Supplemental Digital Content is available in the text
Keywords: antibiotic susceptibility, clinical infection, empyema, Staphylococcus saccharolyticus, treatment
Abstract
Background:
Staphylococcus saccharolyticus is a rare cause of human infectious disease. The clinical characteristics and treatment of patients with S saccharolyticus infections remain largely unknown.
Objectives:
We present the first reported case of empyema due to S saccharolyticus. In addition, a systematic review and pooled analysis of all S saccharolyticus cases were done to summarize the clinical and microbiological characteristics and treatment of this rare pathogen.
Methods:
A case of empyema caused by S saccharolyticus diagnosed in study hospital was reported. This case and those identified from PubMed, EMBASE, and Web of Science were analyzed.
Results:
In total, 8 patients were reviewed. The averages of the white blood cell count, sedimentation rate, and C-reactive protein were 16.8 × 109/L, 72 mm/h, and 176 mg/L, respectively. The average time-to-positivity of the anaerobic cultures was 5 days. The S saccharolyticus was resistant to metronidazole, but susceptible to fluoroquinolones, clindamycin, and vancomycin in all the cases with drug sensitivity tests available for these antibiotics. Two of 7 patients showed resistance to all β-lactams. Both of those patients finally died.
Conclusions:
S saccharolyticus should be added to the list of anaerobic microorganisms that are able to cause empyema. A prolonged anaerobic culture is critical to improve the yield of this possibly underestimated pathogen. The time to positive culture of S saccharolyticus may not help to distinguish true-positive growth from contaminated growth. Acute or subacute courses and systemic evidence of infection may contribute to judge the clinical significance of positive cultures and avoid unnecessary antibiotic treatment. β-Lactam agents plus fluoroquinolones or vancomycin/teicoplanin or clindamycin may be appropriate to achieve full coverage of the β-lactam resistant bacteria.
1. Introduction
Staphylococcus saccharolyticus is the only anaerobic species within the genus Staphylococcus. It belongs to the coagulase-negative staphylococci group, and it is part of the normal bacterial flora of the human skin.[1]S saccharolyticus is a rare cause of human infectious disease, but has previously been reported in endocarditis, spondylodiscitis, bone marrow infections, pneumonia, and pyomyositis cases.[2–8]
Empyema, the term used for a bacterial pleural infection, indicates pus in the pleural space or the presence of bacteria in the pleural fluid, as evidenced by a Gram stain or culture.[9] The common community-acquired pleural infection pathogens include streptococcal species, followed by anaerobic bacteria (20%), and staphylococci (10%).[10] To our knowledge, there have been no published reports of empyema associated with S saccharolyticus. Therefore, we have reported the first such case here. Moreover, no data have been presented regarding the clinical features, microbiological results, and treatment outcomes of an S saccharolyticus clinical infection. Therefore, we have also conducted a review of all the patients infected with S saccharolyticus reported in the literature, including our case, to explore these topics. Addressing these issues may help clinicians improve their understanding and management of this unusual cause of infectious diseases.
2. Case presentation
A 54-year-old man was admitted to our hospital with complaints of 10 days of fever and left-sided chest pain on November 18, 2017. He had noted the onset of a moderate grade fever (38.4°C) and severe left chest pain with a visual analog scale (VAS) score of 6 on November 8, 2017. He was admitted to the local hospital 2 days later. The results of his blood tests were as follows: white blood cell count (WBC) 13 × 109/L, 90% neutrophils, hemoglobin (Hb) concentration 134 g/L, platelets 252 × 109/L, erythrocyte sedimentation rate (ESR) 98 mm/h, and C-reactive protein (CRP) 245 mg/L. A chest computerized tomography (CT) scan (Fig. 1A) showed left pleural effusion. He had been given intravenous antibiotic injections daily for 1 week. However, this patient continued to suffer from a fever, and his chest pain progressively worsened, with a VAS score of 8 to 9. At that point he was referred to our hospital. His physical examination showed the following: body temperature 38.3°C, pulse 90 beats/min, breathing rate 18 breaths/min, blood pressure 99/72 mm Hg, and percutaneous oxygen saturation 95% (room air). His respiratory system examination findings were consistent with mild to moderate left-sided pleural effusion, and no abnormalities were detected in the other systems. He had a history of type 2 diabetes mellitus for 10 years, which was not well controlled and monitored. A repeated CT scan showed increased left pleural effusion (Fig. 1B) and newly formed encapsulated effusion in the left interlobar fissure (Fig. 1C). The results of the laboratory examination were as follows: WBC 12 × 109/L, neutrophils 80%, Hb 134 g/L, platelets 603 × 109/L, ESR 98 mm/h, CRP 143 mg/L, procalcitonin negative, and T-SPOT.TB (tuberculosis test) 0 spot-forming cells/106 peripheral blood mononuclear cells. The rheumatology-associated antibody titers were negative, including the antinuclear, anti-dsDNA, antineutrophil cytoplasmic, and antiextractable nuclear antigen antibodies.
Figure 1.
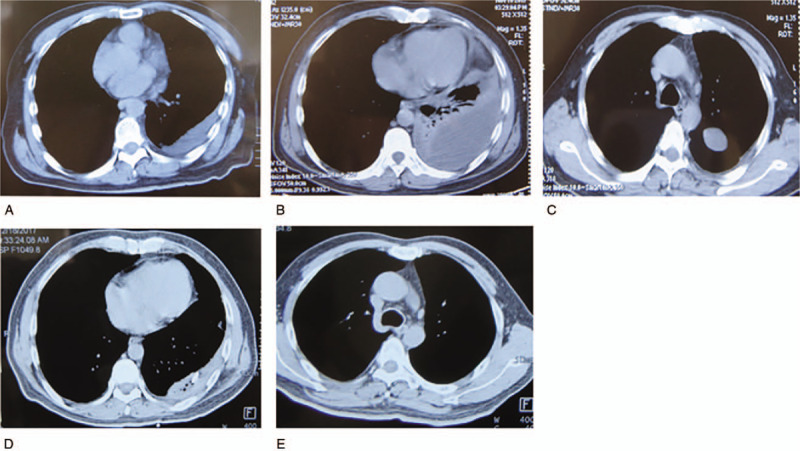
Pleural effusion on chest CT at the local hospital (A), increased pleural effusion (B), and newly formed encapsulated effusion (C) on CT upon admission, improvement in the left pleural effusion (D) and the encapsulated effusion (E) on CT before discharge.
CT-guided therapeutic thoracentesis and chest tube drainage were performed. The pleural aspirate was viscous and purulent. An analysis of the pleural aspirate showed the following values: WBC 1576/mm3, neutrophils 84%, total protein 59 g/L, albumin 30 g/L, lactate dehydrogenase 4240 IU/L, pH 7.0, adenosine deaminase 60.6 IU/L, glucose 5.8 mmol/L, T-SPOT.TB 0 spot forming cells (SFCs)/106 peripheral blood mononuclear cells (PBMCs), normal carcinoembryonic antigen, negative tuberculosis/nontuberculous mycobacterium DNA amplification, and negative acid-fast bacilli staining. The pleural fluid culture was negative for aerobic bacteria, fungi, and mycobacteria. Only the anaerobic bottle was positive after 5 days of incubation. The positive strain was inoculated on blood agar and anaerobically cultured. Visible white, small, smooth, round, neat-edged colonies emerged on the blood sheep agar plate (Fig. 2A), and Gram staining revealed gram-positive cocci under a microscope (Fig. 2B). The matrix-assisted laser desorption/ionization-time of flight examination indicated that the organism was S saccharolyticus [identification rate 99.9%, supplemental digital content (e-Fig. 1)]. Molecular identification via the polymerase chain reaction amplification of the gap gene was performed, and the amplification product was sequenced. The resulting gene sequences were submitted to GenBank for Basic Local Alignment Search Tool (BLAST) alignment. Based on the BLAST analysis of the gap gene, only S saccharolyticus showed 99% homology. The minimum inhibitory concentration of the isolate was <0.002 μg/mL for penicillin, <0.016 μg/mL for amoxicillin-clavulanate, 0.032 μg/mL for ceftriaxone, 0.064 μg/mL for cefotaxime, 0.25 μg/mL for oxacillin, <0.016 μg/mL for cefoperazone, <0.002 μg/mL for ertapenem, 0.006 μg/mL for imipenem, 0.023 μg/mL for moxifloxacin, <0.002 μg/mL for levofloxacin, 0.064 μg/mL for clindamycin, 3 μg/mL for chloramphenicol, 0.5 μg/mL for linezolid, 0.75 μg/mL for vancomycin, and 256 μg/mL for metronidazole.
Figure 2.
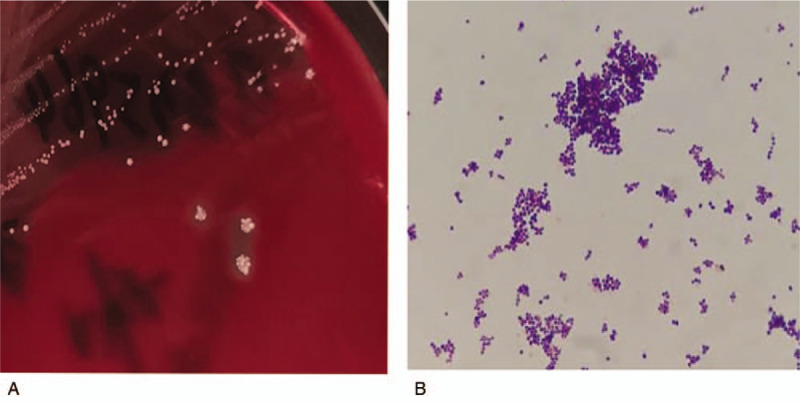
Colony morphology on anaerobic blood sheep agar (A) and Gram stain presentation (oil mirror, ×1000) (B) of the Staphylococcus saccharolyticus clinical isolate.
Before the culture and drug susceptibility test results becoming available, the patient was given an empirical intravenous antibiotic treatment consisting of ceftazidime (2 g two times daily) and moxifloxacin (0.4 g one time daily). At the same time, his blood glucose was monitored and controlled by insulin. This patient responded well, with resolutions of the fever and left-sided chest pain and reductions in the WBC to normal, ESR to 35 mm/h, and CRP to normal. A repeated CT scan before discharge showed significant improvement in the left pleural effusion (Fig. 1D) and disappearance of the encapsulated effusion in the left interlobar fissure (Fig. 1E). At this point, the treatment was switched to the oral administration of moxifloxacin (0.4 g one time daily) for 4 weeks.
3. Materials and methods
3.1. Search strategy and selection criteria
3.1.1. Identification
A full systematic search of the PubMed, Embase, and Web of Science databases using the keyword “Staphylococcus saccharolyticus” in title and abstract was conducted on February 2019. No language limit or time span was set for any of the searches. The overall search yielded 34 abstracts from PubMed, 32 abstracts from Embase, and 8 abstracts from the Web of Science. After removing the duplicates, a total of 45 abstracts were identified. The study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital (No. SK-449). Informed consent was obtained from the patient.
3.1.2. Screening
One full-text article written in Polish was removed; therefore, 44 full-text articles were reviewed independently by 2 authors. All the uncertainties were resolved through a discussion between the 2 reviewers. A case was considered after it met the diagnosis of an infection caused by S saccharolyticus based on the following requirements: systemic and local symptoms of infection; radiological, laboratory, and/or histopathological evidence consistent with infection; and microbiological evidence of S saccharolyticus in a local infectious site. Thus, 37 full-text articles that did not fulfill the diagnosis criteria were excluded. Finally, 7 full-text articles describing a total of 7 cases were included. The publication retrieval procedure and the inclusion and exclusion of the cases are illustrated in a flow chart (Fig. 3).
Figure 3.
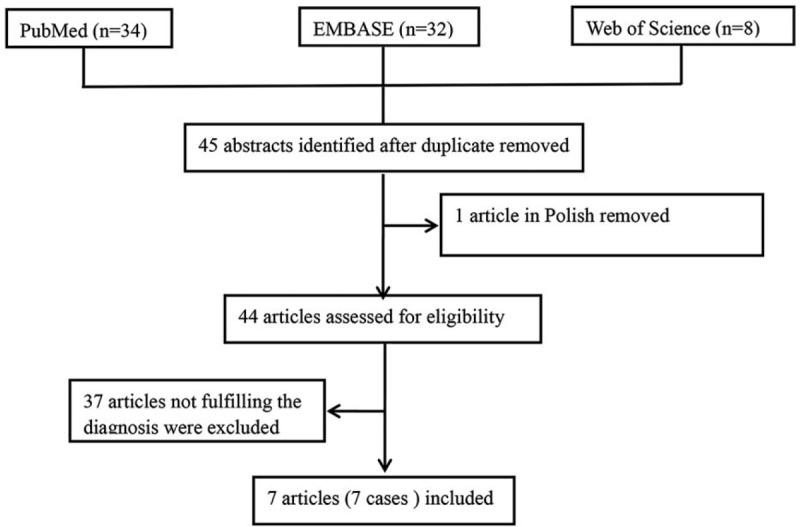
Flow chart of publication retrieval procedure and inclusion and exclusion of cases.
3.1.3. Data extraction
The following data were extracted from the eligible cases and recorded on a standard data extraction form age at diagnosis, sex, diagnosis, underlying diseases, duration between onset of symptoms and final diagnosis, symptoms, ESR, CRP, WBC, specimen and time-to-positivity, drug sensitivity test results, and antibiotic treatment and clinical outcome (categorized as relieved/improved or died).
3.2. Statistical analysis
All the data analyses were carried out with Statistical Package for the Social Sciences, version 17.0 for Windows (SPSS Inc, Chicago, IL). The continuous data were summarized as the mean and range, whereas the categorical variables were presented as the percentage. Because not all of the case reports provided sufficient details about the above-mentioned data, the median, range, and percentage reported here refer only to those cases with available data.
4. Results
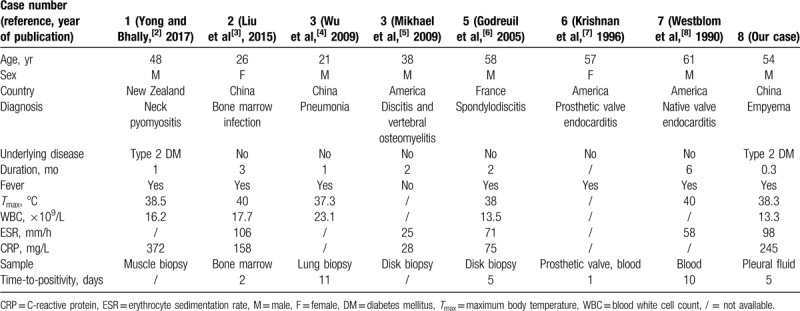
Including our case, we found a total of 8 patients with various infections caused by S saccharolyticus. The demographics, diagnoses, clinical features, and laboratory and microbiological results are summarized in Table 1. The median patient age was 45 years old (range: 21–61). Two of 8 patients had a history of type 2 diabetes mellitus, whereas other patients had no underlying disease. The mean duration of symptoms was 2.2 months (range: 0.3–6). The averages of the WBC, ESR, and CRP values were 16.8 × 109/L, 72 mm/h, and 176 mg/L, respectively. All the patients presented with systemic clinical presentations of infection, such as a fever and/or elevated ESR, CRP, and WBC values. The microbiological evidence of an infection caused by S saccharolyticus came from anaerobic cultures of the blood, bone marrow, pus, or biopsy tissues. The average time-to-positivity of the cultures was 5 days (range: 1–11).
Table 1.
Demographics, diagnosis, clinical features, laboratory, and microbiological results of the 8 cases included in the review.
The drug sensitivity results of the 8 cases are summarized in Figure 4. The saccharolyticus was resistant to metronidazole and susceptible to fluoroquinolones, clindamycin, vancomycin, teicoplanin, chloramphenicol, pristinamycin, erythromycin, and rifampin in all the cases with drug sensitivity tests available for these antibiotics. In the 7 patients with susceptibility results available for β-lactams, 5 patients exhibited sensitivity, and 2 patients exhibited resistance.
Figure 4.
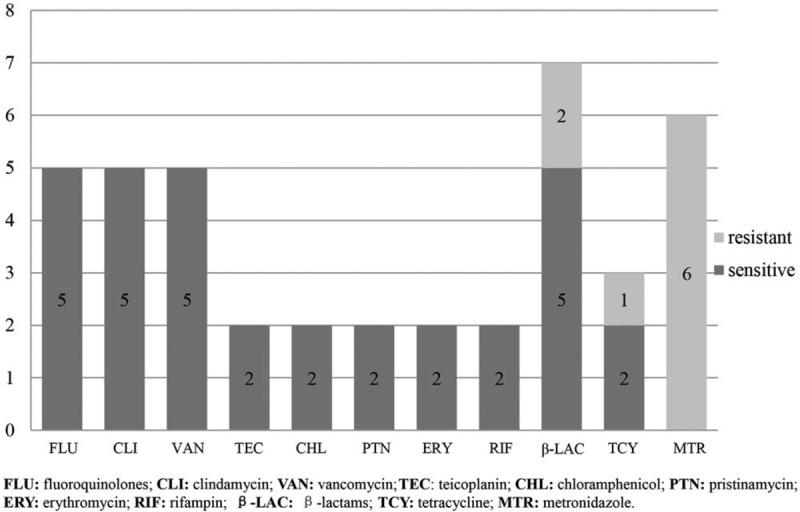
Cases with available drug susceptibility results for each antibiotic. The bars indicate the number of cases with available results. The proportions of the cases sensitive and resistant to each antibiotic are shown in black and gray, respectively, with the number in the bars.
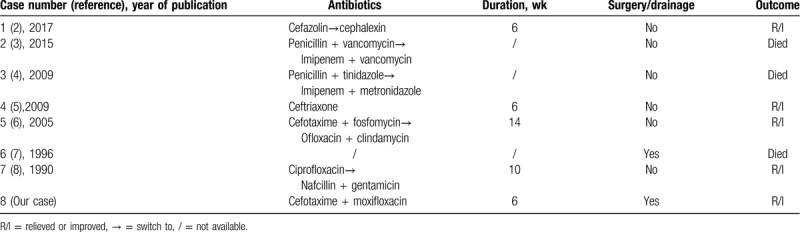
All the patients received intravenous and/or oral antibiotic treatments. As shown in Table 2, the treatment durations were based on the diagnosis, and they varied from 6 to 14 weeks. The antibiotics received by the patients consisted mainly of β-lactams and/or fluoroquinolones. Five of the 8 patients had effective recovery results; however, 3 patients died (cases 2, 3, and 6). Two of them (cases 3 and 6) exhibited resistance to all the β-lactams.
Table 2.
Summary of the treatment and outcomes of 8 cases with various infections caused by S saccharolyticus.
5. Discussion
S saccharolyticus is rarely associated with human disease. In the literature, <10 human infection cases caused by S saccharolyticus have been reported. This organism has also been isolated from blood cultures,[11,12] contaminated platelet concentrates,[13] the sputum of patients with cystic fibrosis,[14] and pancreatic fluid collected from the percutaneous drainage of pseudocysts and abscesses.[15] However, the clinical features were not enough to establish the diagnosis of infection, and the positive cultures were not clinically significant. In our case, the patient presented with the typical systemic and local symptoms of infection and the laboratory and radiological evidence of empyema. Purulent pleural fluid was aspirated via an aseptic procedure, and other organisms were absent in the bacterial, fungal, and mycobacterial cultures. Moreover, the patient responded well to the susceptible antibiotic treatment. These all support the pathogenic role of S saccharolyticus in this case. This is the first reported occurrence of an S saccharolyticus infection in the pleural space, which indicates that S saccharolyticus should be added to the list of anaerobic microorganisms that are able to cause empyema.
Notably, S saccharolyticus was only isolated from the anaerobic culture and our study showed that the average time-to-positivity was 5 days. The longest was 11 days. It was much longer than the time to positivity of clinical isolates which were considered as pathogen.[16] This finding implied that S saccharolyticus could be an underestimated pathogen due to its anaerobic growth and long time required for the positive culture which was usually considered as predicting contaminants. Therefore, a prolonged incubation time should be recommended to improve the yield of this possibly underestimated pathogen. Furthermore, the time to positive culture of S saccharolyticus may not help to distinguish true-positive growth from contaminated growth given the significant delay that the growth time presents.
All of the S saccharolyticus infection cases in our review demonstrated acute or subacute courses and systemic evidence of infection, such as a fever and/or obviously elevated ESR, CRP, and WBC, other than the local evidence of infection. This indicates that an S saccharolyticus infection usually exhibits the clinically typical presentation of a bacterial infection. It may have been suspected that the positive cultures were “contaminants” and not clinically significant when there was a lack of systemic presentation of an infection, because S saccharolyticus is part of the normal bacterial flora of the human skin. Schneeberger et al[17,18] reported 2 case series (17 cases) of patients without typical systemic and local symptoms of a shoulder joint infection (except pain). The S saccharolyticus was isolated from the joint tissue probes during arthroscopy in the 2 cases. The antibiotic treatments were given based on the results of the drug susceptibility tests; however, the effects of the antibiotic treatments were disappointing, even after negative cultures were achieved. Therefore, whether these bacteria were pathogenic organisms and had clinical significance requires further investigation. Clinicians should be aware of the clinical features of infections caused by S saccharolyticus to appropriately judge the clinical significance of positive cultures and avoid unnecessary antibiotic treatment.
It was shown that S saccharolyticus was susceptible to fluoroquinolones, vancomycin, teicoplanin, clindamycin, and erythromycin, whereas it was resistant to metronidazole, even though it is an anaerobe. Resistance to all the β-lactam agents was found in 2 of 7 cases, and both of those patients finally died. One of the pneumonia cases had not been treated with the susceptible antibiotics before death due to the lack of previous experience with this bacterium. The final identification and drug sensitivity results were obtained 11 days after the date of the lung biopsy.[4] Therefore, when considering empirical antibiotic therapy for this bacterium which is usually necessary given the long time required for growth and drug susceptibility test, metronidazole should be avoided, and β-lactam agents plus fluoroquinolones or vancomycin/teicoplanin or clindamycin may be appropriate to achieve full coverage of the β-lactam resistant bacteria.
Five of the patients showed clinical and radiological improvement after the antibiotic therapy, whereas 3 patients died. Case 6 was infected by the β-lactam resistant isolate and died of heart failure caused by endocarditis.[7] One possible reason for the treatment failure in case 3 was the use of insensitive empirical antibiotics.[4] Moreover, Liu et al[3] believed that the antibiotic concentration in the bone marrow was not high enough due to the bone marrow-blood barrier, even though sensitive antibiotics were given, and this was one possible explanation for the unsatisfying treatment effect in case 2. An early diagnosis and early start of sufficient treatment with susceptible antibiotics are crucial for an effective recovery.
There were several limitations to our study. First, this was a retrospective review of the cases reported in the literature. Not all the reports provided sufficient details regarding the clinical symptoms, laboratory results, drug susceptibility test results, and treatment. Second, only 8 cases were included in this systemic review because S saccharolyticus is a rare cause of human infectious disease. Therefore, the extrapolation of our findings is limited, given the small sample size. Further studies with larger sample sizes are needed.
6. Conclusions
S saccharolyticus should be added to the list of unusual organisms capable of causing clinical infectious diseases, including endocarditis, spondylodiscitis, bone marrow infections, pyomyositis, pneumonia, and empyema. A prolonged anaerobic culture is critical to establish the etiological diagnosis and to improve the yield of this possibly underestimated pathogen. The time to positive culture of S saccharolyticus may not help to distinguish true-positive growth from contaminated growth. Acute or subacute courses and systemic evidence of infection may help to judge the clinical significance of positive cultures and avoid unnecessary antibiotic treatment. Clinicians should be aware of the drug susceptibility pattern demonstrated in our study to ensure that the correct empirical antibiotics are selected, because 1 to 2 weeks are required from culture through identification to the final drug sensitivity tests. An early etiological diagnosis and starting a sufficiently susceptible antibiotic treatment in time are necessary to avoid devastating outcomes. We hope our report and review contribute to improving the understanding and management of this rare anaerobic Staphylococcus.
Author contributions
PW and YL contributed to study design; data collection and analysis; statistical analysis; and writing, review and approval of the final manuscript. ZX and YX contributed to oversight of data collection and revised the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Footnotes
Abbreviations: BLAST = Basic Local Alignment Search Tool, CRP = C-reactive protein, CT = computerized tomography, ESR = erythrocyte sedimentation rate, Hb = hemoglobin, S. saccharolyticus = Staphylococcus saccharolyticus, VAS = visual analog scale, WBC = white blood cell count.
How to cite this article: Wang P, Liu Y, Xu Y, Xu Z. Staphylococcus saccharolyticus infection: case series with a PRISMA-compliant systemic review. Medicine. 2020;99:26(e20686).
PW and YL contributed equally to this work.
This research was funded by Key Program of Precision Medicine from National Key Research and Development Plan (2016YFC0905700).
Supplemental Digital Content is available for this article.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Evans CA, Mattern KL, Hallam SL. Isolation and identification of Peptococcus saccharolyticus from human skin. J Clin Microbiol 1978;7:261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yong N, Bhally H. Bilateral neck pyomyositis caused by Staphylococcus capitis and Staphylococcus. Case Rep Infect Dis 2017;2017:3713212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu CJ, Sun B, Guo J, et al. A case of bone marrow infection by Staphylococcus saccharolyticus. Eur Rev Med Pharmacol Sci 2015;19:1161–3. [PubMed] [Google Scholar]
- [4].Wu X, Yu C, Wang X. A case of Staphylococcus saccharolyticus pneumonia. Int J Infect Dis 2009;13:e43–6. [DOI] [PubMed] [Google Scholar]
- [5].Mikhael MM, Bach HG, Huddleston PM, et al. Multilevel diskitis and vertebral osteomyelitis after diskography. Orthopedics 2009;32:60. [DOI] [PubMed] [Google Scholar]
- [6].Godreuil S, Jean-Pierre H, Morel J, et al. Unusual case of spondylodiscitis due to Staphylococcus saccharolyticus. Joint Bone Spine 2005;72:91–3. [DOI] [PubMed] [Google Scholar]
- [7].Krishnan S, Haglund L, Ashfaq A, et al. Prosthetic valve endocarditis due to Staphylococcus saccharolyticus. Clin Infect Dis 1996;22:722–3. [DOI] [PubMed] [Google Scholar]
- [8].Westblom TU, Gorse GJ, Milligan TW, et al. Anaerobic endocarditis caused by Staphylococcus saccharolyticus. J Clin Microbiol 1990;28:2818–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koppurapu V, Meena N. A review of the management of complex para-pneumonic effusion in adults. J Thorac Dis 2017;9:2135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maskell NA, Batt S, Hedley EL, et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 2006;174:817–23. [DOI] [PubMed] [Google Scholar]
- [11].Hitzenbichler F, Simon M, Salzberger B, et al. Clinical significance of coagulase-negative staphylococci other than S. epidermidis blood stream isolates at a tertiary care hospital. Infection 2017;45:179–86. [DOI] [PubMed] [Google Scholar]
- [12].Steinbrueckner B, Singh S, Freney J, et al. Facing a mysterious hospital outbreak of bacteraemia due to Staphylococcus saccharolyticus. J Hosp Infect 2001;49:305–7. [DOI] [PubMed] [Google Scholar]
- [13].Ali H, Rood IG, De Korte D, et al. Strict anaerobic Staphylococcus saccharolyticus isolates recovered from contaminated platelet concentrates fail to multiply during platelet storage. Transfusion 2012;52:916–7. [DOI] [PubMed] [Google Scholar]
- [14].Worlitzsch D, Rintelen C, Böhm K, et al. Antibiotic-resistant obligate anaerobes during exacerbations of cystic fibrosis patients. Clin Microbiol Infect 2009;15:454–60. [DOI] [PubMed] [Google Scholar]
- [15].Malecka-Panas E, Juszynski A, Chrzastek J, et al. Pancreatic fluid collections: diagnostic and therapeutic implications of percutaneous drainage guided by ultrasound. Hepatogastroenterology 1998;45:873–8. [PubMed] [Google Scholar]
- [16].Ruiz-Giardín JM, Martin-Díaz RM, Jaqueti-Aroca J, et al. Diagnosis of bacteraemia and growth times. Int J Infect Dis 2015;41:6–10. [DOI] [PubMed] [Google Scholar]
- [17].Schneeberger AG, Yian E, Steens W. Injection-induced low-grade infection of the shoulder joint: preliminary results. Arch Orthop Trauma Surg 2012;132:1387–92. [DOI] [PubMed] [Google Scholar]
- [18].Schneeberger AG, Gilbart MK, Sheikh R, et al. Non-purulent low-grade infection as cause of pain following shoulder surgery: preliminary results. Chir Organi Mov 2009;93: Suppl 1: S71–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


