Supplemental Digital Content is available in the text
Keywords: acupuncture, network meta-analysis, Premature ovarian failure, systematic review
Abstract
Background:
Premature ovarian failure (POF) is commonly treated with hormone replacement therapy (HRT). Many patients with POF choose acupuncture as a complementary therapy over HRT, due to possible adverse reactions. This systematic review and network meta-analysis (NMA) compares the efficacy of different forms of acupuncture therapies for POF.
Methods:
Seven databases including PubMed, the Cochrane Library, Embase, Wanfang database, China National Knowledge Infrastructure database, VIP Chinese Science, and Chinese Biomedical Database were searched for randomized controlled trials (RCTs) of various acupuncture treatments for POF. This time spanned from the date of database inception to January 13, 2020. RevMan 5.3 was used to assess the bias risk of the studies. A NMA of the included studies was performed using Stata14.0.
Results:
A total of 408 items were searched in this study, and finally this NMA included 16 RCTS, involving 1,307 patients. It showed that acupuncture (OR:1.35,95%1.24 to 1.47) has the best effectiveness among the four acupuncture (standardized mean difference [SMD]-16.30,95% −31.33 to −1.28) is the most effective and the best in reducing follicle-stimulating hormone levels among the four acupuncture treatments. Acupuncture (SMD 26.67,95%5.95 to 47.40) and acupoint embedding (SMD41.14,95%11.90 to 70.37) were ranked in the top 2 positions, in improving estradiol, whereas acupuncture (SMD-4.90,95% −8.10 to −1.70) was than acupoint embedding and HRT, in reducing luteinizing hormone level. In addition, our conclusions have not changed significantly after the sensitivity analysis.
Protocol registration number: CRD42020150508.
Conclusion:
With clinical evidence summarized by NMA, it is observed that acupuncture is the most promising therapy for improving menopausal symptoms, decreasing serum follicle-stimulating hormone and luteinizing hormone level. Therefore, acupuncture could be effective for patients with POF, who are intolerant to the adverse effects of hormone replacement therapy or who would prefer non-drug therapies. Further multi-center and high-quality RCT studies should be conducted to make our conclusion more rigorous.
1. Introduction
Premature ovarian failure (POF) is defined as the cessation of ovarian function before the age of 40, and is associated with elevated serum gonadotropin levels (follicle stimulating hormone (FSH) >40 mIU/mL).[1] Studies have reported that the incidence of POF stands at 0.01% before the age of 20,[2] 0.1% before the age of 30, and 1% to 2% before the age of 40,[3] with an average age of onset of 23.3 years.[4] POF decreases the fertility of women within the reproductive age. In addition, it has serious health consequences, including autoimmune diseases, osteoporosis, infertility, ischemic heart disease, and increased cardiovascular diseases.[5–7]
Thus, early intervention should be initiated to prevent long-term consequences. POF is primarily treated with hormone replacement therapy (HRT).[6] This approach alleviates the symptoms caused by the decline in various ovarian hormones levels in POF. However, HRT has been associated with many serious adverse effects such as breast cancer, heart attacks and stroke.[3,8]
Acupuncture has been accepted by many people and is widely used to treat various diseases including back pain, arthritis, headache, asthma, and other female diseases.[9] It modulates the menstrual cycle and up-regulates uterine electromyography, which in turn improves reproductive function.[3,4,10] For this reason, is the most commonly used complementary therapies in many Western countries.[9,11]
In recent years, acupuncture has been widely used in POF. Clinical studies show that it not only effectively decreases hot flashes and related symptoms,[12] but also decreases serums FSH and luteinizing hormone (LH) level, raise serum estradiol (E2) level, relieve anxiety, reduce mental stress and improve the quality of life.[13] Several systematic reviews[14–16] have shown that acupuncture treatment improve ovarian function and serum sex hormone levels in POF patients significantly. In addition to traditional needle acupuncture, newer forms of acupuncture have been developed, including warm acupuncture, acupoint catgut embedding and moxibustion. Notably, previous systematic reviews[14–16] have largely examined the efficacy of different acupuncture treatments as a whole. Clinically, the choice of acupuncture therapy is often based on the experience of the acupuncturists. The lack of evidence-based concepts that guide the choice of acupuncture often leads to poor outcomes.
It is therefore necessary to develop a more standardized approach to guide the clinical application of acupuncture for POF. Herein, we employed network meta-analysis (NMA) to compare the efficacy of acupuncture, warm acupuncture, acupoint catgut embedding and moxibustion in the treatment of POF.
2. Materials and methods
2.1. Design and registration
This systematic review was reported with the Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA) Statement and was registered on the PROSPERO as CRD42020150508. (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42020150508) This is a systematic review and ethical approval is not necessary.
2.2. Ethic approval
No ethical issues are foreseen because this study does not involve clinical trials. The research data is from published papers.
2.3. Inclusion Criteria
2.3.1. (1) Participants
Patients who were diagnosed and met the criteria of POF;[17–22] mainly with amenorrhea for over four months, FSH above 40 IU/L as detected at least 2 times, and younger than 40 years.
2.3.2. (2) Interventions and Comparison
The treatment group was treated with acupuncture, warm acupuncture, electroacupuncture, moxibustion or acupoint catgut embedding alone. The control group was treated with conventional medicine or 1 of the above-mentioned acupuncture treatments.
2.3.3. (3) Outcomes
The primary outcome measurement was based on the improvement in symptoms and serum index.
Effectiveness rate = (total points of symptom before treatment- total points of symptom after treatment)/ total points of symptom before treatment × 100%.
Total effective rate = (number of healing cases+ number of markedly effective cases + number of effective cases)/ total number of cases × 100%.
-
(1)
Healing: Clinical symptoms and signs disappeared, menstrual cycle, serum FSH, E2 levels returned to normal, and the total effective rate of≥95%;
-
(2)
Markedly effective: Clinical symptoms and signs were significantly improved, menstrual cycle, serum FSH and E2 levels were significantly improved, 70%≤total effective rate < 95%;
-
(3)
Effective: Clinical signs and symptoms were slightly improved, menstrual cycle, serum FSH and E2 levels were slightly improved, 30% ≤ total effective rate < 70%;
-
(4)
Invalid: There was no improvement in clinical signs and symptoms, no improvement in menstrual cycle, no improvement in serum FSH and E2 levels, total effective rate < 30%.
The second outcome measures included Serum FSH, E2, and LH.
The second outcome measure included Serum follicle-stimulating hormone (FSH), E2 and LH.
2.4. Data source and Search strategy
We searched PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, VIP Chinese Science, WanFang, and Chinese Biomedical Literature databases from the date of their inception to January 13, 2020. The languages used for the trials were restricted to English or Chinese. According to the characteristics of each database, MeSH terms were combined with free words:(“acupuncture” OR “moxibustion” OR “electroacupuncture” OR “acupoint catgut embedding” OR “warm Acupuncture”) AND “premature ovarian failure” OR “Premature Menopause” AND “Randomized Controlled Trial” OR “Randomized.”
2.5. Study selection and data extraction
First, all the screened studies were imported into Note Express. Studies that were repeated and did not meet the inclusion criteria were excluded after reading the title and abstract. The full text was screened again according to the inclusion and exclusion criteria. Data extraction was done by 2 authors, independently. The content contained the authors’ names, publication date, diagnostic criteria, the number of the experimental and control group, gender, age, course of the diseases, intervention, control group type, outcome indicators, the evaluation standard, and so on. Study characteristics (author and year of publication); participant characteristics (age, disease course, and cases of each group); intervention information (measures of intervention and control, treatment duration, adverse events) and outcome. In case of inconsistency in the data, a third author was called to verify.
2.6. Risk of bias in individual studies
Two reviewers assessed the risk of bias of the randomized controlled trial (RCTs) using the Cochrane Collaboration tool for assessing the risk of bias.[23] Each study was evaluated for low, unclean and high-risk bias based on 7 items:
-
(1)
random sequence generation;
-
(2)
allocation concealment;
-
(3)
blinding of participants and personnel;
-
(4)
blinding of outcome assessment;
-
(5)
incomplete outcome data;
-
(6)
selective reporting; and
-
(7)
other bias.
Any disagreement was addressed by the third reviewer. The risk of bias assessment was performed using Review Manager 5.3.
2.7. Statistical analysis
Randomized effects model was used for pairwise meta-analysis for each pair of interventions, using Review Manager 5.3.3 (Cochrane Collaboration, Denmark). Odds ratios (OR) were used to analyze dichotomous outcomes, standard mean differences (SMDs) for continuous outcomes and 95% confidence intervals to estimate relative treatment effectiveness.
A NMA was performed in STATA 14.0. The network evidence graph was implemented through the command ‘netplot’. Differences between direct and indirect evidence for the same comparisons were explored according to the results of I2 and P-value of the global consistency, and then the node-splitting analysis method was used to assess inconsistency between direct and indirect comparisons of each pair of interventions.[24] Finally, the inconsistency factor within each closed loop of evidence was used to detect inconsistency among studies, while direct and indirect comparisons were used for the same study.[25] The surface under the cumulative ranking curves (SUCRA) and mean rank (MR) were used as the evaluation indexes. The SUCRA value was the index reflecting the possibility of the intervention measures. The closer MR was to 0, the better the effect of the intervention. P < .05 was considered statistically significant.[26]
We used a comparison-adjusted funnel plot to determine whether there was a small sample effect or publication bias in the network.
3. Results
3.1. Study selection and characteristics
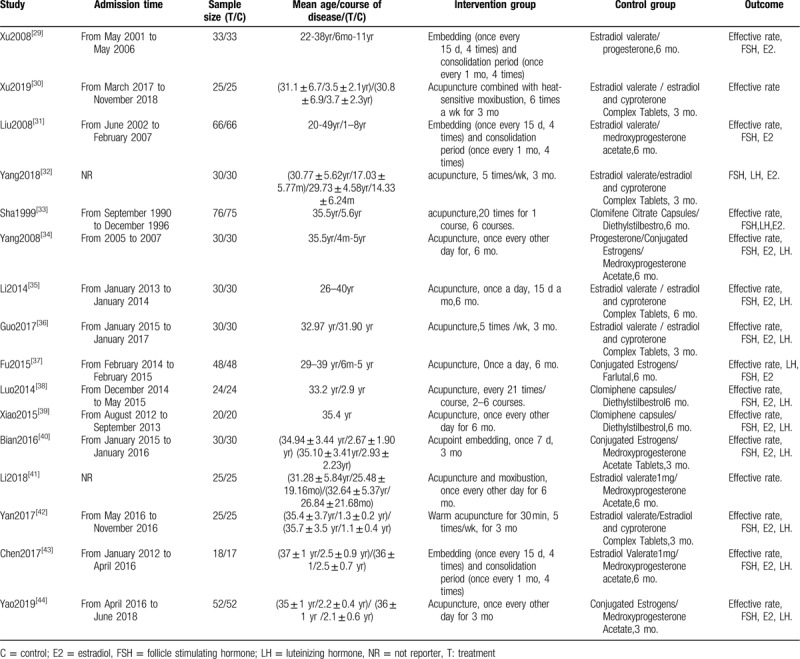
As shown in Figure 1, our systematic search identified 408 articles; 16 studies, published between 1979 and 2019 and involving 1307 patients, were eligible. Patients’ age ranged from 26 to 40 years old; the course of disease ranging from 86 days to 11 years; and the study sample size ranged from 10 to 52 patients. In terms of interventions, 16 studies compared acupuncture and HRT, and all were 2-arm studies; nine involved acupuncture, one warm acupuncture, four acupoint catgut embedding, and two acupuncture and moxibustion. The characteristics of each study are presented in Table 1.
Figure 1.

Flow chart of studies considered for inclusion.
Table 1.
Characteristics of the included RCTs.
3.2. Risk of bias of the Included Studies
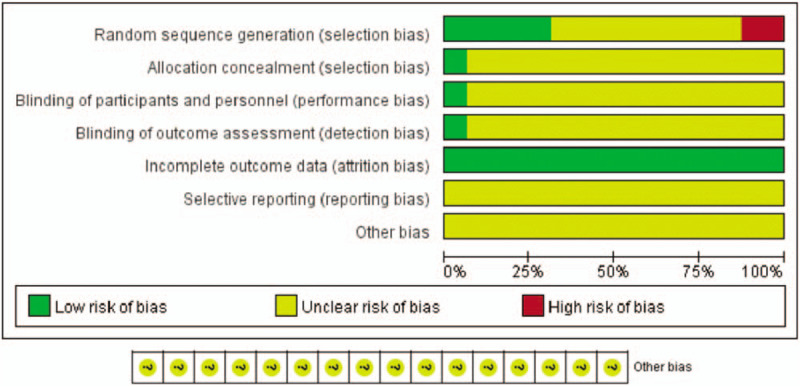
In terms of random sequence generation, four studies (35%)[27,28,30,34,42] reported an appropriate method for randomization, 2 addressed admission[29,41] and therefore high risk, the rest only reported random, so it is not clear. Allocation concealment and blind were only reported in one study,[34] the rest were not reported. None of the studies made efforts to blind the personnel or the participants. All studies were not registered in advance or planned; therefore, selective outcome reporting was not clear. Therefore, the overall risk bias is unclear. The risk of bias summary is listed in Figure 2.
Figure 2.
Network meta-analysis of eligible comparisons.
3.3. Results of NMA
3.3.1. Pairwise meta-analysis
Table 2 and Supplemental Content 1, Available at present the results of the pairwise meta-analysis and heterogeneity estimates. All 16 studies reported effective rate, 10 reported LH, and 14 reported E2 and FSH.
Table 2.
Pairwise meta-analyses.
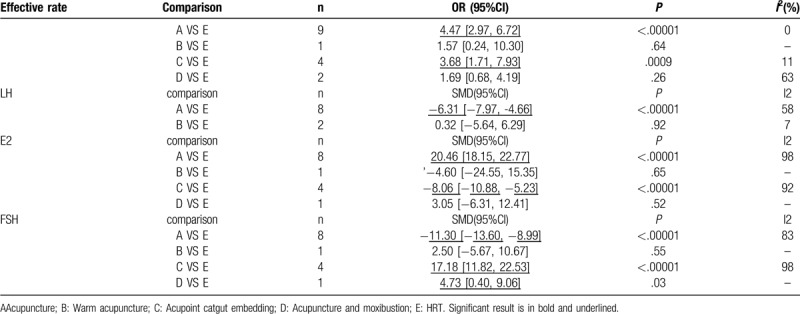
The forest plot of effective rate indicates that acupuncture (OR 4.47, 2.97 to 6.72, I2 =0%) and acupoint catgut embedding (OR 3.68,1.71 to 7.93, I2 =11%) were more efficacious than HRT.
The forest plot of LH indicates that acupuncture (SMD -6.31, -7.97 to -4.66, I2 = 58%) had significantly higher improvement than HRT. were associated with a
The forest plot of E2 indicates that acupuncture (SMD 20.46, 18.15 to 22.77, I2 = 98%) was superior to HRT, however, acupoint catgut embedding (SMD -8.06, -10.88 to -5.23, I2 = 92%) had a worse effect than HRT.
The forest plot of FSH indicates that acupuncture (SMD -11.30, -13.60 to -8.99, I2 = 83%), acupoint catgut embedding (SMD 17.18,11.82 to 22.53, I2 = 98%), acupuncture (SMD 4.73, 0.40 to 9.06) and moxibustion show lower FSH than HRT.
Sensitivity analysis was performed for paired studies with I2 greater than 50%, removing any single study one by one. The results did not change significantly, indicating that the pooled results were stable.
3.3.2. NMA
3.3.2.1. (1) Effective rate
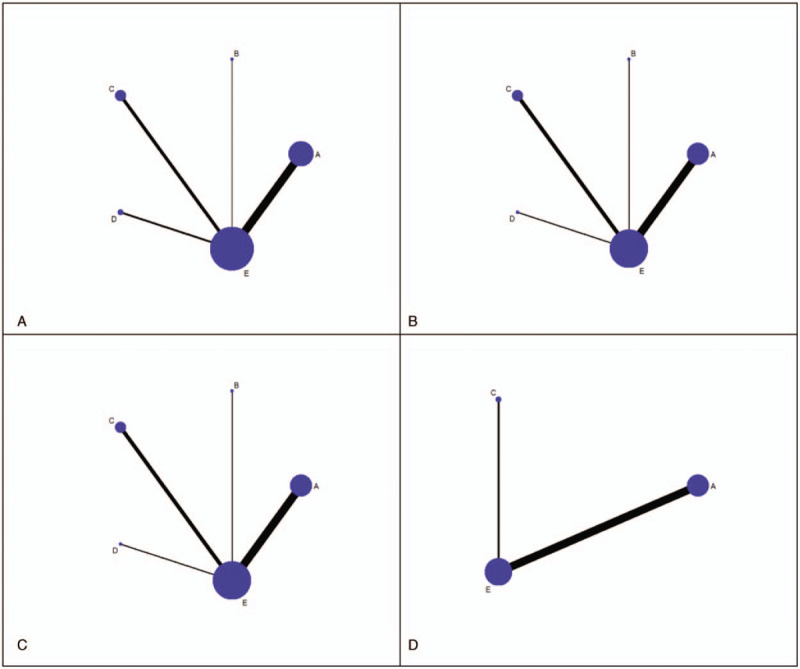
Figure 3A shows network results of treatment comparisons for the effective rate. The results of the NMA for the effective rate are presented in Table 2. A total of 16 RCTs (1307 participants) were analyzed.
Figure 3.
Summary of the risk of bias.
The results of NMA indicate that warm acupuncture and HRT have improved efficiency compared to acupoint embedding, and embedding is more efficient than HRT. The rest of the comparisons had no statistically significant difference (Table 3).
Table 3.
Network meta-analysis comparisons of effective rate and FSH.
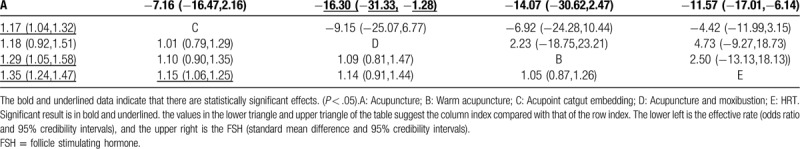
According to SUCRA, acupuncture (97.3%) had the highest effective rate, followed by acupoint catgut embedding (58.8%), acupuncture with moxibustion (53.6%), warm acupuncture (29.2%) and HRT (11.1%) (Supplemental Content 2).
3.3.2.2. (2) FSH
Figure 3b shows the network plot of treatment comparisons for FSH. The results of NMA for the effective rate are presented in Table 2. A total of 14 RCTs (1024 participants) were analyzed for FSH changes. NMA indicated that acupuncture is more effective in reducing FSH than warm acupuncture and HRT. There were no statistically significant differences among other comparisons on FSH (Table 3).
According to the SUCRA, acupuncture (96.7%) had the highest probability of reducing FSH, followed by acupoint catgut embedding (64.9%), HRT (37.5%), warm acupuncture (30.1%) and acupuncture and moxibustion (20.7%) (Supplemental Content 3).
3.3.2.3. (3) E2
Figure 3C, shows network plot of treatment comparisons for E2. The results of NMA for the effective rate are presented in Table 2. A total of 14 RCTs (1024 participants) were analyzed for E2. NMA indicates that acupoint embedding and acupuncture can improve E2 more effectively than HRT. There was no statistically significant difference among other comparisons on the E2 (Table 4).
Table 4.
Network meta-analysis comparisons of E2 and LH.

According to the SUCRA, acupoint catgut embedding (89.3%) and acupuncture (70.6%) ranked in the top 2 positions for effectiveness on E2, followed by acupuncture and moxibustion (36.5%), warm acupuncture (27.9%) and HRT (25.7%) (Supplemental Content 4, Available at).
3.3.2.4. (4) LH
Figure 3D shows the network plot of treatment comparisons for LH. The results of NMA for the LH are presented in Table 2. A total of 10 RCTs (713 participants) were analyzed for LH. NMA indicates that acupuncture is superior to HRT in reducing LH. There was no statistically significant difference between acupoint catgut embedding and HRT on LH (Table 4).
According to SUCRA, acupuncture (94%) had the highest probability in reducing LH, followed by acupoint catgut embedding (30.8%), and HRT (25.3%) (Supplemental Content 5, Available at).
3.3.3. Evaluation of Statistical Inconsistency
Side-splitting did not indicate any statistical inconsistency for any outcomes (Supplemental Content 6, Available at).
3.3.4. Small-study effects
In general, there was no strong evidence effects of the small study across outcomes.
However, among the outcome indicators of FSH and E2, several studies on the lateral side of the funnel plot may be related to the heterogeneity between studies (Supplemental Content 7, Available at).
3.3.5. Sensitivity analysis
Supplemental Content 8, Available at presents the results of the sensitivity analyses. Excluding 2 studies with high-risk bias resulting from the inappropriate random method did not significantly change our results about effective rate and FSH.
4. Discussion
The purpose of this study was to compare the efficacy of different acupuncture treatments in the treatment of POF. Numerous systematic reviews[14–16] have shown that acupuncture-related therapies are superior to western drugs in improving POF, although such conclusion has been obtained for the entire range of acupuncture treatments as a whole. In this study, we ranked the efficacy of different acupuncture therapies using NMA. In so-doing, we provide the specific clinical efficacy of each acupuncture technique.
In study, 16 RCTS, involving 1307 patients were examined. NMA showed that acupuncture was the most effective with the best FSH reduction level. Among the 4 acupuncture treatments; acupuncture and acupoint embedding were the first and second most effective in improving E2; whereas acupuncture was superior to acupoint embedding and HRT in decreasing LH. Most importantly, these conclusions did not change significantly after sensitivity analysis.
Numerous studies have explored the mechanism of action of acupuncture in the treatment of POF. The mechanism of acupuncture therapy for POF is described according to the types of acupuncture. Acupuncture defined as a procedure in which specific body areas, the acupoints (also called meridian points), are pierced with fine needles to induce a therapeutic response. It has been found that acupuncture activates the dopamine system in the brain and finetunes the function of HPOA. In this way, acupuncture alters normalizes the physiological functions.[43] In ovariectomized rats, Cheng et al[44] found that acupuncture increased low estrogen levels and reduced high gonadotropin levels. Li et al compared the effects of acupuncture and diethylstilbestrol on sex hormones in rats with POF.[45] They found that acupuncture significantly increased uterine wall thickness and improved uterine blood circulation in rats. Thus, acupuncture improves the reproductive organs in POF rats, and normalizes sex hormones levels. Acupoint catgut embedding involves the implantation of a sheep gut or other absorbable thread to an acupuncture point in line with the theory of acupuncture and meridian. Acupoint embedding on acupoints reduces FSH and LH levels and increases E2 by stimulating the acting on the hypothalamic-pituitary-ovarian axis system. Acupoint embedding also regulates paracrine and/or autocrine functions through stem cell factors, thereby improving the ovarian microenvironment.[46,47] Acupoint catgut embedding has been observed to improve ovarian blood supply, inhibit follicular atresia, promote follicular development and recruitment, increase the nutrition of ovarian granulosa cells, enhance the activity of aromatase of the follicular membrane cells granulosa cells, convert androgens into estrogen, leading to elevation in E2 levels in blood.[10] Warm acupuncture approach combines acupuncture with moxibustion. In this therapy, a moxa column is added to the end of a needle, ignites it and the heat is transmitted through the needle to the body to treat diseases. Chen[48] found that warm acupuncture not only simultaneously confer acupuncture therapy and moxibustion therapy. Warm acupuncture regulates increases E2 and progesterone, inhibits the secretion of LH, establishing a negative feedback loop between ovary and pituitary. This loop normalizes elevated FSH, balances the HPO axis and enhances the body's immunity.[49]. In acupuncture and moxibustion, is a combination of acupuncture and moxibustion to treat diseases. Moxibustion is a method in which a moxa herb is burned above the skin or on the acupuncture points. Moxibustion can promotes blood circulation and removes blood stasis by regulating the constriction of blood vessels.[50] One study[51] found that moxibustion at guan yuan restored the function of the hypothalamic-pituitary-ovarian axis to normal level mainly by promoting the synthesis of E2. Elsewhere,[52] acupuncture combined with moxibustion improved the estrogen level and estrogen receptor protein levels. In this way, the combination therapy regulated the HPO axis and endocrine function, thereby improving the ovarian function.
Despite the findings mentioned in this study, the following limitations should be mentioned: First, this study was conducted on a relatively small sample size, ranging from 10 to 52 patients. Thus, to increase the reliability of our results, further studies with larger sample size are warranted. Second, the risk of bias for most of the included RCTs is not clear, especially biases related to randomization, allocation, concealment, and blinding, which might decrease the credibility of our conclusions. Future clinical trials compliant to the CONSORT reporting system are advocated to validate our findings.[52] Thirdly, heterogeneity cannot be ruled out given the differences in acupoints, technique and treatment frequency used across studies. In addition, the studies included were conducted in China and all participants were Chinese, which affects sampling and limits extrapolation of our results to other populations. Future clinical trials on acupuncture conforming to the Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA)[53] are advocated across countries and regions to provide more conclusive and reliable results. Fourth, there are only indirect comparisons among acupuncture treatment, which reduces the credibility of our conclusions. Future head-to-head trials on acupuncture therapies should be performed to provide more compelling evidence to guide the clinical application of acupuncture.
5. Conclusion
Clinical evidence, summarized using NMA, indicates that acupuncture has the highest probability of being the most effective therapy for improving menopausal symptoms and decreasing FSH and LH levels. Therefore, acupuncture is an effective alternative for patients with POF, who are intolerant to hormone replacement therapy or who would prefer alternative and complementary therapies. Moreover, further multi-center and high-quality RCT studies should be conducted to contribute more details.
Author contributions
Conceptualization: Jinhuan Zhang.
Data curation: Jinhuan Zhang, Xingxian Huang.
Formal analysis: Jinhuan Zhang.
Investigation: Xingxian Huang.
Methodology: Yongfeng Liu, Yuhai He.
Resources: Yongfeng Liu.
Software: Yuhai He.
Supervision: Haibo Yu.
Writing – original draft: Jinhuan Zhang.
Writing – review & editing: Jinhuan Zhang, Yongfeng Liu, Yuhai He, Xingxian Huang, Haibo Yu.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: E2 = estradiol, FSH = follicle-stimulating hormone, LH = luteinizing hormone, NMA = network meta-analysis, POF = premature ovarian failure, RCT = randomized controlled trial, SMD = standardized mean difference.
How to cite this article: Zhang J, Huang X, Liu Y, He Y, Yu H. A comparison of the effects of Chinese non-pharmaceutical therapies for premature ovarian failure: a PRISMA-compliant systematic review and network meta-analysis. Medicine. 2020;99:26(e20958).
This study was supported by Shenzhen's Sanming Project (SZSM201612001)
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Testa G, Chiaffarino F, Vegetti W, et al. Case-control study on risk factors for premature ovarian failure. Gynecol Obstet Invest 2001;51:40–3. [DOI] [PubMed] [Google Scholar]
- [2].Kokcu A. Premature ovarian failure from current perspective. Gynecol Endocrinol 2010;26:555–62. [DOI] [PubMed] [Google Scholar]
- [3].Shelling AN. Premature ovarian failure. Reproduction 2010;140:633–41. [DOI] [PubMed] [Google Scholar]
- [4].Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol 2010;45:257–79. [DOI] [PubMed] [Google Scholar]
- [5].Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol (Paris) 2003;64:87–92. [PubMed] [Google Scholar]
- [6].Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. [DOI] [PubMed] [Google Scholar]
- [7].Dragojević-Dikić S, Marisavljević D, Mitrović A, et al. An immunological insight into premature ovarian failure (POF). Autoimmun Rev 2010;9:771–4. [DOI] [PubMed] [Google Scholar]
- [8].Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2005;11:391–410. [DOI] [PubMed] [Google Scholar]
- [9].Laissue P, Vinci G, Veitia RA, et al. Recent advances in the study of genes involved in non-syndromic premature ovarian failure. Mol Cell Endocrinol 2008;282:101–11. [DOI] [PubMed] [Google Scholar]
- [10].Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2007;68:196–202. [DOI] [PubMed] [Google Scholar]
- [11].Agarwal S, Alzahrani FA, Ahmed A. Hormone replacement therapy: would it be possible to replicate a functional ovary? Int J Mol Sci 2018;19:3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fan AY. Trial suggests both acupuncture and acupressure are effective at reducing menopausal hot flashes. Acupunct Med 2016;34:320. [DOI] [PubMed] [Google Scholar]
- [13].Chen Y, Fang Y, Yang J, et al. Effect of acupuncture on premature ovarian failure: a pilot study. Evid Based Complement Alternat Med 2014;2014:718675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jian Runzi, He Tiantian, Zhang Qiongdan, et al. Meta-analysis of acupuncture and western medicine in the treatment of premature ovarian failure. Science & technology vision 2017;01:123–4. [Google Scholar]
- [15].Fan Meiling, Yang Pan, Wu Xiaoyan, et al. Meta-analysis of the effect of acupuncture on premature ovarian failure. J Yunnan Coll of Tradit Chin Med 2018;41:63–9. [Google Scholar]
- [16].Luo Xi, Li Qian, Cheng Jie, et al. A systematic review and meta-analysis of the effectiveness of acupuncture for premature ovarian failure. Journal of Traditional Chinese Medicine 2016;57:1027–32. [Google Scholar]
- [17].Xie Xing, Gou Wenli. Obstetrics and Gynecology. Version 8. 2013;Beijing: People's Health Publishing Press, 354–356. [Google Scholar]
- [18].Yu Chuanxin, Li Shuxian. Practical gynecological endocrinology. version 2. 2004;Shanghai: Fudan University Press, 100. [Google Scholar]
- [19].Beckpeccoz P, Persani L. Premature ovarian failure. Orphanet Journal of Rare Diseases 2006;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu Ling, Song Yijun. Clinical manifestations and diagnostic criteria of premature ovarian failure. Journal of Practical Obstetrics and Gynecology 2003;19:195–6. [Google Scholar]
- [21].Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril 1990;53:804–10. [PubMed] [Google Scholar]
- [22].Conway GS. Premature ovarian failure. British Medical Bulletin 2000;56:643–9. [DOI] [PubMed] [Google Scholar]
- [23].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–44. [DOI] [PubMed] [Google Scholar]
- [25].Higgins JPT, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2010;64:163–71. [DOI] [PubMed] [Google Scholar]
- [27].Xu Xuebing, Liu Hongyi. Treatment of 33 cases of premature ovarian failure by embedding sutures at the points of the upper and lower Shu Yu points. Chinese Folk Remedies 2008;16:12–3. [Google Scholar]
- [28].Xu Mengbo, Tian Haiyan. Comparative observation of curative effect of electroacupuncture combined with thermosensitive moxibustion and western medicine on premature ovarian failure. World Journal of Acupuncture-Moxibustion 2017;27:9–14. [Google Scholar]
- [29].Liu Hongjiao, Peng Jianhong, Xu Xuebing. Observation on the curative effect of burying thread in Yuzhao acupoint on premature ovarian failure. Chinese Acupuncture and Moxibustion 2008;28:325–7. [PubMed] [Google Scholar]
- [30].Yang Bize. Clinical study of body acupuncture combined with ear acupuncture in the treatment of premature ovarian failure (kidney deficiency and liver stagnation). Yunnan College of Traditional Chinese Medicine 2018. [Google Scholar]
- [31].Sha Gui’e, Zhao Wenmin, Ma Renhai. Clinical study on acupuncture treatment of 76 cases of premature ovarian failure. Chinese Acupuncture and Moxibustion 1999;19:7–9. [Google Scholar]
- [32].Yang Xiaohong, Lai Xiaomei, Huang Zubo. Clinical observation on acupuncture treatment of 60 cases of premature ovarian failure. Sichuan Traditional Chinese Medicine 2008;26:106–7. [Google Scholar]
- [33].Li Li. Clinical observation of acupuncture points on premature ovarian failure. Nanchang, Jiangxi, China 2014. [Google Scholar]
- [34].Guo Yongjun. Clinical research on the transfer of Tongdu acupuncture for premature ovarian failure. Guangzhou University of Chinese Medicine 2017. [Google Scholar]
- [35].Fu Ming. Clinical observation of acupuncture treatment for premature ovarian failure. World Clinic Medicine 2015;9:173. [Google Scholar]
- [36].Luo Yingyu, Fu Bei. Clinical observation on acupuncture treatment of premature ovarian failure. Hubei Journal of Traditional Chinese Medicine 2014;36:63. [Google Scholar]
- [37].Xiao Qingfeng, Fu Bei, Zhou Huifang. Clinical observation on treatment of premature ovarian failure with bushen quyu acupuncture. Hubei Journal of Traditional Chinese Medicine 2015;37:58–9. [Google Scholar]
- [38].Bian Xinhui, Yun A, Chen Jiajie. Clinical observation on acupoint embedding for treatment of premature ovarian failure. Journal of Guangxi University of Traditional Chinese Medicine 2016;19:19–21. [Google Scholar]
- [39].Li Wenfang, Guo Qinyuan, Xie Lifeng. Clinical observation on acupuncture in treating premature ovarian failure with spleen and kidney yang deficiency. Journal of Practical Traditional Chinese Medicine 2018;34:1239–40. [Google Scholar]
- [40].Yan Jiangtian, Wu Fan. Clinical observation on the treatment of premature ovarian failure with acupuncture points. Beijing, China: 20172. [Google Scholar]
- [41].Chen Min, Chen Lihua, Tian Xiaoping, et al. Clinical observation on acupoint embedding for treatment of premature ovarian failure. Shanghai Journal of Acupuncture and Moxibustion 2017;36:697–701. [Google Scholar]
- [42].Yao Min, Wang Qin, Pan Hongling, et al. Acupuncture treatment of premature ovarian failure and its effect on the expression levels of cytokines TNF-α and IFN-γ in patients. China Acupuncture 2019;39:1181–4. [DOI] [PubMed] [Google Scholar]
- [43].Shi Yun, Zhang Yuzhen. Clinical observation on tonifying the kidney, spleen, regulating liver and activating blood to treat premature ovarian failure. Journal of Traditional Chinese Medicine 2006;24:1174–6. [Google Scholar]
- [44].Cheng Kai, Tian Suling. Effect of counter-acupuncture “Guan Yuanzhang's” Sanyinjiao "on hypothalamic-pituitary-ovarian axis of ovariectomized rats. Acupuncture Research 2012;37:15–9. [PubMed] [Google Scholar]
- [45].Li Mingyue. Effect of Bushen Shugan acupuncture on sex hormones in rat model of premature ovarian failure. Jinan: Shandong University of Traditional Chinese Medicine; 2015. [Google Scholar]
- [46].Ding SS, et al. Acupuncture modulates the neuro-endocrine-immune network. QJM: monthly journal of the Association of Physicians 2014;107:341–5. [DOI] [PubMed] [Google Scholar]
- [47].Bossut DF, Stromberg M w, Malven PV.Electroacupuncture— induced analgesia in sheep: measurement of cutaneous pain thresholds and plasma concentrations of prolactin and beta—endorphin immunoreactivity, Am J Vet Res, l 986,47:669—76. [PubMed] [Google Scholar]
- [48].Mao Yangping. Study on acupuncture treatment of immune infertility and its regulating effect on B-endorphin. Guangzhou: Guangzhou University of Traditional Chinese Medicine; 2010. [Google Scholar]
- [49].Liu JM. The research of mechanism about T lymphocyte immunoregulation of aging model rats by means of electro-acupuncture therapy on Guanyuan (CV4) and Zusanli (ST36) points. 2009;China: Hubei College of Traditional Chinese Medicine, 9–31. [Google Scholar]
- [50].Wang Hao. Effect of Yishen Tiaozhou method on p-endorphin in premature ovarian failure model rats. Journal of Clinical Acupuncture and Moxibustion 2012;28:59–63. [Google Scholar]
- [51].Wang S, Lin S, Zhu M, et al. Acupuncture reduces apoptosis of granulosa cells in rats with premature ovarian failure via restoring the PI3K/Akt signaling pathway. Int J Mol Sci 2019;20:6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Macpherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. J Evid Based Med 2010;3:140–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


