Supplemental Digital Content is available in the text
Keywords: computed tomography scan, pulmonary sclerosing pneumocytoma, thyroid transcription factor-1
Abstract
Rationale:
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign tumor of the lung, mostly presented in Asian middle-aged women. Initially, it was considered as a vascular origin tumor, but then research evidence showed that it was derived from natural epithelial tissue. On imaging, this tumor may be found as a solitary well-circumscribed lung parenchymal lesion, and is often located in juxtapleural or juxtafissural positions. On histopathology, it consists of cuboidal surface cells and stromal round cells, both of which are positive for thyroid transcription factor-1. Here we report a case of a young PSP male patient and review the relevant literature in order to improve our understanding of this disease.
Patient concerns:
An 18-year-old man was referred to our hospital after accidentally finding a lesion on chest X-ray. Contrast-enhanced computed tomography showed a soft tissue mass with homogeneous enhancement in the left lower lobe posterior segment.
Diagnoses:
The diagnosis of PSPs was confirmed by histopathological examination.
Interventions and outcomes:
The patient underwent a thoracoscopic wedge resection and was followed-up after that. One month later, he had good performance status with no recurrent tumors.
Lessons:
PSP in a young man is really uncommon, and is confused with malignant tumors. A histopathological examination is considered as the diagnostic gold standard for this uncommon tumor. Surgery is the main treatment.
1. Introduction
Pulmonary sclerosing pneumocytoma (PSP), which was first described as pulmonary sclerosing hemangioma by Liebow et al, in 1956,[1] is a rare benign neoplasm. It mostly occurs in adults over 50 years old and the incidence in women is 5 times higher than in men.[2,3] This tumor was initially thought to be vascular in origin. However, it is currently considered as epithelial in nature and was named PSP according to the 2015 World Health Organization Classification of lung tumors.[3,4]
On imaging, PSP is a solitary well-circumscribed lung parenchymal lesion, often juxtapleural or juxtafissural in location.[5,6] It often shows homogeneous enhancement.[7,8] These imaging characteristics are not specific, as a result, radiology alone is not an ideal method for the definitive diagnosis.[9]
On histopathology, PSP contains 2 types of cells, cuboidal surface cells and stromal round cells, both of which are regarded as neoplastic.[4,10] In immunohistochemistry, they are positive for thyroid transcription factor-1 (TTF-1).[11,12]
2. Case presentation
An 18-year-old male patient was referred to our hospital in August 2019 after accidentally detecting a solitary round lesion on the lower left lobe on chest X-ray and computed tomography (CT) scan (see Fig. 2, Supplemental Content, which demonstrates the lesion detected on chest X-ray). His medical history was normal. The patient had no symptoms of respiratory disorder, no smoking, and no tuberculosis history (see Fig. 1, Supplemental Content, which demonstrates the patient's status on admission). On admission, his vital signs and respiratory examination were normal. Laboratory tests showed that white blood cells: 6360/mm3, red blood cells: 4,930,000/mm3, hemoglobin: 15.1 g/dL, platelets: 209,000/mm3, urea: 30.8 mg/dL, and creatinine: 1.2 mg/dL. Two tumor markers of carcinoembryonic antigen and Cyfra 21-1 were <1.73 and 4.04 ng/mL, respectively. Contrast-enhanced thorax CT showed a soft-tissue lesion in the size of 37 × 30 mm located in the lower left lobe (below the left greater fissure), which was homogeneous and showed strong enhancement with a ground-glass opacity lesion around (Fig. 1).
Figure 1.
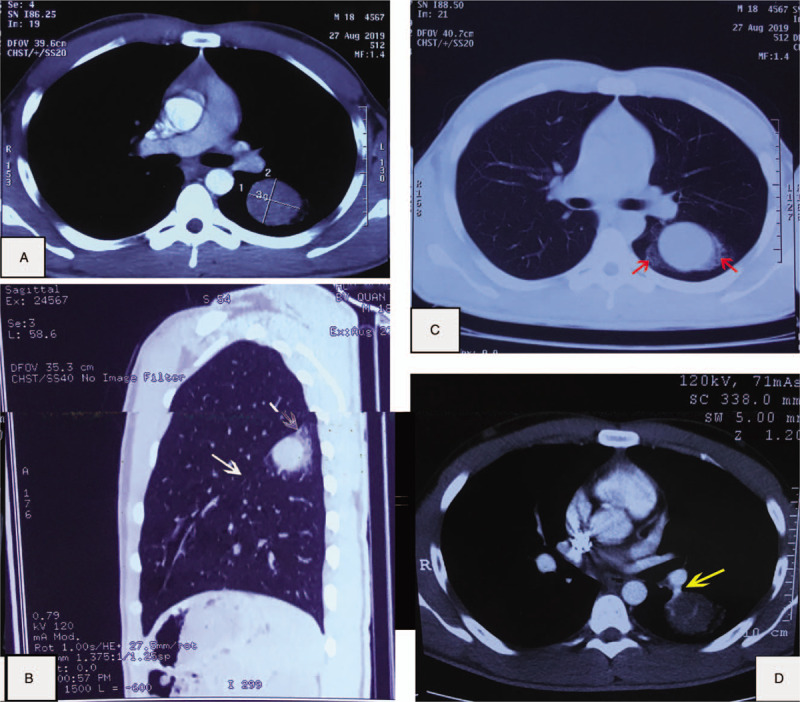
(A and B) A round lesion located in the lower left lobe in thorax CT, below the left greater fissure (white arrows). (C) A mass with surrounding ground-glass opacity (red arrows), defined as the “halo sign.” (D) CT performed 1 mo later revealed that the size had not changed. An obviously enhanced, engorged vascular structure (a yellow arrow) adjacent to the lesion. It was defined as the “overlying vessel sign.” CT = computed tomography.
One month later, he underwent enhanced dynamic CT with a 16-multidetector CT scan. The results showed that the size of this tumor had not changed over time. Mean baseline tumor attenuation was 27.8 ± 8.0 Hounsfield unit (HU) (range, 25–34 HU). The mean tumor peak enhancement value was 69.9 ± 8.0 HU (range, 55–90 HU), and the mean net enhancement value was 42.1 HU. The time to peak enhancement was 60 seconds (see Fig. 3, Supplemental Content, which demonstrates the enhanced dynamic 16-multidetector CT scan with 20 seconds time interval). This tumor was initially considered as a malignant lesion which could be lung cancer or primary pulmonary lymphoma. He was counseled and agreed to be performed bronchoscopy and transthoracic biopsy. The results of bronchoscopy were clear airway and normal bronchial mucosa in both sides. Bronchoalveolar lavage fluid tests for common bacteria, tuberculosis, and fungi were all negative. The patient was diagnosed by CT-guided core-needle biopsy. A histopathological examination on formalin-fixed paraffin-embedded tissue revealed that 2 cellular components of this tumor consisted of surface epithelial cells similar to type II alveolar pneumocytes and round stromal cells, which were organized into 4 main histological patterns: papillary, sclerosing, solid, and hemorrhagic (Fig. 2). Immunohistochemical staining detected both TTF-1 and cytokeratin AE1/AE3 in the surface epithelial cells. TTF-1 was detected in the round stromal cells, but cytokeratin AE1/AE3 was not (Fig. 3). The tests for the other markers were performed. While only the round cells were positive (40%) for progesterone receptor, both types of cells were negative for estrogen receptor, weakly positive (<1%) for Ki67, and strongly positive for vimentin (see Fig. 4, Supplemental Content, which demonstrates the results in immunohistochemical staining for progesterone receptor, estrogen receptor, Ki67, and Vimentin).
Figure 2.
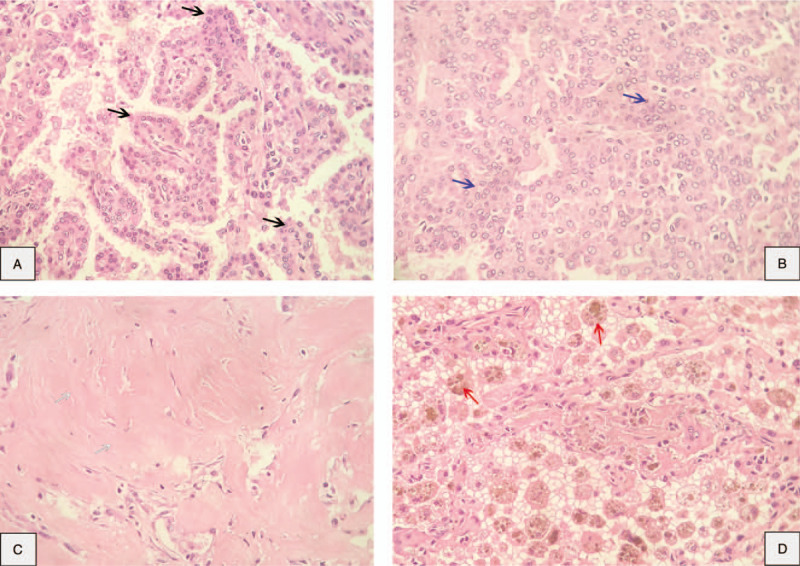
Pathological findings: pulmonary sclerosing pneumocytoma in a core-needle biopsy (hematoxylin-eosin, 40×). Two types of cells, cuboidal surface cells and stromal round cells, were organized into 4 structural patterns. (A) Papillary (black arrows). (B) Solid (blue arrows). (C) Sclerotic (white arrows). (D) Hemorrhagic (red arrows).
Figure 3.
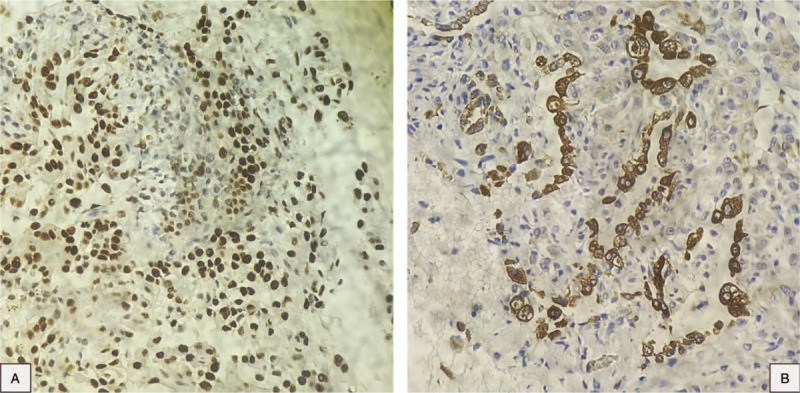
Immunohistochemical staining. (A and B) The surface cells are positive for both TTF-1 and cytokeratin AE1/AE3; the round cells are positive for TTF-1, negative for cytokeratin AE1/AE3. TTF-1 = thyroid transcription factor-1.
This case was reviewed at the multidisciplinary tumor board of our hospital. There was an opinion that the patient should be followed-up periodically on chest X-ray instead of having surgery because of the high risk of bleeding complications. However, his large tumor raised the risk of compressing adjacent structures, we concluded that he should be performed the segmentectomy. The patient underwent a wedge resection of the posteromedial basal segment of the left lung without any complications (see Fig. 5, Supplemental Content, which demonstrates the postoperative tumor). Postoperative histopathological examinations were consistent with the previous biopsy results. After the surgery, he was followed-up, and 1 month later, no recurrent lesions were discovered on chest X-ray (see Fig. 6, Supplemental Content, which demonstrates the 1-month-postoperative chest X-ray image). The patient provided a written informed consent for this publication.
3. Discussion
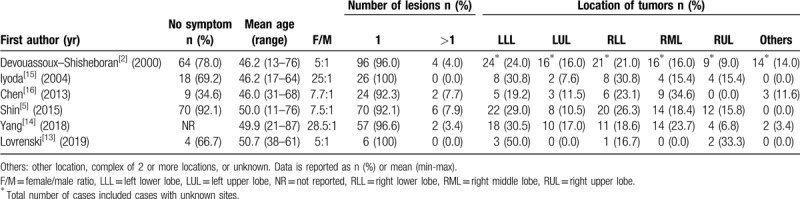
At first, this case was difficult to make a preliminary diagnosis because previous researches had shown that PSP is mainly presented in middle-aged women, and a female-to-male ratio is from 5:1 to 7.7:1 in different populations.[2,5,13] Several reports showed this ratio might be significantly higher.[14,15] PSP is mostly asymptomatic, and ranges from 92.1% to 96.6% of all cases. It is accidentally detected by routine check-ups or follow-up examination of current respiratory diseases.[2,5,13–15] However, Hu et al and Chen et al reported that the percentage of patients having at least 1 symptom (hemoptysis, coughing, sputum, chest pain, or fever) was 63% (29/46), 65.4% (17/26), respectively.[16,17] The minor difference among these reports might be due to both racial differences and small sample sizes. According to a report by Shin et al, most patients had a single lesion (92.1%), smooth boundary (65.8%), and oval shape (65.8%) and the mean diameter was 22.7 mm. The CT signs included marginal pseudocapsule (50%), overlying vessel (26.3%), air gap (2.6%), and halo sign (17.1%). Only 4 patients (5.3%) had 2 lesions (5.3%), and 2 patients (2.6%) had >3 lesions.[5] Similarly, the proportion of the solitary lesion ranges from 96.0% to 100% in several articles. The tumor is commonly located in the left lower lobe, and the presence of this tumor in the other lobes varies from study to study.[2,13–15] Our case is consistent with reports of the number of lesion, the tumor location, and the CT sign. In addition, some tumors have been situated in the fissure between 2 lobes of the lung, the mediastinum, the hilum, and unknown locations [2,14,16] (See Table 1). Some studies reported that PSP could be bilateral, which should be distinguished from metastatic lung tumors.[17–19]
Table 1.
Review of demographic characteristics and clinical information of pulmonary sclerosing pneumocytoma.
The characteristics of PSP in thoracic CT scan has been described as a benign tumor with strong enhancement after intravenous administration of contrast medium,[6] homogeneous enhancement with maximum CT values ranging from 90 to 110 HU.[8] In this case, the mean tumor peak enhancement value was 69.9 ± 8.0 HU (range, 55–90 HU), the mean net enhancement value was 42.1 HU, and the time to peak enhancement was 60 seconds. According to a study by Yi et al, malignant nodules had higher mean peak enhancement values, mean net enhancement values, and lower mean time to peak enhancement than benign nodules. In addition, when 30 HU or more of net enhancement was set as a cutoff value to distinguish between malignant and benign nodules, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 99%, 54%, 71%, 97%, 78%, respectively.[20] These clues led us to initially think about the stage cT2N0Mx lung cancer even though he was very young. Nevertheless, in a study by Chung et al, PSP had even more rapid and stronger enhancement than malignant nodules.[7] These characteristics integrated with morphologic CT findings (ie, round or ovoid shape, smooth margin, and homogeneous attenuation) could allow differentiation between PSP and malignant nodules.
In the current World Health Organization classification of lung tumors from 2015, PSP has been changed from the “Miscellaneous tumors” group, where was previously classified in both the 1999 and 2004 versions, to the “Adenomas” group.[4,21,22] In terms of histopathology, there are 4 possible histological components: papillary, sclerotic, solid, and hemorrhagic.[15,18] A combination of 4 patterns is mostly observed, ranging from 50.0% to 76.9%, a 3-pattern combination followed with 19.2% to 43.8%, and a relatively small percentage of 2-pattern combination. In some literature, no single-pattern tumors have been reported. Observation results also indicated that the proportion of each pattern differs from one study to another[2,15,16,23] (See Table 2). PSP includes a dual population of surface cells similar to type II pneumocytes and round cells, with marginally different histogenetic profiles.[4] Immunohistochemically, the surface cells are positive for both TTF-1 and cytokeratin AE1/AE3, whereas the round cells are only positive for TTF-1. PSP has been recognized as not the tumor of vascular origin for many years. It is believed that origin of this tumor is primitive respiratory epithelium that express TTF-1.[2,7,13,24] The process of this tumor diagnosis can be critically difficult in the frozen section, small biopsies, and cytology because there is the possibility of being confused with adenocarcinoma and carcinoid tumors.[4,14,16]
Table 2.
Review of histological characteristics of pulmonary sclerosing pneumocytoma.
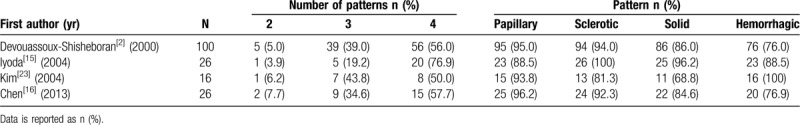
The recurrence rate is negligible, according to Devouassoux-Shisheboran et al, Iyoda et al, Shin et al, and Hu et al, it was 0% (0/30), 3.8% (1/26), 1.3% (1/76), 0% (0/45), respectively, with the follow-up periods ranged from 1 to 228 months.[2,5,15,17] Furthermore, there have been no reports showing that patients died or had any severe complications of the recurrence after surgical treatment.[16,25] Several articles reported that pleural and lymph node metastases occurred in a very small percentage of PSP, but these did not negatively affect prognosis; therefore, it was thought to be benign.[2,26,27] Xu et al suggested that surgical resection was curative for this tumor, and therefore no additional treatment needed after the surgery.[27] In our case, CT showed there were no pleural or hilar lymph node metastases. No recurrences have appeared during follow-up by the last contact and chest X-ray review. Surgery is the basic treatment, including limited resection (enucleation or wedge) and lobectomy.[2,13,15,17] Among these techniques, video-assisted thoracoscopic surgery is the most frequent procedure, in particular video-assisted thoracoscopic surgery lobectomy (50.0%).[16] Additionally, some patients definitively diagnosed with PSP by biopsy were just followed through changes on chest X-ray or cured by radiotherapy for unresectable tumors.[28,29]
4. Conclusions
PSP is a rare benign lung neoplasm that typically affects Asian middle-aged women. The definitive diagnosis requires a histopathological examination with a corresponding immunohistochemical analysis. Also, it is easily mistaken for adenocarcinoma and carcinoid tumors. Surgery is still the best treatment for this disease.
Author contributions
Formal analysis: Huu Y Le.
Investigation: Huu Y Le, Khac Tuyen Nguyen, Van Ai Hoang.
Methodology: Dinh Phuc Pham, Khac Tuyen Nguyen.
Resources: Huu Y Le, Dinh Phuc Pham, The Son Trinh.
Supervision: Quyet Do.
Writing – original draft: Huu Y Le, Van Ai Hoang.
Writing – review & editing: Dinh Phuc Pham, Khac Tuyen Nguyen, The Son Trinh, Quyet Do.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CT = computed tomography, HU = Hounsfield unit, PSP = pulmonary sclerosing pneumocytoma, TTF-1 = thyroid transcription factor-1.
How to cite this article: Le HY, Pham DP, Nguyen KT, Hoang VA, Trinh TS, Do Q. Pulmonary sclerosing pneumocytoma in an 18-year-old male patient: a case report and literature review. Medicine. 2020;99:26(e20869).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9:53–75. [DOI] [PubMed] [Google Scholar]
- [2].Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, et al. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000;24:906–16. [DOI] [PubMed] [Google Scholar]
- [3].Illei PB, Rosai J, Klimstra DS. Expression of thyroid transcription factor-1 and other markers in sclerosing hemangioma of the lung. Arch Pathol Lab Med 2001;125:1335–9. [DOI] [PubMed] [Google Scholar]
- [4].Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- [5].Shin SY, Kim MY, Oh SY, et al. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015;94:e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Im JG, Kim WH, Han MC, et al. Sclerosing hemangiomas of the lung and interlobar fissures: CT findings. J Comput Assist Tomogr 1994;18:34–8. [DOI] [PubMed] [Google Scholar]
- [7].Chung MJ, Lee KS, Han J, et al. Pulmonary sclerosing hemangioma presenting as solitary pulmonary nodule: dynamic CT findings and histopathologic comparisons. AJR Am J Roentgenol 2006;187:430–7. [DOI] [PubMed] [Google Scholar]
- [8].Xie R, Zhou X, Lü P, et al. Diagnosis of pulmonary sclerosing hemangioma with incremental dynamic CT: analysis of 20 cases. Zhonghua Jie He He Hu Xi Za Zhi 2003;26:7–9. [PubMed] [Google Scholar]
- [9].Wang Q-B, Chen Y-Q, Shen J-J, et al. Sixteen cases of pulmonary sclerosing haemangioma: CT findings are not definitive for preoperative diagnosis. Clin Radiol 2011;66:708–14. [DOI] [PubMed] [Google Scholar]
- [10].Niho S, Suzuki K, Yokose T, et al. Monoclonality of both pale cells and cuboidal cells of sclerosing hemangioma of the lung. Am J Pathol 1998;152:1065–9. [PMC free article] [PubMed] [Google Scholar]
- [11].Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol 2014;32:3673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:668–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lovrenski A, Vasilijević M, Panjković M, et al. Sclerosing pneumocytoma: a ten-year experience at a Western Balkan University Hospital. Medicina (Kaunas) 2019;55:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang C-H, Lee L-Y. Pulmonary sclerosing pneumocytoma remains a diagnostic challenge using frozen sections: a clinicopathological analysis of 59 cases. Histopathology 2018;72:500–8. [DOI] [PubMed] [Google Scholar]
- [15].Iyoda A, Hiroshima K, Shiba M, et al. Clinicopathological analysis of pulmonary sclerosing hemangioma. Ann Thorac Surg 2004;78:1928–31. [DOI] [PubMed] [Google Scholar]
- [16].Chen B, Gao J, Chen H, et al. Pulmonary sclerosing hemangioma: a unique epithelial neoplasm of the lung (report of 26 cases). World J Surg Oncol 2013;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hu A-M, Zhao D, Zheng H, et al. Preoperative diagnosis in 46 cases of pulmonary sclerosing hemangioma. Chin Med J (Engl) 2016;129:1377–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hanaoka J, Ohuchi M, Inoue S, et al. Bilateral multiple pulmonary sclerosing hemangioma. Jpn J Thorac Cardiovasc Surg 2005;53:157–61. [DOI] [PubMed] [Google Scholar]
- [19].Maeda R, Isowa N, Miura H, et al. Bilateral multiple sclerosing hemangiomas of the lung. Gen Thorac Cardiovasc Surg 2009;57:667–70. [DOI] [PubMed] [Google Scholar]
- [20].Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004;233:191–9. [DOI] [PubMed] [Google Scholar]
- [21].Travis WD, Brambilla E, Muller-Hermelink HK. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Publications; 2004. [Google Scholar]
- [22].Travis WD, Colby TV, Corrin B, et al. Histological Typing of Lung and Pleural Tumours. 3rd ed.Berlin Heidelberg: Springer-Verlag; 1999. [Google Scholar]
- [23].Kim GY, Kim J, Choi YS, et al. Sixteen cases of sclerosing hemangioma of the lung including unusual presentations. J Korean Med Sci 2004;19:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chan AC, Chan JK. Pulmonary sclerosing hemangioma consistently expresses thyroid transcription factor-1 (TTF-1): a new clue to its histogenesis. Am J Surg Pathol 2000;24:1531–6. [DOI] [PubMed] [Google Scholar]
- [25].Kalhor N, Staerkel GA, Moran CA. So-called sclerosing hemangioma of lung: current concept. Ann Diagn Pathol 2010;14:60–7. [DOI] [PubMed] [Google Scholar]
- [26].Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, et al. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med 2003;127:321–5. [DOI] [PubMed] [Google Scholar]
- [27].Xu H-M, Zhang G. A rare case of pulmonary sclerosing hemagioma with lymph node metastasis and review of the literature. Int J Clin Exp Pathol 2015;8:8619–23. [PMC free article] [PubMed] [Google Scholar]
- [28].Rivera E, Gesthalter Y, VanderLaan P, et al. Pulmonary sclerosing pneumocytoma. J Bronchology Interv Pulmonol 2018;25:e54–6. [DOI] [PubMed] [Google Scholar]
- [29].Fayers RW, Lim TS, Havlat MF. Pulmonary sclerosing pneumocytoma (sclerosing haemangioma): radical radiation therapy. J Med Imaging Radiat Oncol 2016;60:693–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


