this prospective, open-label, single-arm, non-randomized phase II trial, we investigated the activity and safety of regorafenib in patients with advanced, pretreated solid tumors.
The study was conducted at Humanitas Clinical and Research Center, IRCCS, Rozzano (Milan), Italy. Focusing on STS, we enrolled adult patients affected by inoperable and progressive STS, selecting 3 cohorts of patients: leiomyosarcoma, synovial sarcoma, and vascular STS. Failure of, intolerance or contraindications to anthracyclines was required to enter the study.
Other relevant inclusion criteria were:
-
1.
presence of ≥1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1;
-
2.
Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1;
-
3.
life expectancy ≥12 weeks; and
-
4.
adequate bone marrow function (absolute neutrophil count ≥1500 cells/μl, platelets ≥100,000 platelets/μl and hemoglobin ≥9.0 g/dl), international normalized ratio ≤1.5 × the upper limit of normal (ULN), adequate renal function (normal spot urine analysis, serum creatinine ≤1.5 × ULN, and glomerular filtration rate ≥30 ml/minute/1.73 m2), adequate liver function (aspartate aminotransferase [AST] and alanine aminotransferase [ALT] ≤2.5 × ULN and ≤5.0 × ULN for patients with liver involvement of their cancer), bilirubin (≤1.5 × ULN and alkaline phosphatase ≤2.5 × ULN and ≤5 × ULN with liver involvement of their cancer) and pancreatic function (amylase or lipase ≤1.5 × ULN).
The main exclusion criteria were previous treatment with regorafenib and known contraindications to regorafenib (e.g., active cardiac disease, uncontrolled hypertension or arterial or venous thrombotic events).
The study was approved by the local ethical committee. Written informed consent was obtained from all patients.
This study was registered with ClinicalTrials.gov: NCT02307500.
2.2. Procedures
Patients received regorafenib 160 mg orally (4 tablets of 40 mg) once daily, 3 weeks on and 1 week off on a 28-day cycle. Dose interruptions or reductions were required if any of the following adverse events (AEs) occurred: recurrence of grade 3 arterial hypertension despite adequate anti-hypertensive treatment, grade 3 increases in bilirubin or AST/ALT, or any grade 4 toxicity. In case of significant toxicity, the dose of regorafenib had to be reduced stepwise by 40 mg (1 tablet), with 80 mg being the lowest recommended daily dose.
Treatment with regorafenib was administered until any of the following occurred: progressive disease according to RECIST version 1.1, unmanageable or unacceptable toxicity, death, patients refusal or investigators decision. At the discretion of the investigator, patients with progressive disease could continue treatment, in case of evident clinical benefit.
At baseline, the following procedures were performed: a physical examination; hematological coagulation and biochemistry tests; thyroid function tests; urine analysis; and cardiology evaluation with an ECG and echocardiography. Disease status was assessed by a CT scan of the thorax and abdomen. Tumor assessment was repeated every 2 cycles until intolerance and/or progression and 30 days after the end of the treatment. The responses were also evaluated by Choi criteria.[16]
Treatment-related AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
2.3. Outcomes
The primary endpoint was an 8-week PFS in the intention-to-treat (ITT) population. Central radiological review was required. Secondary endpoints were PFS, OS, RR, and safety.
PFS was calculated from the first day of regorafenib treatment to the date of tumor progression or death, whichever occurred first. Data from patients who were alive without tumor progression were censored at the time of their last radiological assessment. OS was measured from the first day of regorafenib administration until the date of death from any cause. Patients without a date of death were censored at the date of the last contact. RR was assessed by the RECIST version 1.1 and Choi criteria. Safety assessments considered toxicity, AEs, and serious AEs related to the study drug.
2.4. Statistical analysis
The study was designed to reject the null hypothesis of the 8-week PFS rate of ≤25% with a type I error of 0.10 and a statistical power of 80% at the alternative hypothesis of an 8-week PFS of ≥50%. A total of 19 patients were required. Further investigation of regorafenib in this setting would be warranted if ≥8 out of 19 patients (42%) were progression-free at 8 weeks.
Data were summarized by descriptive statistics. PFS and OS were estimated using the Kaplan–Meier method. A P value of <.05 was considered as the limit of statistical significance for all secondary evaluations. All analyses were performed using Statistical Analysis System (SAS) version 9.4.
3. Results
3.1. Study population
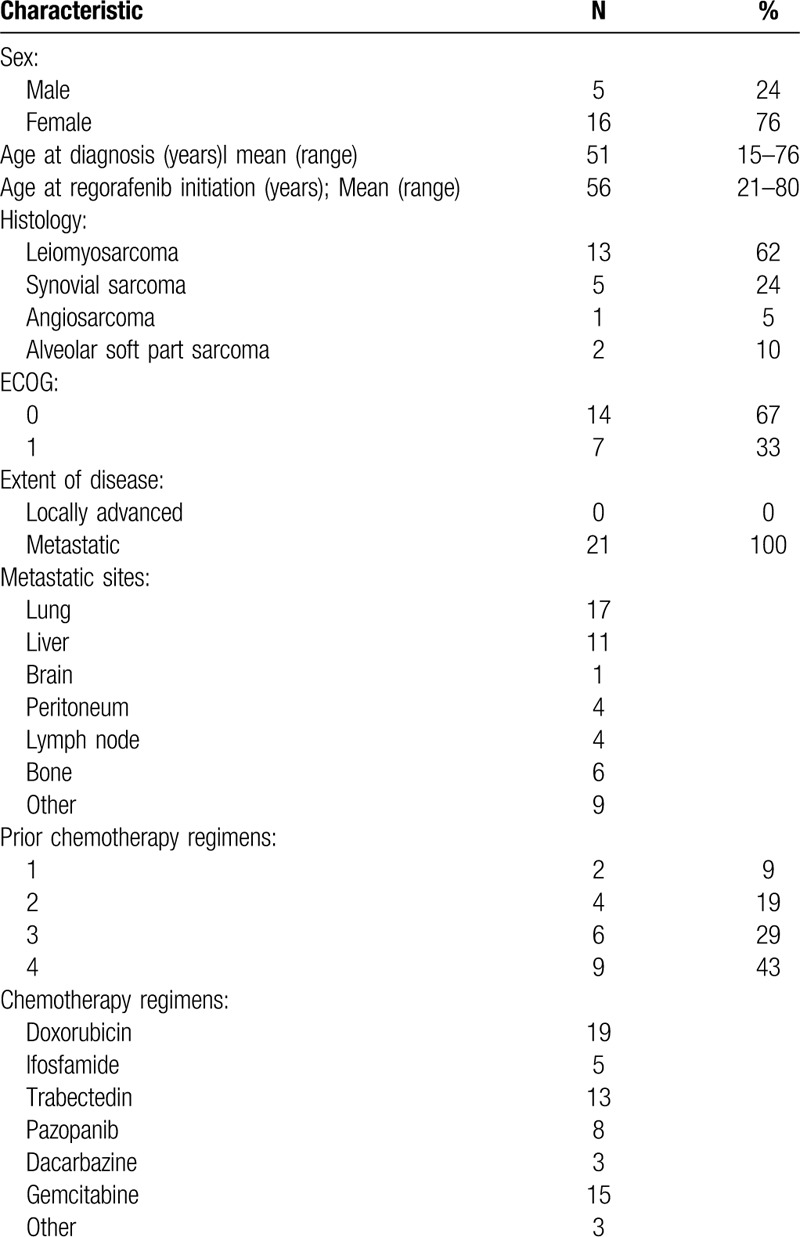
A total of 21 patients were enrolled from April 2015 to November 2016. Patient characteristics are summarized in Table 1, and patients flow is shown in Supplemental Figure 1. In total, 16 patients were female (76%), and the median age at start of treatment was 56 years (range: 21–80). In total, 13 patients had leiomyosarcomas (61%), 5 had synovial sarcomas (24%) and 3 had vascular sarcomas (15%; 1 case of angiosarcoma and 2 cases of alveolar soft part sarcomas). The median number of prior treatments was 3 (range: 1–7). All but 2 patients received prior anthracyclines: patients with alveolar soft part sarcomas did not receive prior chemotherapy because of the known chemo-resistance of the histotype. Seven patients received a prior tyrosine kinase inhibitor (TKI) – specifically, pazopanib (30%).
Table 1.
Patients characteristics.
Out of the 21 patients who were started on regorafenib, 20 were evaluable for safety and 18 for efficacy at 8 weeks. One patient was lost to follow-up after cycle 1 day 1; she died 4 months later and was not evaluable for response and toxicity. Two patients were not evaluable for response: 1 patient discontinued regorafenib during the first cycle due to severe asthenia, anemia, and fever, while the second patient discontinued regorafenib due to severe asthenia and anemia.
3.2. Efficacy
With a median follow-up of 33.5 months (range: 3.5–37.8), 13 out of 21 (62%) patients were progression-free at 8 weeks. In the ITT population, we reported a PFS of 66.7% at 3 months (95% CI: 40.4–83.4) and 16.7% at 12 months (95% CI: 4.1–36.5) (Fig. 1). The median PFS was 3.8 months (95% CI: 2.1–9.4), OS was 100% at 3 months and 57.1% at 12 months (95% CI: 33.8–74.9). Median OS was 14.8 months (95% CI: 7.7–27.8) (Fig. 1).
Figure 1.
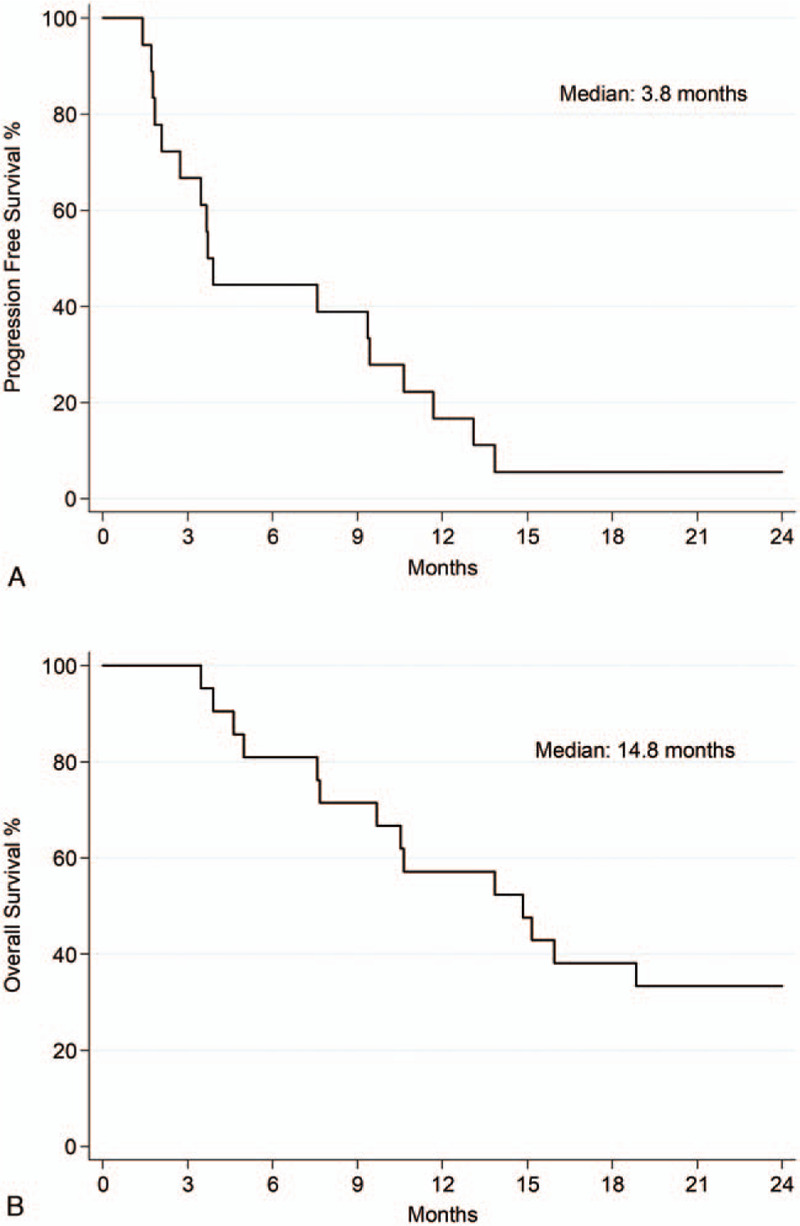
Kaplan–Meier analysis of (A) progression-free and (B) overall survival.
Among the 21 evaluable patients in the ITT population, 1 had partial response (4.8%), 12 had stable disease (57.1%), 5 had progressive disease (23.8%) and no complete responses were seen with a disease control rate of 61.9%. Three out of 21 patients (14%) were not evaluable (see above).
Only 10/21 patients (47%) were evaluable by both RECIST and Choi criteria. This sub-analysis is reported in Figure 2.
Figure 2.
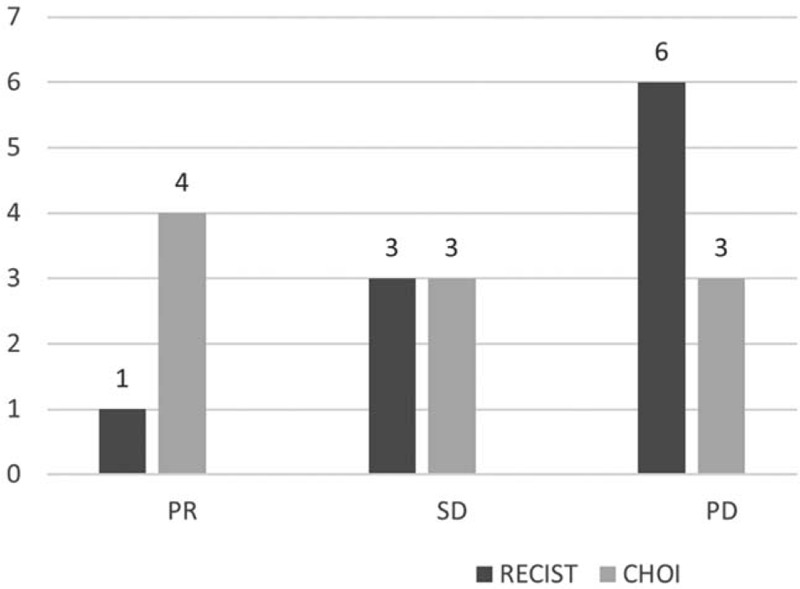
Comparison between RECIST and Choi Criteria. Within this group, 1 PR, 3 SD and 6 PD were seen by RECIST. When Choi criteria were considered, 4 PR, 3 SD and 3 PD were seen. Of note, 1 patient experiencing PD by RECIST had a PR by Choi. PR: partial response; SD: stable disease; PD: progressive disease.
3.3. Safety
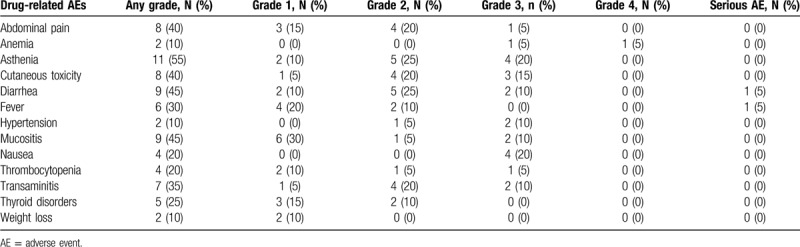
Regorafenib was relatively well tolerated. The median number of cycles per patient was 4 (range: 1–26). The drug-related toxicities occurring in >10% of patients are reported in Table 2. The most common toxicities were asthenia (11/20, 55%), diarrhea (9/20, 45%), mucositis (9/20, 45%), abdominal pain (8/20, 40%), skin toxicity – including hand–foot syndrome (8/20, 40%), and transaminitis (7/20, 35%). Six patients required dose reduction due to hypertension (n = 2), high values of transaminases (n = 1), hyperbilirubinemia (n = 1), allergic reaction (n = 1), and asthenia (n = 2).
Table 2.
Drug-related adverse events.
Reasons for end of treatment were disease progression (11/21, 52.4%), safety (5/21, 23.8%), death (1/21, 4.8%), non-adherence (1/21, 4.8%), medical decisions (1/21, 4.8%), and unknown reasons (2/21, 9.5%).
4. Discussion
Our results show that regorafenib is active in patients with non-adipocytic pretreated advanced STS. We met the primary endpoint, with 13 out of 21 (62%) patients being progression-free at 8 weeks.
The management of advanced STS is still a medical challenge due to the rarity and heterogeneity of the disease. In fact, STS include more than 70 histotypes with peculiar biology, unique molecular characteristics, clinical history, and sensitivity to therapies. Despite these issues, the close collaboration between the medical oncologist, surgeon and radiation oncologist is still improving the OS of metastatic STS.
In the last decade, a few drugs have been introduced, providing the medical oncologist with additional therapeutic options. Among chemotherapeutic agents, trabectedin was approved as a second-line treatment for unresectable or metastatic pretreated STS.[5] Similarly, eribulin mesylate was recently approved in unresectable or metastatic liposarcoma after anthracycline-containing regimen.[6] Beyond damaging DNA, these new drugs act on the microenvironment, angiogenesis, immune infiltrate, and cell differentiation, contributing to tumor control.
Kinase inhibitors also act on the tumor cell and on the tumor microenvironment. Sorafenib has been the first TKI reporting activity in STS, by inhibiting tumor cell proliferation and angiogenesis by RAF, VEGFR2 and VEGFR3, and PDGFR-β. Its activity was reported for the first time in 2009, suggesting a role in recurrent or metastatic STS patients, with a remarkable response rate (14%) in angiosarcoma, a median PFS of 3.2 months and a median OS of 14.3 months.[9] We also conducted a clinical trial with sorafenib in advanced STS involving 101 patients (36 leiomyosarcoma, 19 angiosarcoma, and 46 with other histotypes). The study was positive with a 6-month PFS of 27.1% in leiomyosarcoma, 35% in angiosarcoma, and 35.5% in “other” histologies.[8] In 2012, the FDA approved the first TKI in STS – pazopanib – in patients with non-adipocytic pretreated advanced STS. The approval was based on the improvement in PFS of 3 months vs placebo (4.6 vs 1.6 months), without any difference in OS.[10]
Regorafenib is an oral, small-molecule, multi-kinase inhibitor, targeting signaling pathways implicated in tumor angiogenesis (VEGFR1–3 and TIE2), oncogenesis (KIT, RET, RAF1, and BRAF), and homeostasis of the tumor microenvironment (PDFGR and FGFR). Given the multi-kinase inhibitory profile of regorafenib, we decided to explore its activity in pretreated STS in a monoinstitutional phase II study. At the time of the planning of our clinical trial, a large placebo-controlled, double-blind, randomized phase II study was already ongoing carried out by the French Sarcoma Group.[14] In this trial, 182 advanced pretreated STS were enrolled in 4 cohorts – namely, liposarcoma, leiomyosarcoma, synovial sarcoma, and “other”, with a PFS benefit in all but the liposarcoma cohort.
In our trial, we decided to enroll patients with leiomyosarcoma, synovial sarcoma, and vascular sarcoma, histotypes known to be sensitive to kinase inhibition. According to the study objective, we met the primary endpoint with a PFS of 62% at 8 weeks. We also met the STS-EORTC criteria to select compounds worth studying in the setting of metastatic disease.[17] Specifically, regorafenib showed a 3-month PFS of 66.7% and a 6-month PFS of 44.4%. Noteworthy, some of the responses were long lasting (>24 months), even in patients who received prior therapy with a different TKI. In the ITT population, we reported an objective RR of 4.7% (1 PR/21 patients), in line with previous data on regorafenib and pazopanib. However, these results were not representative of the observed clinical benefit. Therefore, in a small subgroup of patients (10 of 21), we tried to assess response by both RECIST and Choi criteria. If RECIST criteria considers dimensional changes only, the Choi criteria also takes into account the modification in tissue density as expected by TKIs.[16] We reported a RR by RECIST of 10% (1 of 10) compared with a RR by Choi of 40% (4 of 10) (Supplemental Figs. 2 and 3).[18] Thus, RECIST criteria down-estimate the response rate, suggesting that RECIST criteria should be routinely integrated by Choi in the setting of TKI.
In terms of safety, regorafenib proved to be manageable and well tolerated. Its toxicity did not seem to differ from the REGOSARC trial,[14] with the exception of a patient with an allergic reaction and 1 patient experiencing grade 2 alopecia. One death due to unknown reason was registered in a patient experiencing stable disease on regorafenib.
5. Conclusion
In the complex scenario of STS, which includes over 70 histological subtypes with unique biology, natural history, and chemosensitivity, regorafenib appears to be a promising option in patients with advanced disease. Further studies are needed to confirm our results in terms of activity. The identification of predictive characteristics of clinical benefit, such as histology or molecular signatures, as well as an early tissue response assessed by Choi criteria, is strongly needed to personalize the treatment of STS.
Acknowledgments
Editorial assistance was provided by Luca Giacomelli, PhD, and Aashni Shah (Polistudium SRL, Milan, Italy) and was supported by internal funds.
Author contributions
AS conceived and designed the study; analyzed and interpreted data; prepared, edited and reviewed the manuscript. AM, AB, and SB conceived and designed the study; acquired, controlled, analyzed and interpreted data and algorithms; prepared, edited and reviewed the manuscript. NG, RDS and SS acquired, controlled, analyzed and interpreted data, edited and reviewed the manuscript. VQ and LB interpreted data and reviewed the manuscript; prepared, edited and reviewed the manuscript. LG controlled, analyzed and interpreted data and algorithms, performed statistical analysis, and helped in preparing, editing and reviewing the manuscript.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AE = adverse events, ALT = alanine aminotransferase, AST = aspartate aminotransferase, ECOG = Eastern Cooperative Oncology Group, EMA = European Medicines Agency, EORTC = European Organisation for Research and Treatment of Cancer, ITT = intention-to-treat, OS = overall survival, PFS = progression-free survival, PS = performance status, RECIST = Response Evaluation Criteria in Solid Tumors, RR = response rate, SAS = Statistical Analysis System, STS = soft tissue sarcoma, TKI = tyrosine kinase inhibitor, ULN = upper limit of normal.
How to cite this article: Marrari A, Bertuzzi A, Bozzarelli S, Gennaro N, Giordano L, Quagliuolo V, De Sanctis R, Sala S, Balzarini L, Santoro A. Activity of regorafenib in advanced pretreated soft tissue sarcoma: results of a single-center phase II study. Medicine. 2020;99:26(e20719).
AM and AB contributed equally.
SB Reprint requests.
This investigator-sponsored trial (IST) was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from every patient at study inclusion. This study is registered at ClinicalTrials.gov NCT02307500.
Data are available from the corresponding author upon reasonable request.
Dr. Santoro reports personal fees and other from BMS, Servier, Gilead, Pfizer, Eisai, Bayer, MSD, and Arqule, other from Takeda, Roche, Abbvie, Amgen, Celgene, AstraZeneca, Lilly, Sandoz, and NovarTIS, outside the submitted work. R. De Sanctis: EISAI, Novartis, Kyowa-Kirin. All the others have no conflicts of interest to report.
Bayer S.p.A (Milan, Italy) provided the drug for the study and financial support. The company had no role in the conception of the trial, data collection, and analysis, interpretation of results, in the writing of the manuscript or the decision to publish. All drafts of the report were prepared by the corresponding author with input from all co-authors. All authors had full access to study data upon request and all authors had final responsibility for the decision to submit for publication.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Casali PG, Abecassis N, Aro HT, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv51–67. [DOI] [PubMed] [Google Scholar]
- [2].Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15:415–23. [DOI] [PubMed] [Google Scholar]
- [3].Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol 2017;18:1397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812–22. [DOI] [PubMed] [Google Scholar]
- [5].Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629–37. [DOI] [PubMed] [Google Scholar]
- [7].George S, Merriam P, Maki RG, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol 2009;27:3154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Santoro A, Comandone A, Basso U, et al. Phase II prospective study with sorafenib in advanced soft tissue sarcomas after anthracycline-based therapy. Ann Oncol 2013;24:1093–8. [DOI] [PubMed] [Google Scholar]
- [9].Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009;27:3133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. [DOI] [PubMed] [Google Scholar]
- [11].Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- [12].Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- [14].Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:1732–42. [DOI] [PubMed] [Google Scholar]
- [15]. Regorafenib in Patients with Metastatic Solid Tumors Who Have Progressed After Standard Therapy (RESOUND). https://clinicaltrials.gov/ct2/show/NCT02307500. [Google Scholar]
- [16].Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753–9. [DOI] [PubMed] [Google Scholar]
- [17].Van Glabbeke M, Verweij J, Judson I, et al. EORTC soft tissue and bone sarcoma group. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer 2002;38:543–9. [DOI] [PubMed] [Google Scholar]
- [18].Martin-Broto J, Stacchiotti S, Lopez-Pousa A, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2019;20:134–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


