Abstract
Purpose:
Oxidative stress has been shown to reflect on the development of sepsis and disease severity. In the present study, we evaluated the effects of increased levels of oxidative stress and decreased antioxidant coactivity in patients with sepsis, and the importance of oxidative stress on treatment outcomes.
Methods:
Biomarkers of oxidative stress (thiobarbituric acid-reactive substances [TBARS]) and antioxidant capacity (glutathione peroxidase [GPx] and glutathione content [thiol]) were prospectively evaluated along with biochemical and clinical data in 100 patients with sepsis on days 1, 4, and 7 after admission.
Results:
The TBARS level of the non-survivor group was significantly higher than that of the survivor group on day 1 and day 4 and negatively correlated with thiol upon admission. However, thiol was positively correlated with lactate concentration. The TBARS and lactate levels upon admission were independent predictors of fatality.
Conclusions:
We conclude that a TBARS cut-off value of 18.30 μM can be used to predict fatality, and an increase in the TBARS concentration by 1 μM will increase the fatality rate by 0.94%. In the panel of biomarkers, the TBARS assay can be considered as a prognostic biomarker for the treatment of patients with sepsis.
Keywords: outcome, oxidative stress, sepsis, TBARS
1. Introduction
Sepsis has been a great challenge and is a topic of interest in critical care medicine despite improvements in modern treatment owing to its high mortality rate. Current studies have shown that pro-inflammatory mediators and oxidative stress directly caused a dysfunction of the enzyme complexes of the respiratory chain and thereby nucleic acid, protein, and lipid metabolism disorders and various pathophysiological impairments.[1] The increased superoxide production contributed to the remission stage of oxidative damage in several ischemic organs or tissues, mainly in the early hours or days after the diagnosis of sepsis, causing high oxidative stress and low antioxidant potential activity.[2]
Thiobarbituric acid-reactive substances (TBARS), which are lipoperoxidation markers for multi-organ failure or disease progression, have been used to indicate oxidative stress status in several experiments.[3–7] In addition, the antioxidant system in critically ill patients, such as some endogenous antioxidants (e.g., glutathione peroxidase [GPx] and reduced glutathione [thiol]), have been used to indicate antioxidative status. These biomarkers are considered indicators of disease severity. However, the majority of studies investigating this had a small sample size,[3–5,8] enrolled neonatal patients, and did not use precise methods in selecting patients who were admitted to the emergency department (ED) or intensive care unit (ICU).[9,10] Moreover, the studies included patients with several underlying diseases[4] with only 1 blood sample from each patient was obtained, and significant variations among the patients were observed, which may be explained by rapid changes in the balance of the production and clearance of antioxidants and oxidative contents. Consequently, the results of these studies are conflicting. If the variation followed a standard pattern and temporal relationship, our ability to predict the prognosis of sepsis would improve. In this prospective study, we hypothesized that oxidative stress is the excessive breakdown of immune regulation during sepsis and leads phagocytes to progressively induce cytokines or directly cause host toxicity. Moreover, the effects of increased oxidative stress and decreased antioxidant coactivity in patients with sepsis and the importance of oxidative stress in predicting treatment outcomes were evaluated.
2. Methods
2.1. Study participants and definition
In this prospective study, we enrolled 100 non-trauma and non-surgical adult patients with complete data at the Kaohsiung Chang Gung Memorial Hospital, an acute care teaching hospital in Taiwan between January 2015 and December 2018. For comparison, we also enrolled 27 healthy volunteers without clinical evidence of infection for the control group. Our study was approved by the hospitals Institutional Review Committee on Human Research and all patients provided written consent after a thorough explanation of the study. All patients were aged ≥18 years and were screened daily in the ED for the presence of sepsis or septic shock, according to the sepsis criteria defined by the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).[11] All patients with sepsis or septic shock were enrolled in this study if they had a Sequential (Sepsis-related) Organ Failure Assessment (SOFA) score of 2 points or more. Septic shock was defined by a vasopressor requirement to maintain a mean arterial pressure of at least 65 mm Hg and serum lactate concentration of more than 2 mmol/L (>18 mg/dl) in the absence of hypovolemia. The following exclusion criteria were applied:
-
1.
hematologic disease or undergoing chemotherapy;
-
2.
simultaneous multiple comorbidities that could affect our results, such as combined tumor and diabetes mellitus;
-
3.
admission before the past 28 days,
-
4.
signed a do-not-resuscitate order within 24 hours upon ED admission, and
-
5.
discharged against medical advice and not in a critical condition or transferred to another hospital within 28 days.
Chronic lung disease refers to disorders that affect the lungs and other parts of the respiratory system; they usually develop slowly, deteriorate over time, and include chronic obstructive pulmonary disease, asthma, cystic fibrosis, lung cancer, chronic pneumonia, pulmonary hypertension, or interstitial lung disease, etc.[12] Chronic heart disease refers to various types of chronic diseases that influence heart function, including coronary artery heart disease, valvular heart disease, cardiomyopathy, and chronic arrhythmia.
2.2. Clinical evaluation and therapy
We recruited patients within 24 hours of admission to the ED of the Kaohsiung Chang Gung Memorial Hospital. The medical records were prospectively recorded using standardized evaluation forms to collect demographic data, Acute Physiology and Chronic Health Evaluation (APACHE) II score,[13] and the SOFA score, which were estimated within the first 24 hours while the admitted patient was in the ED (day 1) to identify the disease severity and organ malfunction. Data on the infection origin, blood culture pathogens, administration of antibiotics, and the progression of various organ dysfunctions and supportive management were recorded. Consultation with an infectious disease specialist to identify an appropriate anti-microbial therapy for infectious etiologies during the early 24 hours, which is an institutional practice, was performed. Although the benefits of steroid administration for septic shock are not clear,[14–16] we nonetheless prescribed low-dose hydrocortisone to critically ill patients if their conditions did not be improved after fluid resuscitation or vasopressor use. The worst value for selected vital signs, such as systolic blood pressure and pulse rate, were calculated as parameters in SOFA and APACHE II scores within 24 hours upon ED admission. Moreover, vitamin C and E, N-acetylcyceine, thiamine, or other recognized as anti-oxidant were forbidden in our patients.
2.3. Assessment of infection biomarkers
All of our infection biomarker tests were conducted at the hospitals central laboratory. Based on well-established methods,[17,18] the lactate level as well as the level of several inflammatory markers, including white blood cells (WBC), platelets, bilirubin, plasma C-reactive protein (CRP), and procalcitonin, were measured early in the ED. CRP was assessed using enzyme immunoassay, procalcitonin using enzyme-linked fluorescent assay, and lactate using a serum-based assay that was catalyzed by lactate oxidase.
2.4. Blood sampling and assessment of oxidative stress status biomarkers
After enrollment, blood samples of patients were collected on the day of admission (day 1), day 4, and day 7. Furthermore, blood samples were collected once from subjects in the control group. Specimens were collected by venipuncture of forearm veins from patients in both groups. Markers of oxidative stress (TBARS) were assessed using blood samples and quantified with commercially available kits. Blood samples were collected in sterile tubes and then centrifuged at 3000 rpm for 10 minutes. The serum was stored at −70°C until processing.
2.4.1. Glutathione peroxidase (GPx) activity
We measured GPx activity with a commercially available kit (Ransel, Randox Laboratories, Crumlin, UK). Erythrocyte samples were diluted to convert GPx to its reduced form. The samples were incubated for 5 to 10 minutes and diluted with Drabkins Reagent (Sigma-Aldrich, France) to avoid false elevation due to the presence of peroxidases in human blood. The diluted sample was mixed with reagents (including glutathione, glutathione reductase, and nicotinamide-adenine dinucleotide phosphate [NADPH]) and cumene hydroperoxide. GPx catalyzed the oxidation of reduced glutathione using cumene hydroperoxide. In the presence of glutathione reductase and NADPH, the oxidized glutathione (GSSG) was immediately converted to its reduced form, with a concomitant oxidation of NADPH to NADP+. Decreased absorbance was measured at a wavelength of 340 nm after 1 to 2 minutes and expressed in units per liter of hemolysate and multiplied by the appropriate dilution factor to obtain units per liter of whole blood.
2.4.2. Reduced glutathione (thiol) content
The patients antioxidative defense in response to increased oxidative damage was evaluated by measuring the serum level of reduced glutathione (thiol) as serum glutathione is a physiological free radical scavenger. The total serum protein glutathione was estimated by directly reacting glutathione with 5,5′-dithiobis (2-nitrobenzoic acid, DTNB) to form 5-thio-2-nitrobenzoic acid (TNB). The amount of glutathione in each sample was calculated from the absorbance using the extinction coefficient of TNB (A412 = 13,600 M−1 cm−1).
2.4.3. Thiobarbituric acid-reactive substances (TBARS)
As the measurement of TBARS is a well-established method for detecting lipid peroxidation, this study used the TBARS Assay Kit for the rapid photometric detection of the thiobarbituric acid-malondialdehyde (TBA-MDA) adduct at 532 nm, as described by the manufacturer (CAT No. 10009055; Cayman Chemical, Ann Arbor, MI, USA). Briefly, 100 ml of serum was added in duplicates to 100 ml of sodium dodecyl sulfate and 4 ml of color reagent. The reaction mixtures were then incubated for 1 hour in boiling water. After cooling on ice, samples were centrifuged at 1600 g for 10 minutes at 4°C. After warming at 25°C for 5 minutes, the absorbance of the supernatants was measured on a microplate spectrophotometer at 532 nm. Blanks for each sample were prepared and assessed in the same way to correct for the contribution of background noise to A532 values of the samples. The values of the samples were calculated from a linear calibration curve that was prepared using pure MDA-containing samples (range: 0–50 μmol/L).
2.5. Outcome determination
Patients with sepsis-associated diseases were divided into 2 groups (survivors and non-survivors) according to the endpoint of 28-day mortality rate. Physicians evaluated the relationship between the concentration of oxidative stress biomarkers, disease severity scores, and mortality rate daily.
2.6. Statistical analysis
We performed a normality test on our data to establish the variable distribution. Data were expressed as mean ± standard deviation (SD). We conducted univariate analyses using the Students t test, while categorical variables were evaluated using the χ2 test or Fisher exact test. We carried out a correlation analysis with the Pearson correlation test to detect the relationship between 24-hour APACHE II scores, CRP, lactate, procalcitonin, GPx activity, thiol, and TBARS levels on admission. Repeated measurements through analysis of variance (ANOVA) were conducted to compare results at 3 intervals (within 24 hours or day 1, and on days 4 and 7). We used stepwise logistic regression to explore the relationships between significant variables and outcomes, adjusting for potential interfering factors. Then we determined variables with no cell count in a 2 × 2 table in a logistic analysis, and only those that were significantly associated with mortality (P < .05 was considered significant) were retained in the final statistical test. Receiver operating characteristic (ROC) curves were produced for the predictors of death while in the hospital (mortality). The area under the curve (AUC) was determined and compared for significant parameters.
3. Results
3.1. Baseline characteristics of patients with sepsis and patients in the control group
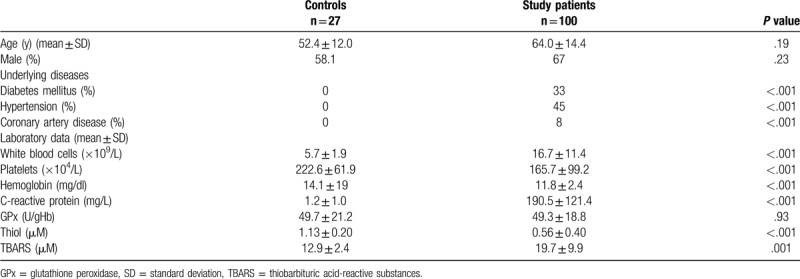
As described in Figure 1, although 149 patients met our criteria, 49 patients were excluded for the following reasons: 9 patients signed a do-not-resuscitate order within 24 hours of ED admission, 12 patients were transferred to another hospital or discharged against medical advice (not in critical condition), 16 patients withdraw from our study, and 8 patients died within 7 days with loss series data. A total of 100 sepsis cases and 27 healthy controls were enrolled in the study. Demographic data of the patients enrolled in the study are listed in Table 1. Patients with sepsis had significantly higher levels of WBC and C-reactive protein and lower levels of platelets and hemoglobin than the controls. Furthermore, patients with sepsis had significantly higher median serum TBARS levels (19.7 ± 9.9 vs 12.9 ± 2.4 μM; P < .001) and lower median serum thiol levels (.56 ± .40 vs 1.13 ± .20 μM; P = .001) than patients in the control group.
Figure 1.
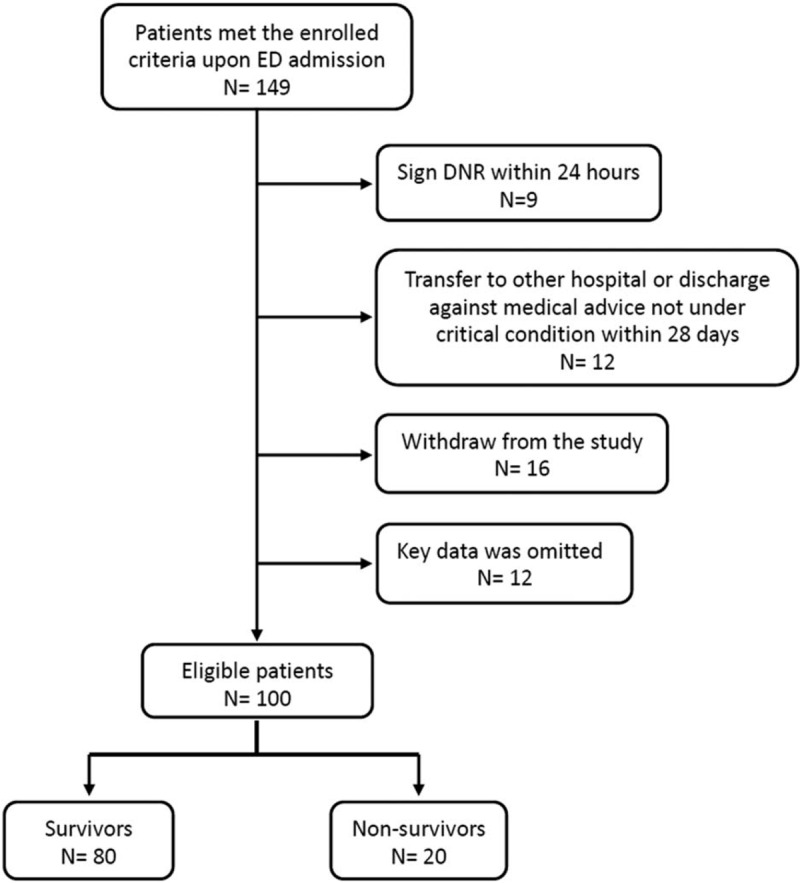
Flow chart of the patients selected for the study.
Table 1.
Baseline characteristics of patients with sepsis and controls.
3.2. Comparison of the survival and non-survival characteristics in sepsis patients
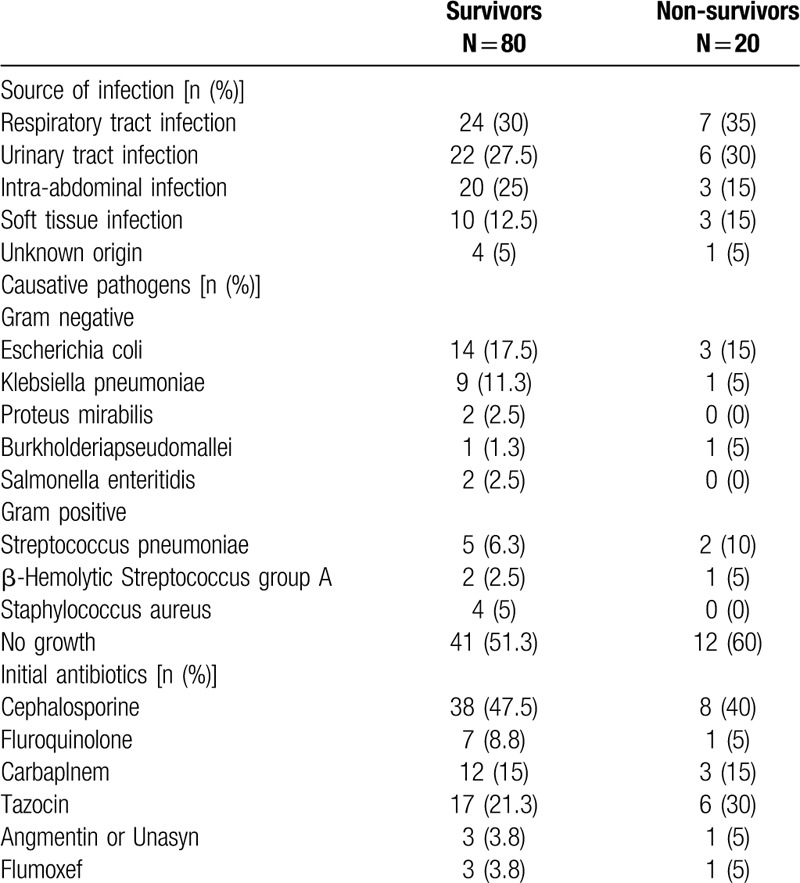
The baseline characteristics of the survivor and non-survivor groups are shown in Table 2. Among the 80 survivors with sepsis, 69.9% (54/80) had septic shock after admission, and among the 20 non-survivors, 75% (15/20) had septic shock. Moreover, 48.8% (39/80) of patients in the survivor group and 40% (8/20) of patients in the non-survivor group had bacteremia. As listed in Table 3, the most common primary infection site was the respiratory tract in both survivors and non-survivors (30% [24/80] and 35% [7/20], respectively). The microbiologic findings showed that the majority of the isolates cultured were gram-negative microorganisms. Escherichia coli was found in both survivors and non-survivors (17.5% [14/80] vs 15% [3/20], respectively), although the cultures in 41% (51.3) of the survivors and 60% (12/20) of the non-survivors were devoid of any pathogen. Antimicrobic agent analysis showed that cephalosporine was the most prescribed antibiotic in both survivors and non-survivors (47.5% [38/80] vs 40% [8/20], respectively). Based on the laboratory findings, non-survivors had significantly higher TBARS levels and plasma lactate levels upon admission than the survivors (25.0 ± 9.7 vs17.0 ± 8.8 μM, P = .001; 51.4 ± 37.7 vs 31.5 ± 18.8 mg/dl, P = .03, respectively).
Table 2.
Characteristics of survival and non-survival groups among patients with sepsis.
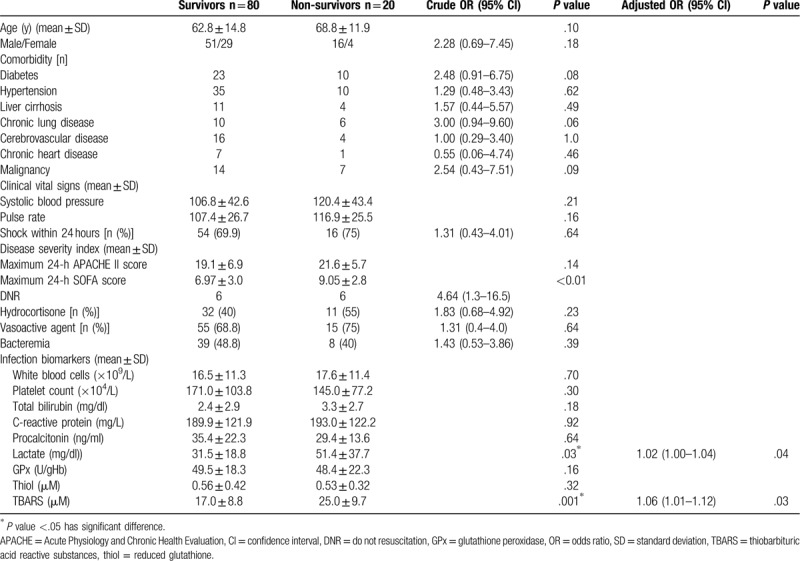
Table 3.
Infection sources, bacteremia pathogens and initial antibiotics in sepsis group.
3.3. Correlation between oxidative stress status and antioxidative capacity, other infection parameters, and disease severity score
A correlation analysis was carried out to evaluate the effects of TBARS on the biomarkers of antioxidative capacity, infection parameters, and disease severity score in patients with sepsis. The statistical results (correlation coefficient, P-value) are shown in Table 4. The mean TBARS level was significantly negatively correlated with thiol level (γ = −.22, P < .05) and positively correlated with lactate concentration (γ = .26, P < .05) and CRP (γ = .23, P < .05). No significant correlation was observed between TBARS level and GPx (γ = −.23, P = .07), WBC (γ = .04, P = .69), platelet count (γ = −.12, P = .28), procalcitonin (γ = −.20, P = .07), and the maximum 24-hour APACHE score (γ = .09, P = .40).
Table 4.
Correlation analysis between TBARS levels, other biomarkers, and clinical severity indexes.
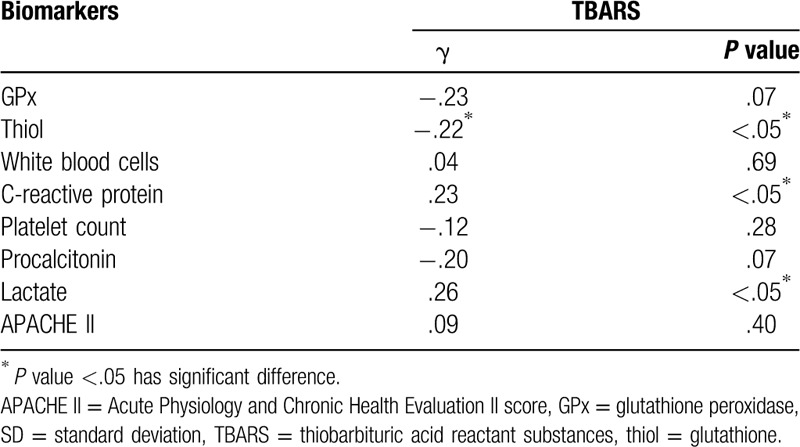
3.4. Serial changes in circulating oxidative stress and antioxidative capacity between survivors and non-survivors
Serial changes in the biomarkers of oxidative status and antioxidative capacity of the survivors and non-survivors among patients with sepsis and those in the control groups are presented in Figure 2. Results show that non-survivors had significantly higher TBARS concentrations than survivors on day 1 (26.1 ± 10.1 vs 18.0 ± 9.2 μM, P < . 001) and day 4 (24.8 ± 8.6 vs 18.3 ± 11.1 μM, P = .03) but not on day 7 (23.8 ± 11.5 vs 17.0 ± 10.1 μM, P = .12). Among patients with sepsis, both survivors and non-survivors had significantly lower thiol levels than the controls (0.56 ± 0.42 vs 0.53 ± 0.32 vs 1.13 ± 0.20 μM, P < .05, respectively) on admission. However, there was no significant difference in the thiol concentration between survivors and non-survivors at any point in time. In addition, no significant differences in the GPx concentration were observed on day 1 or at any other point among the survivors, non-survivors, and controls. Moreover, repeated measurements using ANOVA with Scheffe multiple comparison test demonstrated that TBARS levels of the 2 severely infected groups (survivors vs non-survivors) were significantly different (P < .001) at different times (days 1, 4, and 7)
Figure 2.
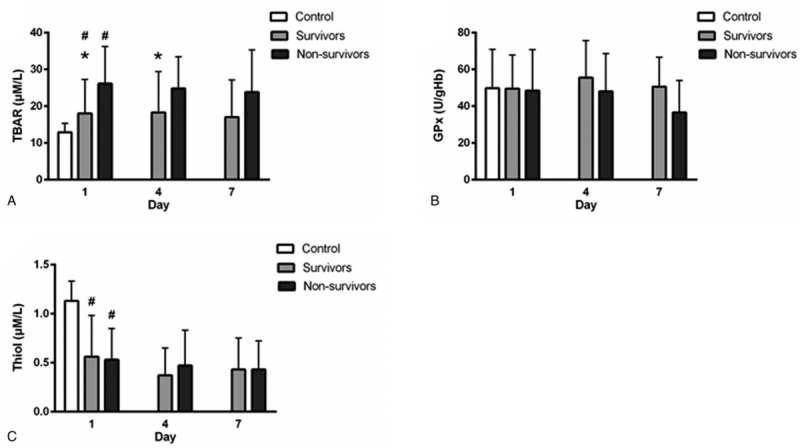
Levels of oxidative stress parameters on diverse days in patients with sepsis and patients in the control group. #P < .05, patients with sepsis vs controls; ∗P < .05, survivors vs non-survivors. (a) Serum TBARS (thiobarbituric acid-reactive substances), (b) Serum GPx (glutathione peroxidase), (c) Thiol (glutathione).
3.5. Predictive factors of the clinical outcomes
Twenty of the 100 (20%) patients with sepsis died in the hospital. The potential prognostic factors of the 100 patients with sepsis are listed in Table 2. Statistical analysis of the clinical manifestations and laboratory data of the survivors and non-survivors measured on admission showed that only 2 variables were significant: TBARS (P = .001) and lactate level (P = .03). After performing a stepwise logistic regression analysis, the TBARS and lactate concentrations measured on admission were determined to be both independently predictive of fatality. The AUC for TBARS and lactate levels were 0.74 (95% confidence interval [CI], .64–.85; P = .001) and 0.69 (95% CI, .56–.82; P = .01), respectively (Fig. 3). Moreover, the cut-off values for predicting mortality were 18.30 μM (sensitivity, 75%; specificity, 62.5%) and 32.6 mg/dl (sensitivity, 75%; specificity, 64%), respectively.
Figure 3.
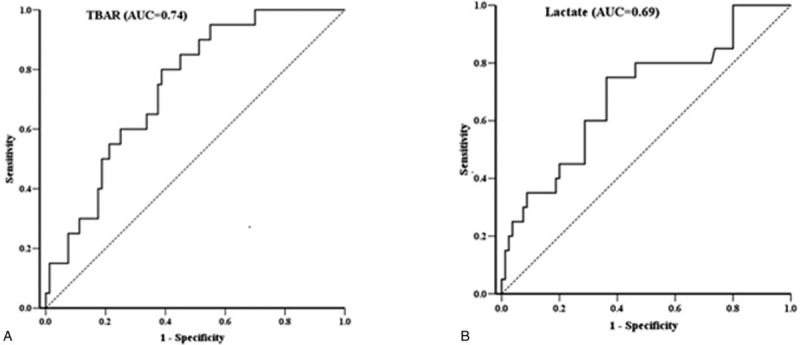
Receiver operating characteristic (ROC) curve for antioxidant and inflammatory parameters. The diagnostic accuracy of biomarkers displayed after analyzing the ROC curve. (a) Serum TBARS (thiobarbituric acid-reactive substances), (b) Plasma lactate; area under the curve.
4. Discussion
This study evaluated the serial changes in oxidative stress status as changes in biomarkers among patients with sepsis. Consequently, 5 major findings were obtained. First, patients with sepsis displayed significantly higher serum TBARS and lower serum-reduced glutathione and thiol levels in comparison to the healthy controls. Second, TBARS and lactate concentration were significantly higher in non-survivors with sepsis than in survivors. Third, TBARS levels were significantly negatively correlated with thiol levels and positively correlated with lactate level and CRP concentration. Fourth, the serial TBARS concentrations between the non-survivor and survivor groups were significantly different, with the non-survivor group displaying significantly higher TBARS levels on day 1 and day 4 in comparison to the survivor group. Finally, TBARS and lactate levels measured on admission were shown to be independent predictors of fatality. The cut-off value of TBARS that predicted fatality was 18.30 μM (sensitivity, 75%; specificity, 62.5%), and an increase in the TBARS concentration by 1 μM increased the fatality rate by .94%. Based on our results, the levels of the lipid peroxidation biomarker, TBARS, seem to meet the requirements for outcome prediction in the treatment of sepsis. Thus, the TBARS assay can be considered a prognostic marker that can be added to the panel of conventional sepsis parameters.
4.1. Oxidative stress and antioxidative capacity during sepsis and relevant biomarkers
Several complex mechanisms are involved in sepsis pathogenesis. When the pathogen invades the host, neutrophils and macrophages produce inflammatory cytokines, reactive oxygen species (ROS), and reactive nitrogen species in response to inflammation.[19] A resulting imbalance between oxidant and antioxidant defenses will create oxidative stress, trigger inflammatory responses[20,21] and superoxide-induced endothelial cell dysfunction, and increase vascular permeability,[22] resulting in organ cytotoxicity.[1]
Due to the concentrations of free radical products being difficult to examine, measurements of these biomarkers have relied on quantifying byproducts of macromolecule oxidation, such as lipid peroxidation or protein oxidation. Considering that previous studies have shown a good prognostic predictive ability and stable end products of oxidative biomarkers, and that they are clinically easy to detect with high validity, we selected TBARS to evaluate oxidative stress status in septic patients. Although there is little evidence that biomarkers of antioxidative capacity are better predictors of the severity or outcome of sepsis, we selected GPx and thiols as biomarkers in this study as they both play important roles in antioxidative capacity. In addition, GPx is a major antioxidant in eliminating H2O2 and reducing lipid oxidation, and thiol provides antioxidative capacity via several mechanisms and is available in serum or plasma.
4.2. TBARS levels are associated with patient outcomes
On admission, TBARS and lactate concentrations were shown to be higher in non-survivors than in survivors, both in our previous study and in other investigations.[4,23,24] Such observations support 2 scenarios. First, sepsis may initiate an inflammatory pathway and induce an imbalance of oxidants and antioxidants, causing oxidative stress. Second, oxidative stress may involve an imbalance in oxygen supply and consumption during sepsis, resulting in anaerobic glycolysis, mitochondrial dysfunction, and lactate production.[25,26]
The absence of significant differences in the serial analysis of thiol and GPx concentrations between survivors and non-survivors in our study may indicate that antioxidants are involved in complex sepsis mechanisms and rapid alternation in multiple cells; thus, biomarkers are often poor predictors of sepsis-related fatalities.[2] There are multiple limitations to using these 2 antioxidative biomarkers. First, the baseline levels of antioxidant activity are influenced by tissue and host heterogeneity. Additionally, antioxidant levels and activity are short-lived and variable. Finally, the methodology for precisely measuring levels of these antioxidants requires expensive instrumentation and lengthy analysis and is highly time-consuming. All these factors combined affect the results of studies examining the effects of antioxidant activity.
In the serial analysis, there was significant discriminative power between survivors and non-survivors on day 1 and day 4, but not on day 7 TBARS concentrations. Additionally, the ANOVA test showed that non-survivors had significantly higher TBARS concentrations than survivors. As a consequence, we could conclude that there were significantly higher TBARS concentrations in non-survivors than in survivors in the serial analysis, and that TBARS could be used to discriminate between survivors and non-survivors in the early course of sepsis.
Although TBARS and lactate levels on admission to the ER were both shown to be independent predictive factors of the outcome in septic patients admitted to the ED, we concluded that the TBARS concentration is a more powerful index than the lactate levels after conducting an AUC analysis. Thus, TBARS is a good prognostic and clinical severity parameter at the time of sepsis diagnosis and marker of response to treatment during septic patients first days in the ED. Similarly, Motoyama et al observed that the TBARS levels in systemic inflammatory response syndrome (SIRS) patients with multiple organ failure (MOF) are significantly higher than those in the SIRS patients without MOF,[4] which is in accordance with the results of other trials.[3,5,6] Many other studies support similar study findings to ours; however, several reports showed no significant changes in the oxidative stress biomarker.[27] Furthermore, although TBARS was most frequently used as a biomarker for lipid peroxidation, it lacked specificity. The discrepancy may also be attributed to the varying methodologies (e.g., enrollment criteria, population group or size, and heterogeneous treatments) and follow-up periods (1 blood sample vs serial measurements) used.
4.3. Comparisons of TBARS and lactate in prognostic ability
With regard to the ability of TBARS and lactate values to predict fatality, Liu et al reviewed 8 prospective and 14 retrospective observational studies and concluded that increased initial lactate concentrations were significantly associated with an elevated risk of mortality (odds ratio [OR], 2.92; 95% CI, 2.40–3.55; P < .00001).[28] Furthermore, Michael et al observed that lactate normalization within 6 hours had a better in-hospital prognostic ability than other measures of lactate kinetics, including initial lactate and clearance rate (AUC, 0.67 vs 0.64 vs 0.58, respectively), within 24 hours of ED admission. Several additional studies have demonstrated that early lactate normalization or initial level on ED admission have good prognostic ability.[29,30] Nonetheless, fewer trials have explored the prognostic ability of TBARS, and comparisons between TBARS and lactate values are rarely made. In our study, the TBARS and lactate concentrations on ED admission were both independent prognostic factors, with TBARS concentration revealing a better ability to predict patient outcome than the lactate concentration, based on the AUC analysis (AUC, 0.74 vs 0.69, respectively). We believe this is because the TBARS concentration increased at the early stage of oxidative stress-related injury during infection. In contrast, lactate concentrations gradually increased downstream in the sepsis pathway, which was evoked by not only ROS but also inflammatory cytokine stimulus and greatly elevated while tissue hypoperfusion developed. In our study, the TBARS concentration had better prognostic accuracy, perhaps owing to its rapidly triggered change in concentrations during early sepsis and uncontrolled sepsis before lactate formation. Nevertheless, additional research studies should focus on exploring the differences between the prognostic abilities of these 2 biomarkers.
4.4. Signal transmission pathways and mechanisms of oxidative stress production and modulation during sepsis
Increasing evidence suggests that there are links between oxidative stress increases during sepsis and inflammatory reaction. Such links are relevant for modulation of numerous intracellular pathways and the nuclear translocation and binding of transcription factors, including nuclear factor kappa-B (NFκB). NFκB appears to have a crucial role in sepsis pathophysiology, and convincing evidence exists for the upregulation of NFκB activity during sepsis.
Lipopolysaccharide binding to inflammatory cells activates a number of intracellular signaling pathways, including the NFκB pathway and mitogen-activated protein kinase pathways. These pathways activate various transcription factors, including NFκB/Rel proteins, activator protein 1 (AP-1), and nuclear factor–interleukin 6.[31] In general, in intact cells, antioxidants and ROS have opposing effects, with antioxidants causing decreases in NFκB activation by increasing AP-1 activation (with AP-1 acting as a secondary antioxidant response factor) and ROS causing NFκB activation.[32] Oxidative stress may thus be involved in direct or indirect mechanisms resulting in cellular injury during sepsis. Numerous biomarkers have been investigated to identify the roles of oxidative stress and modulating transcription factor activity on sepsis; however, to date, no antioxidant therapy has been shown to improve survival in sepsis patients. This may be a result of oxidative biomarkers being the intermediate products of inflammatory pathways with unclear roles.[33]
Currently, it is difficult to utilize potential biomarkers of oxidative stress and antioxidant levels for diagnostic or prognostic applications in sepsis, likely because most of these biomarkers are intermediate products. Moreover, complicated cellular mechanisms that occur during sepsis contribute to the lack of accurate and easily applicable diagnostic and prognostic methods. Further exploration of potential biomarkers of oxidative stress or antioxidative capacity by standard validated methods should be conducted.
Our study demonstrated that a high TBARS concentration upon admission is a powerful predictor of fatality in patients with sepsis. Nonetheless, our study had several limitations. First, erythrocyte, plasma, or serum are used to measure oxidative stress status and antioxidants in critically ill patients and evaluate different pathways. As ROS have no specific targets and a short half-life, we measured the oxidant and antioxidant markers using the patients serum and erythrocytes. Second, cellular damage indicated by TBARS may occur in different tissues and is probably affected by several mechanisms of DNA release, due to the heterogeneity of pathogens and critical condition of the patients. Third, some confounding factors may have influenced our results, including the treatment method used (antibiotic preference, low-dose steroid use) and several comorbidities in immunocompromised patients, such as diabetes (despite attempting to exclude such patients from our study population). Last, the sample size of our study was relatively small; thus, similar studies with larger sample sizes need to be performed to further clarify the role of these biomarkers in predicting the outcome of patients with sepsis.
5. Conclusions
Our study confirmed the hypothesis that oxidative stress increases substantially during sepsis and decreases after therapy, and that the biomarkers of oxidative stress can predict disease severity and treatment outcomes. Furthermore, TBARS and lactate concentrations on admission were independently associated with patient mortality. Finally, we conclude that the TBARS levels at admission can be a more powerful prognostic index of mortality for patients with acute sepsis than conventional infection biomarkers and clinical scores. Thus, the use of serial plasma TBARS seems promising as a prognostic predictor in the treatment of patients with sepsis.
Author contributions
Sheng-Yuan Hsiao analyzed results, generated figures, and wrote most of the manuscript. Cheng-Hsien Lu performed and designed experiments. Chia-Te Kung and Cheng-Hsien Lu also conceived and supervised the study and designed experiments. Chia-Te Kung, Nai-Wen Tsai and Chih-Min Su contributed to formal analysis and software. Chao Tung Chen, Chin-Cheng Huang, Hung-Chen Wang, Ben-Chung Cheng, Yu-Jih Su, Wei-Che Lin, Ya-Ting Chang, Yun-Ru Lai, and Yi-Fang Chiang all assisted in enrolling study cases and healthy volunteers, examining their laboratory data, analyzing the results, and writing part of the manuscript. All authors revised the final version of the manuscript.
Conceptualization: Chia-Te Kung, Cheng-Hsien Lu.
Data curation: Sheng-yuan Hsiao, Chih-Min Su MD, Chin-Cheng Huang, Nai-Wen Tsai, Hung-Chen Wang, Ben-Chung Cheng.
Formal analysis: Chih-Min Su MD, Nai-Wen Tsai.
Investigation: Sheng-yuan Hsiao, Chia-Te Kung, Yun-Ru Lai, Yi-Fang Chiang.
Methodology: Yi-Fang Chiang.
Project administration: Sheng-yuan Hsiao, Chin-Cheng Huang.
Resources: Chin-Cheng Huang, Ben-Chung Cheng, Yu-Jih Su, Wei-Che Lin.
Software: Chih-Min Su MD, Nai-Wen Tsai, Ben-Chung Cheng.
Supervision: Chia-Te Kung, Cheng-Hsien Lu.
Validation: Yun-Ru Lai, Hung-Chen Wang, Yu-Jih Su, Wei-Che Lin.
Visualization: Yun-Ru Lai, Hung-Chen Wang, Yu-Jih Su, Wei-Che Lin.
Writing – original draft: Sheng-yuan Hsiao.
Writing – review & editing: Cheng-Hsien Lu.
Footnotes
Abbreviations: ANOVA = analysis of variance, AP-1 = activator protein 1, APACHE = Acute Physiology and Chronic Health Evaluation, AUC = area under the curve, CI = confidence interval, CRP = C-reactive protein, ED = emergency department, GPx = glutathione peroxidase, MDA = malondialdehyde, NADPH = nicotinamide-adenine dinucleotide phosphate, NFκB = nuclear factor kappa-B, ROS = reactive oxygen species, SD = standard deviation, SOFA = Sequential Organ Failure Assessment, TBARS = thiobarbituric acid-reactive substances, thiol = glutathione, TNB = 5-thio-2-nitrobenzoic acid, WBC = white blood cells.
How to cite this article: Hsiao SY, Kung CT, Su CM, Lai YR, Huang CC, Tsai NW, Wang HC, Cheng BC, Su YJ, Lin WC, Chiang YF, Lu CH. Impact of oxidative stress on treatment outcomes in adult patients with sepsis: a prospective study. Medicine. 2020;99:26(e20872).
This work was supported by grants from Chang Gung Memorial Hospital (Chang Gung Medical Research Project CMRPG891341). The funding sources had no role in the development of the research and manuscript.
The authors declare no conflict of interest.
The datasets generated during and/or analyzed during the current study are publicly available.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Kaymak C, Basar H, Sardas S. Reactive oxygen species (Ros) generation in sepsis. FABAD J Pharm Sci 2011;36:41–7. [Google Scholar]
- [2].Karapetsa M, Pitsika M, Goutzourelas N, et al. Oxidative status in ICU patients with septic shock. Food Chem Toxicol 2013;61:106–11. [DOI] [PubMed] [Google Scholar]
- [3].Goode HF, Cowley HC, Walker BE, et al. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Criti Care Med 1995;23:646–51. [DOI] [PubMed] [Google Scholar]
- [4].Motoyama T, Okamoto K, Kukita I, et al. Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome. Crit Care Med 2003;31:1048–52. [DOI] [PubMed] [Google Scholar]
- [5].Ogilvie AC, Groeneveld AB, Straub JP, et al. Plasma lipid peroxides and antioxidants in human septic shock. Intensive Care Med 1991;17:40–4. [DOI] [PubMed] [Google Scholar]
- [6].Katundu KGH, Hill LT, Davids LM, et al. An observational study on the relationship between plasma vitamin C, blood glucose, oxidative stress, endothelial dysfunction and outcome in patients with septic shock. South Afr J Crit Care 2016;32:21–7. [Google Scholar]
- [7].Cancelier AC, Petronilho F, Reinke A, et al. Inflammatory and oxidative parameters in cord blood as diagnostic of early-onset neonatal sepsis: a case-control study. Pediatr Crit Care Med 2009;10:467–71. [DOI] [PubMed] [Google Scholar]
- [8].Asci A, Surmeli-Onay O, Erkekoglu P, et al. Oxidant and antioxidant status in neonatal proven and clinical sepsis according to selenium status. Pediatr Int 2015;57:1131–7. [DOI] [PubMed] [Google Scholar]
- [9].Luchtemberg MN, Petronilho F, Constantino L, et al. Xanthine oxidase activity in patients with sepsis. Clin Biochem 2008;41:1186–90. [DOI] [PubMed] [Google Scholar]
- [10].de Vega JMA, Díaz J, Serrano E, et al. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit Care Med 2002;30:1782–6. [DOI] [PubMed] [Google Scholar]
- [11].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hakim AD, Littner MR. Barkoukis TJ, Matheson JK, Ferber R, Doghramji K. Chapter 21 - Sleep-Related Disorders in Chronic Pulmonary Disease. Therapy in Sleep Medicine. Philadelphia: W.B. Saunders; 2012. 270–85. [Google Scholar]
- [13].Wagner DP, Draper EA. Acute physiology and chronic health evaluation (APACHE II) and medicare reimbursement. Health Care Financ Rev 1984;Suppl: Suppl: 91–105. [PMC free article] [PubMed] [Google Scholar]
- [14].Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009;301:2362–75. [DOI] [PubMed] [Google Scholar]
- [15].Rochwerg B, Oczkowski S, Siemieniuk RA, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis (protocol). BMJ Open 2017;7:e016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Volbeda M, Wetterslev J, Gluud C, et al. Glucocorticosteroids for sepsis: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2015;41:1220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kung CT, Su CM, Chang HW, et al. Serum adhesion molecules as outcome predictors in adult severe sepsis patients requiring mechanical ventilation in the emergency department. Clin Biochem 2014;47:38–43. [DOI] [PubMed] [Google Scholar]
- [18].Kung C-T, Su C-M, Chang H-W, et al. The prognostic value of leukocyte apoptosis in patients with severe sepsis at the emergency department. Clin Chim Acta 2015;438:364–9. [DOI] [PubMed] [Google Scholar]
- [19].Babior BM. Phagocytes and oxidative stress. Am J Med 2000;109:33–44. [DOI] [PubMed] [Google Scholar]
- [20].Ghosh R, Mitchell DL. Effect of oxidative DNA damage in promoter elements on transcription factor binding. Nucleic Acids Res 1999;27:3213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000;279:L1005–28. [DOI] [PubMed] [Google Scholar]
- [22].Boisrame-Helms J, Kremer H, Schini-Kerth V, et al. Endothelial dysfunction in sepsis. Curr Vasc Pharmacol 2013;11:150–60. [PubMed] [Google Scholar]
- [23].Takezawa J, Taenaka N, Nishijima MK, et al. Amino acids and thiobarbituric acid reactive substances in cerebrospinal fluid and plasma of patients with septic encephalopathy. Crit Care Med 1983;11:876–9. [DOI] [PubMed] [Google Scholar]
- [24].Tsai NW, Chang YT, Huang CR, et al. Association between oxidative stress and outcome in different subtypes of acute ischemic stroke. Biomed Res Int 2014;2014:256879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brunelle JK, Chandel NS. Oxygen deprivation induced cell death: an update. Apoptosis 2002;7:475–82. [DOI] [PubMed] [Google Scholar]
- [26].L’her E, Sebert P. A global approach to energy metabolism in an experimental model of sepsis. Am J Respir Crit Care Med 2001;164:1444–7. [DOI] [PubMed] [Google Scholar]
- [27].Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta 2007;380:50–8. [DOI] [PubMed] [Google Scholar]
- [28].Liu G, Haijin L, An Y, et al. Early actate levelsfor prediction of mortality in patients with sepsis or septic shock: a meta-analysis. Int J Clin Exp Med 2017;10:37–47. [Google Scholar]
- [29].Puskarich MA, Trzeciak S, Shapiro NI, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013;143:1548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chertoff J, Chisum M, Garcia B, et al. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol 1997;17:3–9. [DOI] [PubMed] [Google Scholar]
- [32].Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J 1993;12:2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim JS, Kwon WY, Suh GJ, et al. Plasma glutathione reductase activity and prognosis of septic shock. J Surg Res 2016;200:298–307. [DOI] [PubMed] [Google Scholar]


