Abstract
To identify the clinical risk factors and investigate the efficacy of a classification model based on the identified factors for predicting 2-year recurrence after ischemic stroke.
From June 2017 to January 2019, 358 patients with first-ever ischemic stroke were enrolled and followed up in Shenzhen Traditional Chinese Medicine Hospital. Demographic and clinical characteristics were recorded by trained medical staff. The outcome was defined as recurrence within 2 years. A multivariate logistic regression model with risk factors and their interaction effects was established and evaluated.
The mean (standard deviation) age of the participants was 61.6 (12.1) years, and 101 (28.2%) of the 358 patients were female. The common comorbidities included hypertension (286 patients, 79.9%), diabetes (148 patients, 41.3%), and hyperlipidemia (149 patients, 41.6%). The 2-year recurrence rate was 30.7%. Of the 23 potential risk factors, 10 were significantly different between recurrent and non-recurrent subjects in the univariate analysis. A multivariate logistic regression model was developed based on 10 risk factors. The significant variables include diabetes mellitus, smoking status, peripheral artery disease, hypercoagulable state, depression, 24 h minimum systolic blood pressure, 24 h maximum diastolic blood pressure, age, family history of stroke, NIHSS score status. The area under the receiver operating characteristic curve (ROC) was 0.78 (95% confidence interval: 0.726–0.829) with a sensitivity of 0.61 and a specificity of 0.81, indicating a potential predictive ability.
Ten risk factors were identified, and an effective classification model was built. This may aid clinicians in identifying high-risk patients who would benefit most from intensive follow-up and aggressive risk factor reduction.
The clinical trial registration number: ChiCTR1800019647
Keywords: ischemic stroke, recurrence, risk estimation, risk factors
1. Introduction
Patients who survive an ischemic stroke are at a high risk of recurrence within 2 years. Approximately 80% to 85% of patients survive the first ischemic stroke, and of these, 15% to 30% experience a recurrent stroke within the first 2 years.[1] Recurrent stroke, as one of the clinical endpoints, is the main cause of death, re-hospitalization, and long-term disability. Compared with the first stroke, neurological impairment caused by recurrence is more serious, more difficult to treat, and has higher mortality. Therefore, secondary prevention after the first stroke is of great significance to reduce the recurrence of stroke.
The factors leading to high risk of recurrent stroke are still poorly understood.[2] The epidemiologically determined predictors of stroke recurrence include age,[3–5] arterial hypertension,[5–7] atrial fibrillation,[7,8] diabetes mellitus or impaired glucose tolerance,[4,5,9,10] and hyperlipidemia.[4] These factors all play important roles in secondary prevention. In addition to these factors, there may be other risk factors that related to stroke recurrence.
Some differences between studies based on medical care data and community access data may be explained by differences in changes in lifestyles and risky behaviors, including smoking alcohol abuse, poor nutrition and sedentary lifestyles, and the role of long-term medical treatment.
The ability to predict the risk of stroke recurrence through a clinical prediction algorithm would help tailor future preventive and therapeutic measures according to the estimated risk of recurrence for each individual patient. Thus, the main objectives of this study were:
-
1.
to determine significant clinical features for patients admitted to the hospital after the first episode of ischemic stroke;
-
2.
to establish a risk model based on the significant clinical features and predict recurrence events within 2 years after the ischemic stroke.
2. Methods
2.1. Study population
This was a prospective cohort study to determine the causes of recurrent stroke within 2 years. The ethics committee of the Shenzhen Traditional Chinese Medicine Hospital (Shenzhen, China) approved the study. All patients or their legal representatives provided written informed consent.
2.2. Data source
Upon admission, baseline data, including age, gender, medical history, physical examination, and laboratory test results were collected. To diagnose ischemic stroke, all patients underwent detailed radiographic evaluation, including cranial magnetic resonance (MR) scan and duplex color Doppler ultrasound or contrast-enhanced MRA (CEMRA).
The inclusion criteria include the onset of ischemic stroke within 14 days of admission, age between 30 and 80 years. Patients were excluded if they were clinically unstable, required close monitoring, were moribund, or were physically or subjectively unable to comply with MR examination. The patients suspected to suffer from cerebral diseases other than stroke and those with diseases that influence vessel morphology were also excluded.
2.3. Study design, data collection, and observation period
Clinically risk factors commonly found in the literature, were collected before and during hospitalization. The risk factors included patient demographic characteristics (age, sex, height, and weight), medical history (hypertension, diabetes mellitus, hyperlipidemia, coronary heart disease, atrial fibrillation, and family history of stroke), lifestyle factors (smoking and drinking), clinical biochemical examination (blood pressure, serum lipid metabolism, glycosylated hemoglobin, and plasma homocysteine), and imaging examination (magnetic resonance imaging).
Diabetes mellitus (defined as a history of diabetes mellitus or diagnosis at discharge), hyperlipidemia (defined as low-density lipoprotein cholesterol of 2.6 mmol/L upon admission, a history of hyperlipidemia, having undergone lipid-lowering treatments, or diagnosed at discharge), hyperhomocysteinemia (defined as homocysteine of above 15 mmol/L upon admission or diagnosed at discharge), history of coronary heart disease (defined as a history of myocardial infarction or angina pectoris), current or previous smokers (patients who continuously smoked ≥1 cigarette per day for 6 months), and heavy drinkers (patients who consumed >2 drinks per day on average for men or >1 drink per day on average for women; a standard drink was defined as a glass of wine, a bottle of beer, or a shot of spirits, ∼10 to 12 g of ethanol)[11] were noted.
All the subjects completed a 24 h ambulatory blood pressure monitoring within 7 days of hospitalization. The interval between consecutive blood pressure measurements was set to 30 min during the day (6:00–22:00), 60 min overnight (22:00–6:00 the next day), and no less than 32 effective measurements were recorded within 24 h. The effective measurement range was 70 to 260 mm Hg for systolic blood pressure and 40 to 150 mm Hg for diastolic blood pressure. The effective blood pressure value had to be not <80% of the number of times to be measured, otherwise, it was considered invalid. The 24 h mean systolic pressure (24hMEANSBP), 24 h mean diastolic pressure (24hMEANDBP), 24 h maximum systolic pressure (24hMAXSBP), 24 h minimum systolic pressure (24hMINSBP), 24 h maximum diastolic pressure (24hMAXDBP), 24 h minimum diastolic pressure (24hMINDBP), 24 h systolic pressure variation coefficient (24hCVSBP), and 24 h diastolic pressure variation coefficient (24hCVDBP) were recorded. Hypertension was defined according to World Health Organization criteria: systolic blood pressure (SBP) ≥ 140 mm Hg, diastolic blood pressure (DBP) ≥90 mm Hg, or the patient on antihypertensive treatment.[12] For the purpose of this analysis, we characterized blood pressure by the SBP/DBP as means ± standard deviations (SD). The range, lowest, and highest SBP/DBP within 24 h were also investigated.
The patients had been tested by Hamilton Depression (HAMD) Scale and the National Institutes of Health Stroke Scale (NIHSS) by trained physicians. HAMD scale is a 17-item scale for depression measure. A score of <7 represents no depression, a score of 8 to 17 represents mild depression, a score of 18 to 24 represents moderate depression, and a score of higher than 25 represents severe depression. NIHSS is a 15-item impairment scale for stroke severity measurement, the scoring range is 0 to 42 points, the higher the number, the greater the severity. A score of less than 5 represents no stroke symptoms or minor stroke, a score of 5 to 15 represents moderate stroke, a score of 16 to 20 represents moderate to severe stroke, and a score of 21 to 42 represents severe stroke.
The patients were followed up for at least 2 years after the first stroke, or until the earliest occurrence of any of the following events: cerebral infarction, cerebral hemorrhage, acute myocardial infarction, death, and lost to follow-up.
2.4. Statistical analysis
The qualitative variables were expressed as absolute frequencies and percentages. The continuous variables were described with mean standard deviation. A Chi-square test and an independent T test were performed for the univariate analysis of the qualitative and quantitative characteristics, respectively. The P-value < .05 was considered to be statistically significant. A multivariate logistic regression model was built with the significant risk factors derived from the univariate analysis and also other risk factors shown to be clinically relevant in previous studies using a significance level of <0.1. The odds ratio (OR) and 95% confidence interval (95% C.I.) were calculated. The sensitivity, specificity, and the area under the curve (AUC) of the receiver operating characteristic (ROC) curve were calculated for model evaluation. All data analyses were performed using SPSS 22.0 software (IBM, New York, NY).
3. Result
3.1. Study population
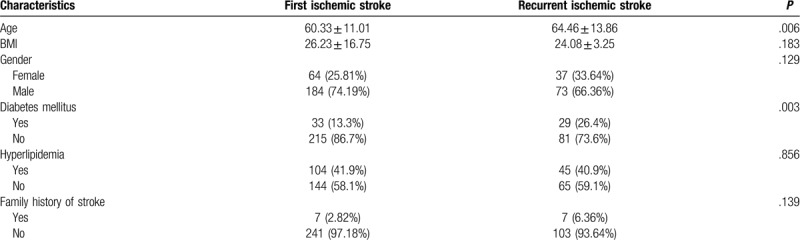
A total of 422 ischemic stroke patients enrolled in our study; 358 patients finished a 2-year follow-up, out of which 110 (30.7%) had more than one recurrent event. The general clinical characteristics of the patients are presented for each group in Table 1 . When compared to patients with first stroke, recurrent stroke patients were significantly older (64.46 vs 61.33, P = .006) and had a higher proportion of diabetes mellitus (26.4% vs 13.3%, P = .003), carotid artery stenosis (35.45% vs 23.39%, P = .018), peripheral artery disease (19.1% vs 33.1%, P = .007), hypercoagulable state (6.36% vs 0.40%, P = .001), history of gastrointestinal ulcer (0 vs 5.2%, P = .012), depression for severe to moderate (17.27% vs 13.31%, P = .002) and NIHSS score status for normal to mild stroke (35.4% vs 54.8%, P = .003). Moreover, patients with recurrent stroke had a higher mean arterial systolic blood pressure (132.8 vs 129.2, P = .033), minimum systolic blood pressure (118.2 vs 114.7, P = .042), and maximum diastolic pressure (90.1 vs 93.7, P = .009) levels in 24 h.
Table 1.
Baseline characteristics of the patients with the first stroke and recurrent stroke.
3.2. Cumulative risk of ischemic events
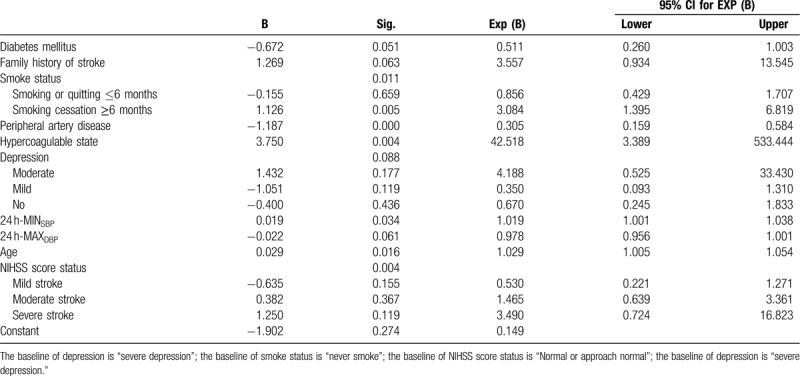
To investigate the efficacy of these factors for predicting the accuracy of stroke recurrence, a multivariate logistic model was built. The accuracy of recurrent stroke prediction was 74.6%. The significant variables include diabetes (P = .051), smoking status (P = .011), peripheral artery disease (P < .001), hypercoagulable state (P = .004), depression (P = .088), 24 h minimum systolic blood pressure (P = .034), 24 h maximum diastolic blood pressure (P = .061), age (P = .016), family history of stroke (P = .063), NIHSS score status (P = .004) (Table 2).
Table 1 (Continued).
Baseline characteristics of the patients with the first stroke and recurrent stroke.
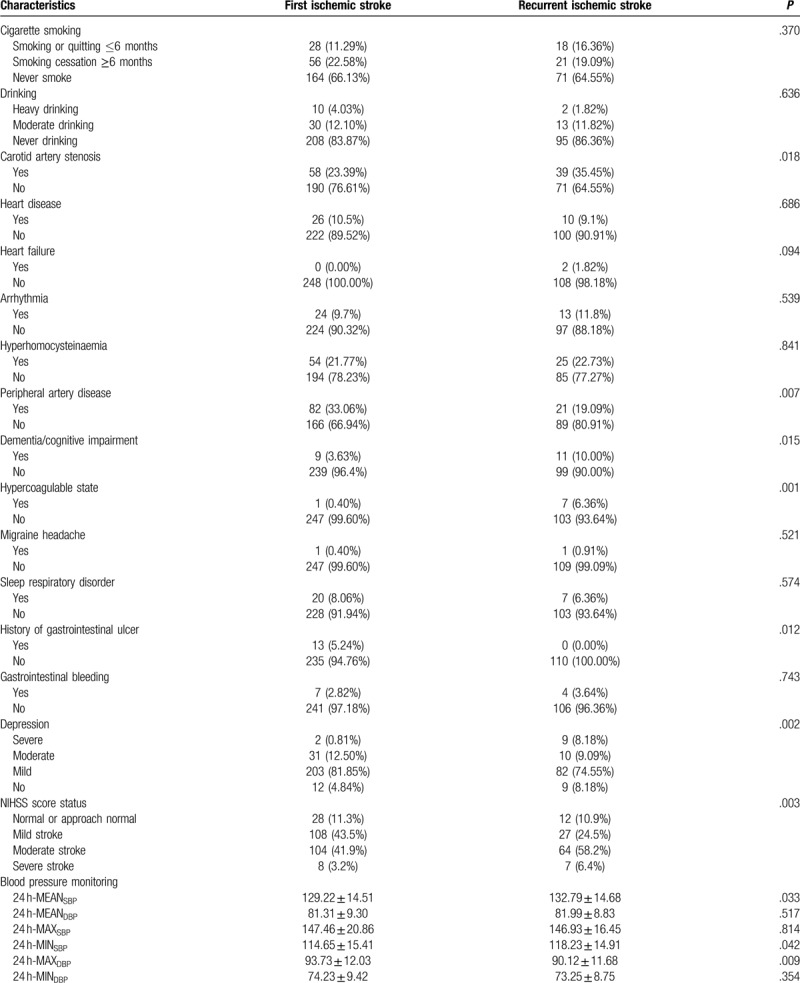
Table 2.
Logistic regression for recurrent ischemic stroke with clinical risk factors.
In order to evaluate the prediction ability of the significant clinical risk factors, the area under the ROC curve of the multivariate logistic regression model was calculated. The AUC is 0.777 (95% CI: 0.726–0.829), with a sensitivity of 0.61 and a specificity of 0.81, indicating a good potential predictive ability (Fig. 1).
Figure 1.
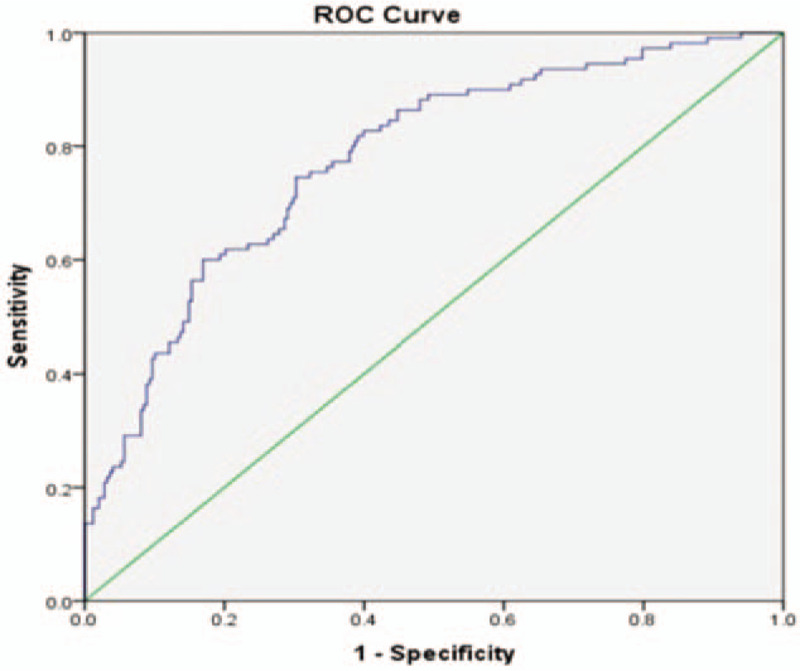
The ROC curve of the multivariate logistic model with clinical risk factors.
4. Discussion
Patients who suffered an ischemic stroke remain at a high risk of developing recurrent stroke in recent years. For patients who have suffered a stroke, an accurate risk assessment is crucial, as recurrent stroke is associated with greater damage to patients and a more serious burden on families compared with the first stroke. The Framingham Heart Study was launched in the 1940s in the United States and the concept of “risk factor” was proposed.[12] Risk factors such as gender, age, smoking, blood pressure, and lipid metabolism were included in the cardiovascular disease risk assessment model.[13] Tsai et al[14] reviewed systematically and provided an overall summary that risk factor associations with ischemic stroke were mostly similar among Chinese and Caucasian. Understanding and taking action to attenuate related risk factors are of utmost importance for recurrent stroke patients.
A few tools such as the Essen Stroke Risk Score (ESRS) are available for the assessment and early warning of recurrence in ischemic stroke.[15,16] In 2005, Rothwell PM and Giles MF of Oxford University proposed the first method (ABCD) to easily assess the risk of stroke recurrence.[17] In 2007, Johnston SC and Rothwell PM added diabetes to the variables of the ABCD index and proposed an improved index (ABCD2), which had certain predictive value for the recurrence of stroke.[18] However, the stability, accuracy and repeatability of these prediction models for recurrent stroke need to be improved. As this study has shown, several clinical risk factors are associated with stroke recurrence. A multivariate logistic model was built based on these factors for recurrent stroke with first stroke patients taken as the reference group. We found that the area under the ROC curve of the model were moderately high.
Various researchers have identified the risk factors for stroke recurrence. Hypertension was found to be the highest risk for recurrent stroke in Kariasa IM's study.[19] Significantly elevated risk ratios were found by Hviid et al[20] for inherited or acquired thrombophilia. Lee [21] et al found a significantly higher stroke recurrence rate in men, elder, and a prior history of ischemia stroke than in women, younger, and no history of stroke. Zheng and Yao[22] suggested that hypertension, diabetes mellitus, atrial fibrillation, and coronary heart disease are associated with a high risk of stroke recurrence. Lei Zhao et al[23] suggested that strict glycemic control might improve prognosis after stroke. A large cohort study on the association between etiologic subgroups and recurrent events, which found that large artery atherosclerosis and cardio embolism were important predictors of recurrent events.[18] The NIHSS scale, which is a standardized measure of stroke severity, can be used to predict short-term outcome.[24] In this study, we examined many possible predictors of stroke recurrence, including demographic variables, medical history, neurological history, aspects of the neurologic examination, stroke diagnosis, and laboratory results. Among them, the results indicated that age, family history of stroke, smoking status, diabetes mellitus, peripheral artery disease, hypercoagulable state, depression, and NIHSS score status were significant associated with recurrent stroke. This is likely to be related to the fact that patients with these stroke etiologies were more likely to have traditional risk factors than patients with an undetermined cause.
In our study, we have used 24 h ambulatory blood pressure monitoring and identified that the risk factors such as 24 h minimum systolic blood pressure, 24 h maximum diastolic blood pressure were associated with a high risk of stroke recurrence. Similar outcomes were found in previous studies demonstrating that blood pressure variability such as maximum, minimum, or mean systolic/diastolic blood pressure within 24 h along were key risk factors of stroke.[25,26] Compared to the first stroke, we found that the critical of the 24 h minimum systolic blood pressure (OR 0.019, P = .034) was higher and the 24 h maximum diastolic blood pressure (OR −0.022, P = .061) was lower in recurrent stroke. This suggests that strict long-term secondary prevention strategies should be encouraged.
The ability to predict the risk of stroke recurrence through clinical characteristics would help to improve the predictive value and to tailor future preventive and therapeutic measures. The findings of this study might have implications for therapeutic trials designed to prevent stroke recurrence and suggest that certain patients (those who are older, smoker, with diabetes, peripheral artery disease, hypercoagulation, depression, higher NIHSS score, higher 24 h minimum systolic blood pressure, lower 24 h maximum diastolic blood pressure) are at a somewhat higher risk for recurrence.
In conclusion, with the prediction model for recurrent cerebral infarction we would be able to use the clinical risk factors for the management of patients with stroke, and to use this model in a community primary healthcare setting or in large population screening. In future study, we will recruit more clinical cases and incorporate more risk factors that are associating to recurrent stroke, in order to predict the occurrence of recurrent stroke comprehensively.
Author contributions
Conceptualization: Yuanyuan Zhuo, Haibo Yu.
Data curation: Yimin Qu, Xingxian Huang, Jack Lee, Benny Zee
Formal analysis: Jack Lee, Benny Zee, Zhuoxin Yang
Investigation: Yuanyuan Zhuo,.
Methodology: Zhuoxin Yang
Project administration: Haibo Yu, Zhuoxin Yang
Resource: Yuanyuan Zhuo, Zhuoxin Yang
Supervision: Zhuoxin Yang
Writing – original draft: Yuanyuan Zhuo, Jiaman Wu.
Writing – review & editing: Yuanyuan Zhuo, Yimin Qu, Haibo Yu, Benny Zee
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, CVDBP = diastolic pressure variation coefficient, CVSBP = systolic pressure variation coefficient, DBP = diastolic blood pressure, ESRS = Essen Stroke Risk Score, HAMD = Hamilton Depression, Hg = hydrargyrum, MAXDBP = maximum diastolic pressure, MAXSBP = maximum systolic pressure, MEANDBP = mean diastolic pressure, MEANSBP = mean systolic pressure, MINDBP = minimum diastolic pressure, MINSBP = minimum systolic pressure, MR = magnetic resonance , MRA = magnetic resonance angiography, NIHSS = National Institutes of Health Stroke Scale, OR = odds ratio, ROC = receiver operating characteristic, SBP = systolic blood pressure, SD = standard deviations.
How to cite this article: Zhuo Y, Wu J, Qu Y, Yu H, Huang X, Zee B, Lee J, Yang Z. Clinical risk factors associated with recurrence of ischemic stroke within two years: A cohort study. Medicine. 2020;99:26(e20830).
YZ and JW contributed equally to this work.
Supported by the National Natural Science Fund of China (81803952), the Shenzhen Municipal Science and Technology Bureau (JCYJ20170412174025934), Scientific Research Projects of Guangdong Traditional Chinese Medicine Bureau (20173013), Sanming Project of Medicine in Shenzhen (SZSM201612001) and the Technology and Business Development Fund of the Chinese University of Hong Kong (TBF17MED004).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001;32:2559–66. [DOI] [PubMed] [Google Scholar]
- [2].Jiali Zhao, Fudi Chen, Lin Lu, et al. Effect of 106PEAR1 and 168PTGS1 genetic polymorphisms on recurrent ischemic stroke in Chinese patient. Medicine 2019;98:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Modrego PJ, Mainar R, Turull L. Recurrence and survival after first-ever stroke in the area of Bajo Aragon, Spain. A prospective cohort study. J Neurol Sci 2004;224:49–55. [DOI] [PubMed] [Google Scholar]
- [4].Lee AH, Somerford PJ, Yau KK. Risk factors for ischaemic stroke recurrence after hospitalisation. Med J Aust 2004;181:244–6. [DOI] [PubMed] [Google Scholar]
- [5].Sacco RL, Shi T, Zamanillo MC, et al. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology 1994;44:626–34. [DOI] [PubMed] [Google Scholar]
- [6].Lai SM, Alter M, Friday G, et al. A multifactorial analysis of risk factors for recurrence of ischemic stroke. Stroke 1994;25:958–62. [DOI] [PubMed] [Google Scholar]
- [7].Leoo T, Lindgren A, Petersson J, et al. Risk factors and treatment at recurrent stroke onset: results from the Recurrent Stroke Quality and Epidemiology (RESQUE) Study. Cerebrovasc Dis 2008;25:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jerrgensen HS, Nakayama H, Reith J, et al. Stroke recurrence: predictors, severity, and prognosis. The Copenhagen Stroke Study. Neurology 1997;48:891–5. [DOI] [PubMed] [Google Scholar]
- [9].Burn J, Dennis M, Bamford J, et al. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke 1994;25:333–7. [DOI] [PubMed] [Google Scholar]
- [10].Prencipe M, Culasso F, Rasura M, et al. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke 1998;29:126–32. [DOI] [PubMed] [Google Scholar]
- [11].Foerster M, Marques-Vidal P, Gmel G, et al. Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am J Cardiol 2009;103:361–8. [DOI] [PubMed] [Google Scholar]
- [12].Aronowitz RA. The Framingham heart study and the emergence of the risk factor approach to coronary heart disease, 1947-1970. Revue Dhistoire Des Sci 2011;64:263–95. [Google Scholar]
- [13].Whitworth J, Chalmers J. World health organization-international society of hypertension (WHO/ISH) hypertension guidelines. Clin Exp Hypertens 2004;26:747–52. [DOI] [PubMed] [Google Scholar]
- [14].Tsai CF, Anderson N, Thomas B, et al. Risk factors for ischemic stroke and its subtypes in Chinese vs. Caucasians: systematic review and meta-analysis. Int J Stroke 2015;10:485–93. [DOI] [PubMed] [Google Scholar]
- [15].Ling X, Yan SM, Shen B, et al. A modified Essen Stroke Risk Score for predicting recurrent ischemic stroke at one year. Neurol Res 2018;40:1–7. [DOI] [PubMed] [Google Scholar]
- [16].Andersen SD, Gorst-Rasmussen A, Lip GYH, et al. Recurrent stroke: the value of the CHA2DS2VASc score and the Essen stroke risk score in a nationwide stroke cohort. Stroke 2015;46:2491–7. [DOI] [PubMed] [Google Scholar]
- [17].Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005;366:29–36. [DOI] [PubMed] [Google Scholar]
- [18].Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283–92. [DOI] [PubMed] [Google Scholar]
- [19].Kariasa IM, Nurachmah E, Setyowati, et al. Analysis of participants’ characteristics and risk factors for stroke recurrence. Enferm Clin 2019;2:286–90. [Google Scholar]
- [20].Hviid CVB, Simonsen CZ, Hvas AM. Recurrence risk in patients with cryptogenic stroke, Patent Foramen Ovale, and Thrombophilia: A Systematic Review and Meta-Analysis; 2019. Aug 4. doi: 10.1055/s-0039-1693739. [DOI] [PubMed] [Google Scholar]
- [21].Lee JD, Hu YH, Lee M, et al. High risk of one-year stroke recurrence in patients with a younger age and prior history of ischemic stroke. Curr Neurovasc Res 2019;doi: 10.2174/1567202616666190618164528. [DOI] [PubMed] [Google Scholar]
- [22].Zheng S, Yao B. Impact of risk factors for recurrence after the first ischemic stroke in adults: a systematic review and meta-analysis. J Clin Neurosci 2019;60:24–30. [DOI] [PubMed] [Google Scholar]
- [23].Zhao L, Wang L, Lu M, et al. Hyperglycemia is associated with poor in-hospital outcome in elderly patients with acute ischemic stroke. Medicine 2019;98:e16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tu WJ, Dong X, Zhao SJ, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol 2013;25:771–8. [DOI] [PubMed] [Google Scholar]
- [25].Wolf PA, D’Agostino RB, Belanger AJ, et al. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–8. [DOI] [PubMed] [Google Scholar]
- [26].Lumley T, Kronmal RA, Cushman M, et al. A stroke prediction score in the elderly: validation and web-based application. J Clin Epidemiol 2002;55:129–36. [DOI] [PubMed] [Google Scholar]


