Abstract
No-reflow is an important complication among patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI).
A retrospective study of 1658 STEMI patients undergoing direct PCI was performed. Patients were randomly assigned at a 7:3 ratio into development cohort and validation cohort and into no-reflow and normal blood flow groups. Clinical data and laboratory examinations were compared to identify independent risk factors and establish a no-reflow risk scoring system.
In the development cohort (n = 1122), 331 (29.5%) had no-reflow. Multivariate analysis showed age ≥ 65 years (OR = 1.766, 95% confidence interval (CI): 1.313–2.376, P < .001), not using angiotonase inhibitor/angiotensin receptor antagonists (OR = 1.454, 95%CI: 1.084–1.951, P = .013), collateral circulation <grade 2 (OR = 3.056, 95%CI: 1.566–5.961, P = .001), thrombosis burden ≥4 points (OR = 2.033, 95%CI: 1.370–3.018, P < .001), diameter of target lesion ≥3.5 mm (OR = 1.511, 95%CI: 1.087–2.100, P = .014), thrombosis aspiration (OR = 1.422, 95%CI: 1.042–1.941, P = .026), and blood glucose >8 mmol/L (OR = 1.386, 95%CI: 1.007–1.908, P = .045) were related to no-reflow. Receiver operating characteristic (ROC) area under the curve was 0.648 (95%CI: 0.609–0.86). At 0.349 cutoff sensitivity was 42.0%, specificity was 79.3%, positive predictive value (PPV) was 44.7%, negative predictive value (NPV) was 77.4%, P < .001. The resulting risk scoring system was tested in the validation cohort (n = 536), with 30.1% incidence of no-reflow. The area under the ROC curve was 0.637 (95%CI: 0.582–0.692). At a cutoff of 0.349 sensitivity was 53.2% and specificity was 66.7%, PPV was 41.2%, NPV was 76.4%, P < .001.
The no-reflow risk scoring system was effective in identifying high-risk patients.
Keywords: no-reflow phenomenon, percutaneous coronary intervention, risk factors, ST-segment elevation myocardial infarction
1. Introduction
Worldwide, cardiovascular disease (CVD) is frequent, and one of the leading causes death.[1] The latest reports have shown that the number of patients with CVD in China is increasing. It is estimated that there are 230 million CVD patients in China, and approximately 3 million people die of CVD every year, accounting for 41% of the total cause of death.[2] Reducing the mortality of myocardial infarction depends largely on early diagnosis and effective treatment. Early active initiation of effective coronary reperfusion therapy is an important part of the treatment of myocardial infarction. The most serious form of acute coronary syndrome is ST-segment elevation myocardial infarction (STEMI).[2] In China, data from 2001 to 2011 showed hospital admissions for STEMI had risen and mortality had remained constant.[3]
Currently, direct percutaneous coronary interventions (PCIs) are the preferred choice for the treatment of STEMI. This can reduce the mortality of acute myocardial infarction (AMI).[4] However, on occasion while the infarction related artery (IRA) of STEMI patients has been successfully opened during PCI, the myocardial tissue may not have reached effective reperfusion; this phenomenon is called “no-reflow.”[5] At present, the exact mechanism of no-reflow remains unclear, but clinical and laboratory findings suggest that it is related to the embolism of the capillary bed, ischemic injury, vascular endothelial dysfunction, production of oxygen free radical, inflammatory reaction, stress response, calcium overload, and other factors.[5]
Although reperfusion techniques for STEMI are constantly improving, no-reflow can still lead to poor prognosis.[6] The incidence of no-reflow after routine PCI, measured by thrombolysis in myocardial infarction (TIMI) grade, is 1% to 5%, and the incidence of no-reflow in AMI patients is 2.3% to 41%.[7–12] However, even with good TIMI grade, myocardial perfusion is less than effective in 15% to 40% of cases with TIMI myocardial perfusion grade (TMPG) at grade 0 to 1.[13] Grade 3 TMPG is necessary to achieve adequate and effective myocardial perfusion.[14] Preoperative evaluation of the risk of no-reflow in patients allows early intervention and active implementation of a treatment strategy to prevent no-reflow occurring.
Various studies have shown different factors are related to the occurrence of no-reflow in the treatment of AMI,[7,15,16] but the larger studies tend to concentrate on the whole AMI population and do not distinguish patients with STEMI. Meta-analysis of studies into the no-reflow phenomenon in STEMI suggests that TIMI flow ≤1 and high thrombus burden are the most impacted no-reflow risk factors.[17] These and other risk factors could be used to establish a risk score for patients with STEMI undergoing PCI for no-reflow and some studies have tried to establish a risk score on this basis.[18–21] However, the simple risk scoring systems have shown different results, and some either need expensive medical equipment, are cumbersome to use, or delay the prediction and so are unsatisfactory. A simple scoring system that comprehensively integrates common clinical risk factors, surgical procedure parameters, and grades risk level while being convenient and practical is needed.
The objective of this study was to retrospectively analyze the clinical data of patients with acute STEMI undergoing direct PCI. This information was then used to screen clinical risk factors related to the no-reflow phenomenon at IRA; to establish a no-reflow risk scoring system; and verify the authenticity and reliability of the risk scoring system. This scoring system may help prevent reperfusion injury in clinical practice, reducing the no-reflow phenomenon and complications, and improving the prognosis of patients with STEMI.
2. Methods
2.1. Participants
This was a retrospective cohort study. Patients who underwent direct PCI in the Department of Cardiology of Tianjin Chest Hospital from January 2010 to May 2016 were collected. The inclusion criteria were as follows:
-
1)
patients aged ≥18 years;
-
2)
patients with onset time ≤24 hours;
-
3)
patients diagnosed with acute STEMI;
-
4)
patients with successful patency of culprit vessels.
The exclusion criteria were:
-
1)
patients who were allergic to antiplatelet drugs, anticoagulants, or iodine-containing contrast agents;
-
2)
patients with contraindications for anticoagulant therapy, such as active visceral hemorrhage, hemorrhagic stroke, or ischemic stroke within half a year (including transient ischemic attack), or aortic dissection, or patients with hematological diseases complicated with coagulation disorders;
-
3)
patients who had undergone coronary artery bypass grafting;
-
4)
patients who had valvular disease or cardiomyopathy;
-
5)
patients with complete left bundle branch block, pre-excitation syndrome, pacemaker electrocardiogram (ECG), and other factors affecting ST-segment changes in ECG;
-
6)
patients with severe liver and renal dysfunction;
-
7)
patients with malignant tumors or autoimmune diseases;
-
8)
patients who had severe infectious diseases recently.
The diagnosis of STEMI was based on the Chinese 2015 Guidelines for the Management of Acute ST-segment Elevation Myocardial Infarction.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Ethics Committee of Tianjin Chest Hospital. Informed consent was waived because of the study's retrospective nature.
2.2. Treatments and evaluations of no-reflow phenomenon
All coronary angiography, PCI, and reperfusion therapy strategies were performed by experienced cardiologists. The patients underwent coronary angiography and PCI through standard radial/femoral artery approaches. According to the IRA lesions, thrombus aspiration, percutaneous transluminal coronary angioplasty (PTCA), or stenting were performed, and successful vascular patency was determined by the residual stenosis ≤10%.
Coronary angiography images were read and evaluated by 2 cardiologists with more than 10 years of clinical experience, and the TIMI flow grading and TMPG grading before and after PCI, including IRA, number of lesion vessels, degree of coronary stenosis, thrombus burden, and collateral circulation were evaluated. The degree of stenosis, lesion length, and lumen diameter of the coronary lesions before stent implantation were measured by quantitative coronary angiography (QCA).
The thrombus burden[22] was defined from grade 0 to 5. The collateral circulation[9] was defined as from grade 0 to 3. The TIMI flow grading[23] was from grade 0 to 3. TMPG[24] was from grade 0 to 3.
TIMI ≤grade 2, or TIMI grade 3 with TMPG grade 0 to 1, was diagnosed as no-reflow. Patients were assigned to no-reflow group and normal blood flow group according to whether there was no-reflow phenomenon.
Perioperative essential medications followed clinical guidelines.[20] The application of an angiotensin converting enzyme inhibitor or angiotensin II receptor antagonist (ACEI/ARB), beta blockers and statins, and the types and doses were determined by the clinicians according to the patient's condition. The use of tirofiban was controlled by the physicians according to the patient's condition.
2.3. Data collection
Demographic data, preoperative findings were collected. Perioperative serological examinations included creatine phosphokinase isoenzyme-MB (CK-MB), troponin I, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triacylglycerol, lipoprotein (LP), percentage of neutrophils (NEUT%), neutrophil count (NEUT#), lymphocyte count (LYMP#), serum creatinine (Cr), blood uric acid (UA), blood glucose (GLU), and other biochemical indicators. The 18-lead ECG was performed once on admission and 90 minutes after surgery. ST-segment resolution was classified as complete (>70%), partial (70–30%), or no improvement (<30%) based on the maximum single lead of ST-segment elevation.[21] The characteristics of the coronary artery lesions were also measured and recorded during PCI, as well as PCI-related parameters.
2.4. Statistical analysis
Statistical treatments were performed using statistical software SPSS Statistics 19.0 (IBM Corp., Armonk, NY). All patients were randomly assigned at a 7:3 ratio to a development cohort and a validation cohort. The measurement data with normal distribution were expressed as mean ± standard deviation, and comparison between the 2 groups was performed using unpaired t test; the measurement data with non-normal distribution were represented by median (range), and comparison between the 2 groups was performed using Mann–Whitney U test. The count data were expressed by absolute value and percentage, and the Chi-square test or Fisher exact probability was used for statistical analysis.
Multivariate logistic regression (backward regression method) was used to screen independent risk factors of no-reflow during operation in patients with STEMI undergoing PCI. By referring to the methods in the literature, based on the multivariate logistic regression in the development cohort, the odds ratio (OR) was calculated, based on which the assignment to each risk factor was performed, and the corresponding scores were obtained. The risk scoring system was constructed, and the area under the receiver operating characteristic (ROC) curve was used to calculate the sensitivity, specificity, and area under the curve of the scoring system. P < .05 was considered statistically significant.
3. Results
3.1. Baseline clinical data
A total of 1658 patients with STEMI undergoing direct PCI were enrolled in the study, including 1122 in the development cohort and 536 in the validation cohort (Table 1).
Table 1.
Baseline data of all patients included in the study in the model and validation cohorts.
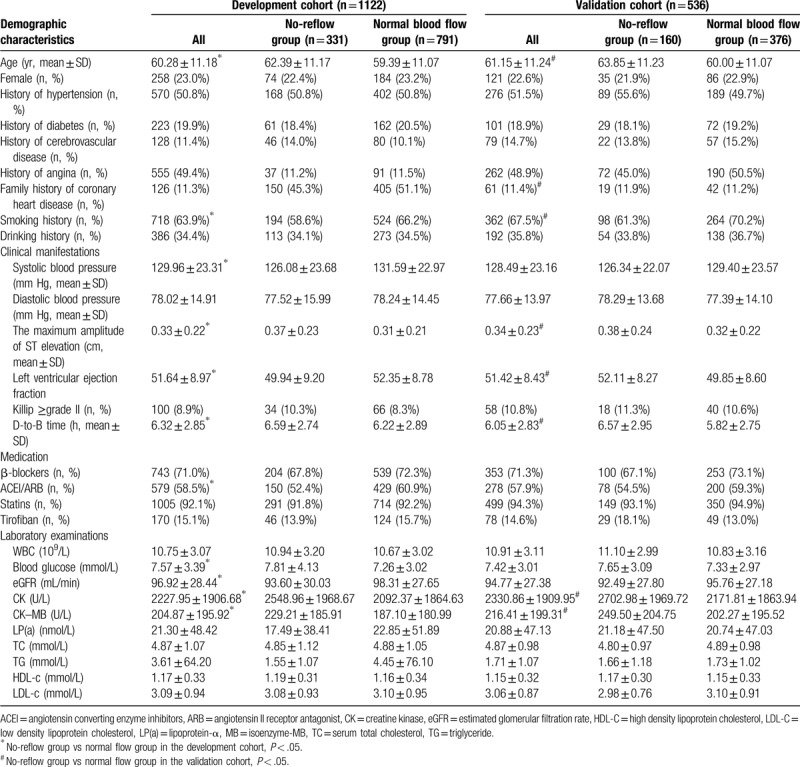
In the development cohort, there were 791 patients with normal blood flow, including 607 males and 184 females, with a mean age of (59.39 ± 11.07) years; there were 331 patients with no-reflow, including 257 males and 74 females, with a mean age of (62.39 ± 11.17) years. Therefore, the incidence of no-reflow was 29.5%.
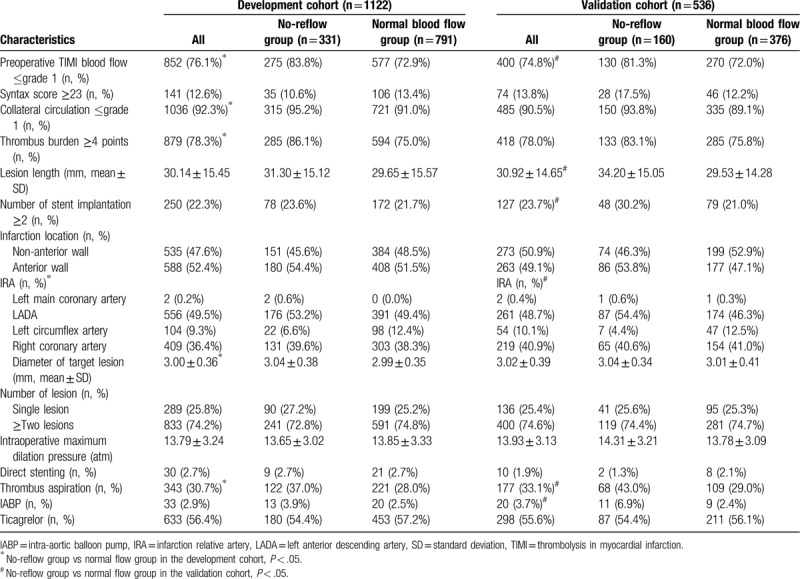
3.2. Coronary angiography characteristics
There was a significant difference in the distribution of the IRA between the no-reflow group and the normal blood flow group (P < .01). The target lesion vessel being the left anterior descending artery (LADA) occurred more often in the no-reflow group: 53.2% vs 49.4%. The diameter of target lesion was significantly greater in the no-reflow group than in the normal blood flow group (P < .05), which was (3.04 ± 0.38 mm) vs (2.99 ± 0.35 mm), respectively. Thrombus aspiration in the no-reflow group was significantly higher than that in the normal blood flow group (P < .05, Table 2).
Table 2.
Coronary angiography results in the model and validation cohorts.
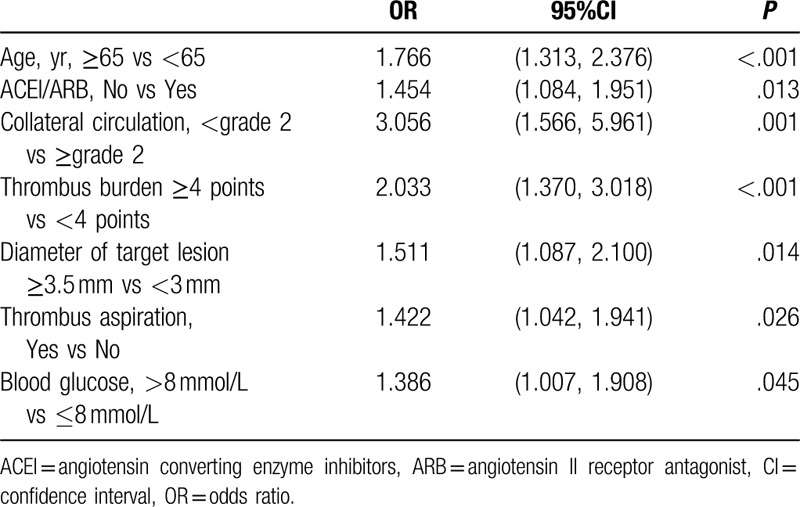
3.3. Screening predictors for no-reflow
Multivariate analysis showed that age ≥65 years (OR = 1.766, 95% confidence interval (CI): 1.313–2.376, P < .001), not using ACEI/ARB (OR = 1.454, 95%CI: 1.084–1.951, P = .013), collateral circulation <grade 2 (OR = 3.056, 95%CI: 1.566–5.961, P = .001), thrombosis burden ≥4 points (OR = 2.033, 95%CI: 1.370–3.018, P < .001), diameter of target lesion ≥3.5 mm (OR = 1.511, 95%CI: 1.087–2.100, P = .014), thrombosis aspiration (OR = 1.422, 95%CI: 1.042–1.941, P = .026), and blood glucose >8 mmol/L (OR = 1.386, 95%CI: 1.007–1.908, P = .045) were independent factors related to no-reflow (Table 3).
Table 3.
Multivariate regression analysis for no-reflow in the development cohort.
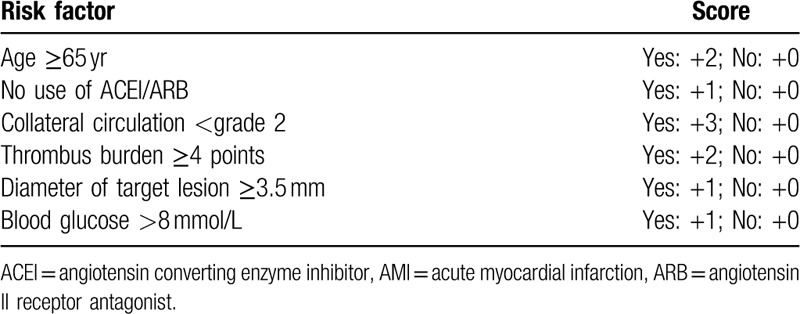
3.4. Establishing a risk scoring system for no-reflow and verification of the model
The multivariate logistic regression results of the development cohort were then used to establish a risk scoring system for the no-reflow during direct PCI for STEMI. According to the clinical value, age, no use of ACEI/ARB, collateral circulation, thrombus burden, diameter of target lesion, and blood glucose were all included (Table 4).
Table 4.
The risk scoring system of no-reflow during the intervention in baseline population with AMI.
The ROC curve for the model for no-reflow in the development cohort is shown in Figure 1. The area under the curve was 0.648 (95%CI: 0.609–0.686). At a cutoff value of 0.349, the sensitivity of the model was 42.0%, the specificity was 79.3%, positive predictive value (PPV) was 44.7%, negative predictive value (NPV) was 77.4%, P < .001.
Figure 1.
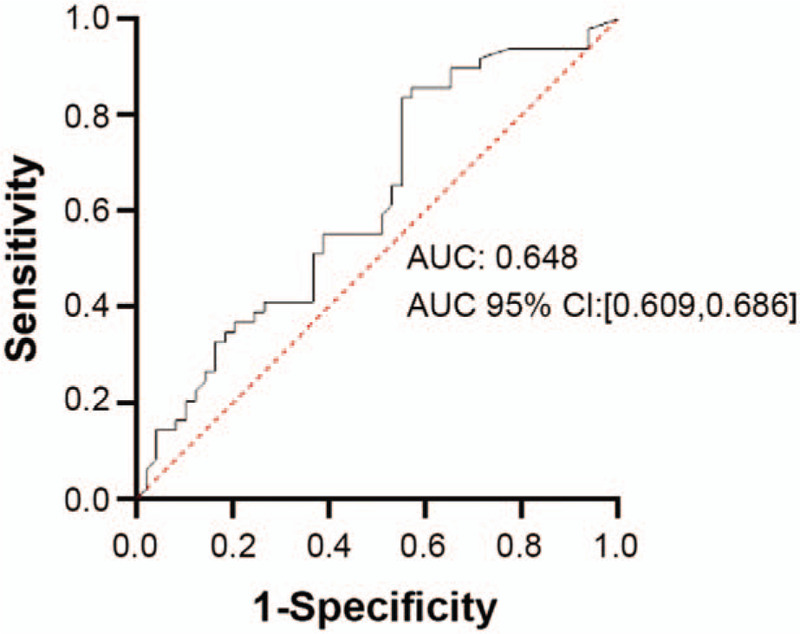
Receiver operating characteristic (ROC) curve of factors related to no-reflow after percutaneous coronary intervention for ST-segment elevation myocardial infarction identified by multivariate logistic regression in the development cohort. Cutoff = 0.349, sensitivity: 42.0%, specificity: 79.3%, PPV: 44.7%, NPV: 77.4%, P < .001.
The model was tested in the 536 patients in the validation cohort, of whom 160 reported no-reflow, and the incidence of no-reflow was 29.9% (Table 1). Figure 2 shows the ROC curve for the verification group. The area under the curve was 0.637 (95%CI: 0.582–0.692). At a cutoff of 0.349, sensitivity was 53.2%, specificity was 66.7%, PPV was 41.2%, NPV was 76.4%, P < .001.
Figure 2.
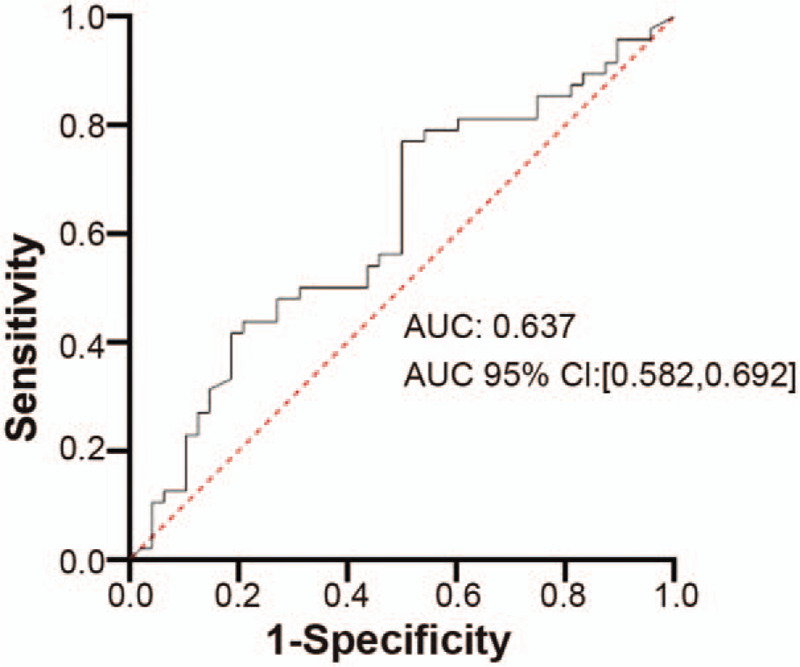
Receiver operating characteristic (ROC) curve of factors related to no-reflow after percutaneous coronary intervention for ST-segment elevation myocardial infarction identified by multivariate logistic regression in the development cohort. When cutoff = 0.349, sensitivity: 53.2%, specificity: 66.7%, PPV: 41.2%, NPV: 76.4%, P < .001.
4. Discussion
This was a retrospective study of the no-reflow phenomenon in patients with STEMI undergoing direct PCI, and its objective was to find a scoring system to evaluate the risk of no-reflow. The results showed that age ≥65 years, no use of ACEI/ARB, collateral circulation <grade 2, thrombosis burden ≥4 points, diameter of target lesion ≥3.5 mm, thrombosis aspiration and blood glucose >8 mmol/L were independent factors related to no-reflow. The resulting risk scoring system was tested in the validation cohort with a sensitivity of 53.2%, a specificity of 66.7%, a PPV of 41.2%, and an NPV of 76.4%, indicating that the scoring system had a good predictive ability for excluding patients at low risk of no-reflow, and was clinically simple and practical.
Previous reports have also established scoring systems for no-reflow phenomenon.[18–21] But these studies show some conflicting results. Harrison et al[8] conducted a large-sample (n = 291,380) study, showing that old age, STEMI, delay in time from symptom to visit, cardiogenic shock, long lesions, high-risk of type C lesions, bifurcation lesions, and poor TIMI blood flow were independent factors associated with no-reflow. However, in that study the proportion of patients with non-STEMI was high, there were more confounding factors in the analysis of coronary lesions, and the thrombus was not included in the analysis, so the practicality was not good. Our study established a simple risk scoring system of no-reflow including 8 rapidly and easily obtained variables, which provided a simple and practical method for rapid and accurate risk stratification of no-reflow for STEMI patients by assigning a score of 0 or 2 to each factor. This system was then validated in the validation cohort and showed good sensitivity and specificity. As the resulting NPV was more than 70% the model showed a good ability to identify patients at low risk of no-reflow who will undergo PCI. Therefore, this identifies people would get benefit from PCI without a high-risk of no-reflow occurring.
Harrison et al[8] in a large-sample study of 291,380 patients also found that older age was associated with no-reflow. The results of this study also showed that age was associated with no-reflow phenomenon, and age ≥65 years was an independent risk factor, which could be used as a predictor of no-reflow.
Related studies found that AMI patients with systolic blood pressure (SBP) <120 mm Hg on admission had significantly higher mortality than those with SBP >120 mm Hg.[25] Animal studies have shown that reduced SBP reduced both coronary anterior and collateral blood flow, significantly increasing coronary microcirculation resistance, thus leading to poor myocardial perfusion and increased infarct size.[26] In addition, the reduction in coronary blood flow can also promote leukocyte aggregation, adhesion, and capture by capillaries, aggravating no-reflow. Although in this study the proportion of patients with SBP ≤100 mm Hg in the no-reflow group of the development cohort was significantly higher than that in the normal blood flow group it was not an independent risk factor associated with no-reflow. Also, in the validation group there was no significant difference in the proportion of patients with SBP ≤ 100 mm Hg between the no-reflow and normal blood flow group. Therefore, we conclude that SBP was not an important factor in this study.
The results of this study showed that the door to balloon (D-to-B) time in the no-reflow group was longer than that in the normal blood flow group. The D-to-B time reflects the degree of myocardial injury and necrosis, and long-term ischemia can cause the swelling of distal capillary endothelia, neutrophil occlusion, and damage to the microcirculatory structure of myocardial tissue, resulting in hypoperfusion of myocardial tissue or even no perfusion. Reffelmann et al[27] found that changes in ultrastructure of myocardial capillary endothelia were directly related to the occurrence of no-reflow, and the destruction of microvascular bed was an important pathological mechanism of no-reflow.[28] Therefore, the longer the D-to-B time, the more severe the damage to the microcirculation of the myocardial tissue, and the higher the likelihood of no-reflow. Generally, the pathological process of myocardial cell necrosis in the infarcted area was basically completed after 6 hours of coronary artery occlusion. The longer the time of vascular occlusion, the worse the reperfusion.
The renin–angiotensin–aldosterone system activation results in increased production of angiotensin II which subsequently increases vascular resistance, myocardial workload, and myocardial oxygen demand. Use of agents, such as ACEI and ARB, to disrupt renin–angiotensin–aldosterone signaling inhibits these downstream detrimental pathways.[29] A small study in 259 consecutive patients who underwent primary angioplasty for a first acute myocardial infarction found that the 47 patients receiving chronic ACEI treatment before admission had lower incidence of no-reflow.[30] Another small study showed 51 patients receiving chronic ARB treatment before admission had lower incidence of the no-reflow phenomenon from 276 consecutive patients with acute myocardial infarction undergoing successful PCI.[31] These studies support the results of this study of no use of ACEI/ARB being an independent factor related to no-reflow and for inclusion in the scoring system.
Recent studies have shown that collateral circulation is one of the independent predictors of no-reflow.[32] The results of this study showed that the collateral circulation grade in the normal blood flow group was statistically different from that in the no-reflow group, and collateral circulation ≥grade 2 was an independent risk factor related to no-reflow. Good collateral circulation can increase microvascular perfusion by opening the microvascular bed. Collateral blood flow is present before ischemic myocardial reperfusion, which can maximize the time of myocardial ischemia. If there is good collateral circulation (≥grade 2) before emergency PCI for AMI, it can protect the coronary microcirculation of AMI patients and significantly reduce the incidence of no-reflow.[33]
Numerous studies have demonstrated that high thrombus burden in coronary artery is an important risk factor of no-reflow phenomenon, and the incidence of myocardial microcirculation disturbance can be significantly reduced by thrombus aspiration.[34,35] A study of 794 patients with AMI who underwent emergency PCI showed that high thrombus burden was an independent predictor of no-reflow.[12] The results of this study showed that the proportion of patients with high thrombus burden before surgery in the no-reflow group was higher than that in the normal blood flow group. After balloon dilation or stent implantation, high thrombus burden was more likely to cause microthrombus to be washed to the distal end of the coronary artery and embolize the myocardium, resulting in poor perfusion of myocardial tissue. Amabile et al[36] predicted the risk of microcirculatory embolization after interventional therapy in patients with AMI by referring to magnetic resonance imaging and concluded that thrombus burden ≥4 was an independent predictor of microembolization in the first year of STEMI. Recent studies have shown that pre-PCI thrombus burden score ≥4 is one of the independent predictors of no-reflow.[16] This study also found that thrombus burden ≥4 points was an independent risk factor for no-reflow. Therefore, for AMI patients of high thrombus burden with pre-PCI thrombus burden ≥4 points, manual thrombus aspiration is recommended to reduce the incidence of no-reflow before the direct PCI, and reduce microvascular obstruction, so that the myocardial tissues can receive effective reperfusion and thrombus aspiration was also an independent factor related to no-reflow.
It is not clear if the culprit artery has a significant influence on no-reflow. In this study there was a significant difference in the distribution of the IRA between the 2 groups. The target lesion vessel being the LADA occurred more often in the no-reflow group. Other studies have shown the presence of sigma shaped RCA is associated with more severe form of coronary artery disease and worse clinical outcome in patients with inferior STEMI.[37] However, a study of 233 patients with inferior wall STEMI undergoing primary PCI found no significant difference between groups associated with major adverse cardiac events, target-vessel revascularization and mortality in patients grouped according to RCA and left circumflex artery culprit artery.[38] This area may need more investigation in terms of the IRA being the RCA as included in the scoring system of this study.
The diameter of the target lesion ≥3.5 mm was another independent factor related to no-reflow identified in this study and included in the scoring system. The size of the target lesion has also been identified in other studies as being important for the occurrence of no-reflow. But, in most studies it is the length of the lesion not the diameter that has been identified as the important factor.[15,16,39]
High blood glucose level was also identified in other studies aiming to provide a simple clinical risk score for STEMI patients at high-risk in terms of no-reflow during PCI.[20,21] One of these studies included age, neutrophil count, admission plasma glucose, β-blocker treatment, time-to-hospital admission, and Killip classes to produce a risk score system with good risk prediction ROC analysis showed c-statistic of 0.757 (95%CI 0.732–0.781).[20] While 1 study used high values of blood glucose at reference alongside long symptom-onset-to-balloon-time and low lymphocyte count as their scoring system to achieve an area under the ROC curve of 0.734 with sensitivity of 46.7% and specificity of 88.28%.[21] High blood glucose is already established as a risk factor for mortality in patients with STEMI and diabetes.[40] In patients without diabetes hyperglycemia can be seen in the course of acute MI and is associated with increased mortality after MI.[41] Another study showed a clear association between hyperglycemia and no-reflow in 146 patients with patients with a first AMI and suggested that impaired microvascular function after AMI may be the result of hyperglycemia which in turn results in a larger infarct size and worse functional recovery.[42]
This study has some limitations, as it was a single-center retrospective case analysis and the scoring system was not investigated and further validated in a prospective cohort. A risk score can only have limited application, as currently, there are no effective therapies available for the treatment of no-reflow. Sample size and multicenter prospective studies will be expanded in the future, further investigating the pathogenesis of no-reflow at the cellular and molecular levels, and exploring effective prevention strategies for no-reflow, interventions, and their internal mechanisms of action of no-reflow.
5. Conclusions
The risk scoring system of no-reflow is effective in early identification of no-reflow phenomenon during direct PCI in low-risk STEMI patients. The parameters selected in this scoring system were easy to obtain, which was convenient for early and rapid identification of high-risk groups; at the same time, risk stratification was performed in low-risk patients for the development of appropriate treatment strategies.
Author contributions
Li Yang and Hongliang Cong conceived and supervised the study; Li Yang and Yin Liu designed experiments; Li Yang, and Xiaolin Chen performed experiments; Yali Lu developed new software and analysed data; Li Yang wrote the manuscript;Hongliang Cong and Yin Liu made manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Footnotes
Abbreviations: ACEI = angiotensin converting enzyme inhibitors, AMI = acute myocardial infarction, ARB = angiotensin II receptor antagonist, CI = confidence interval, CK-MB = creatine kinase-MB, Cr = creatinine, CVD = cardiovascular disease, D-to-B = door to balloon, eGFR = estimated glomerular filtration rate, GLU = glucose, HDL-C = high density lipoprotein cholesterol, IRA = infarction relative artery, LADA = left anterior descending artery, LDL-C = low density lipoprotein cholesterol, LP(a) = lipoprotein-α, LYMP# = lymphocyte count, NEUT# = neutrophil count, ONPV = negative predictive value, PCI = percutaneous coronary intervention, PPV = positive predictive value, PTCA = percutaneous transluminal coronary angioplasty, QCA = quantitative coronary angiography, R = odds ratio, ROC = receiver operating characteristic, SBP = systolic blood pressure, STEMI = ST-segment elevation myocardial infarction, TC = serum total cholesterol, TG = triglyceride, TIMI = thrombolysis in myocardial infarction, TMPG = TIMI myocardial perfusion grade, UA = uric acid.
How to cite this article: Yang L, Cong H, Lu Y, Chen X, Liu Y. Prediction of no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine. 2020;99:26(e20152).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Okwuosa IS, Lewsey SC, Adesiyun T, et al. Worldwide disparities in cardiovascular disease: challenges and solutions. Int J Cardiol 2016;202:433–40. [DOI] [PubMed] [Google Scholar]
- [2].Li H, Ge J. Cardiovascular diseases in China: current status and future perspectives. Int J Cardiol Heart Vasc 2015;6:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li J, Li X, Wang Q, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2015;385:441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Luca G, Suryapranata H, Marino P. Reperfusion strategies in acute ST-elevation myocardial infarction: an overview of current status. Prog Cardiovasc Dis 2008;50:352–82. [DOI] [PubMed] [Google Scholar]
- [5].Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol 2009;54:281–92. [DOI] [PubMed] [Google Scholar]
- [6].Gupta S, Gupta MM. No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J 2016;68:539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaffe R, Charron T, Puley G, et al. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008;117:3152–6. [DOI] [PubMed] [Google Scholar]
- [8].Harrison RW, Aggarwal A, Ou FS, et al. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol 2013;111:178–84. [DOI] [PubMed] [Google Scholar]
- [9].Rentrop KP, Cohen M, Blanke H, et al. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5:587–92. [DOI] [PubMed] [Google Scholar]
- [10].Ndrepepa G, Mehilli J, Schulz S, et al. Prognostic significance of epicardial blood flow before and after percutaneous coronary intervention in patients with acute coronary syndromes. J Am Coll Cardiol 2008;52:512–7. [DOI] [PubMed] [Google Scholar]
- [11].Mehta RH, Harjai KJ, Boura J, et al. Prognostic significance of transient no-reflow during primary percutaneous coronary intervention for ST-elevation acute myocardial infarction. Am J Cardiol 2003;92:1445–7. [DOI] [PubMed] [Google Scholar]
- [12].Yip HK, Chen MC, Chang HW, et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest 2002;122:1322–32. [DOI] [PubMed] [Google Scholar]
- [13].Tomaszuk-Kazberuk A, Sobkowicz B, Kaminski K, et al. Myocardial perfusion assessed by contrast echocardiography correlates with angiographic perfusion parameters in patients with a first acute myocardial infarction successfully treated with angioplasty. Can J Cardiol 2008;24:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Horszczaruk GJ, Kwasiborski P, Rdzanek A, et al. TIMI myocardial perfusion grade and ST-segment resolution in the assessment of coronary reperfusion after primary angioplasty. Kardiol Pol 2014;72:27–33. [DOI] [PubMed] [Google Scholar]
- [15].Zhou H, He XY, Zhuang SW, et al. Clinical and procedural predictors of no-reflow in patients with acute myocardial infarction after primary percutaneous coronary intervention. World J Emerg Med 2014;5:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kirma C, Izgi A, Dundar C, et al. Clinical and procedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J 2008;72:716–21. [DOI] [PubMed] [Google Scholar]
- [17].Fajar JK, Heriansyah T, Rohman MS. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: a meta-analysis. Indian Heart J 2018;70: Suppl 3: S406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang D, Song X, Lv S, et al. Predicting coronary no-reflow in patients with acute ST-segment elevation myocardial infarction using Bayesian approaches. Coron Artery Dis 2014;25:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Avci E, Yildirim T, Aydin G, et al. Combining clinical predictors to better predict for the no-reflow phenomenon. Eur Rev Med Pharmacol Sci 2018;22:4987–94. [DOI] [PubMed] [Google Scholar]
- [20].Wang JW, Chen YD, Wang CH, et al. Development and validation of a clinical risk score predicting the no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Cardiology 2013;124:153–60. [DOI] [PubMed] [Google Scholar]
- [21].Dogan NB, Ozpelit E, Akdeniz S, et al. Simple clinical risk score for no-reflow prediction in patients undergoing primary percutaneous coronary intervention with acute STEMI. Pak J Med Sci 2015;31:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation 2001;103:2550–4. [DOI] [PubMed] [Google Scholar]
- [23].Group TS. The thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med 1985;312:932–6. [DOI] [PubMed] [Google Scholar]
- [24].Gibson CM, Cannon CP, Murphy SA, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 2000;101:125–30. [DOI] [PubMed] [Google Scholar]
- [25].Jorapur V, Steigen TK, Buller CE, et al. Distribution and determinants of myocardial perfusion grade following late mechanical recanalization of occluded infarct-related arteries postmyocardial infarction: a report from the occluded artery trial. Catheter Cardiovasc Interv 2008;72:783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nanas JN, Tsolakis E, Terrovitis JV, et al. Moderate systemic hypotension during reperfusion reduces the coronary blood flow and increases the size of myocardial infarction in pigs. Chest 2004;125:1492–9. [DOI] [PubMed] [Google Scholar]
- [27].Reffelmann T, Kloner RA. The “no-reflow” phenomenon: basic science and clinical correlates. Heart 2002;87:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shi J, Jin H, Ou S. Clinical and angiographic features for predicting no-reflow phenomenon in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Shanghai Med J 2010;33:430–3. [Google Scholar]
- [29].Soukoulis V, Boden WE, Smith SC, Jr, et al. Nonantithrombotic medical options in acute coronary syndromes: old agents and new lines on the horizon. Circ Res 2014;114:1944–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhao JL, Yang YJ, Zhang YH, et al. Chronic pretreatment of ACEI reduces no-reflow in patients with acute myocardial infarction treated with primary angioplasty. Clin Cardiol 2007;30:130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hu T, Wang HC, Wang RT, et al. Effect of chronic pretreatment of angiotensin-converting receptor blocker on no-reflow phenomenon in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc Ther 2013;31:e7–11. [DOI] [PubMed] [Google Scholar]
- [32].Albertal M, Cura F, Escudero AG, et al. Relationship between collateral circulation and successful myocardial reperfusion in acute myocardial infarction: a subanalysis of the PREMIAR trial. Angiology 2008;59:587–92. [DOI] [PubMed] [Google Scholar]
- [33].Desch S, Eitel I, Schmitt J, et al. Effect of coronary collaterals on microvascular obstruction as assessed by magnetic resonance imaging in patients with acute ST-elevation myocardial infarction treated by primary coronary intervention. Am J Cardiol 2009;104:1204–9. [DOI] [PubMed] [Google Scholar]
- [34].Galiuto L, Garramone B, Burzotta F, et al. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention: a myocardial contrast echocardiography substudy of the REMEDIA Trial. J Am Coll Cardiol 2006;48:1355–60. [DOI] [PubMed] [Google Scholar]
- [35].Sardella G, Mancone M, Bucciarelli-Ducci C, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol 2009;53:309–15. [DOI] [PubMed] [Google Scholar]
- [36].Amabile N, Jacquier A, Gaudart J, et al. Value of a new multiparametric score for prediction of microvascular obstruction lesions in ST-segment elevation myocardial infarction revascularized by percutaneous coronary intervention. Arch Cardiovasc Dis 2010;103:512–21. [DOI] [PubMed] [Google Scholar]
- [37].Gungor B, Alper AT, Ozcan KS, et al. Presence of sigma shaped right coronary artery is an indicator of poor prognosis in patients with inferior myocardial infarction treated with primary percutaneous coronary intervention. Catheter Cardiovasc Interv 2014;84:965–72. [DOI] [PubMed] [Google Scholar]
- [38].Ayhan E, Isik T, Ghannadian B, et al. The relationship between culprit artery and the clinical outcomes in patients undergoing primary percutaneous coronary intervention for inferior wall ST segment elevation myocardial infarction. Minerva Cardioangiol 2016;64:367–74. [PubMed] [Google Scholar]
- [39].Abdi S, Rafizadeh O, Peighambari M, et al. Evaluation of the clinical and procedural predictive factors of no-reflow phenomenon following primary percutaneous coronary intervention. Res Cardiovasc Med 2015;4:e25414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cao JJ, Hudson M, Jankowski M, et al. Relation of chronic and acute glycemic control on mortality in acute myocardial infarction with diabetes mellitus. Am J Cardiol 2005;96:183–6. [DOI] [PubMed] [Google Scholar]
- [41].Stranders I, Diamant M, van Gelder RE, et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med 2004;164:982–8. [DOI] [PubMed] [Google Scholar]
- [42].Iwakura K, Ito H, Ikushima M, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol 2003;41:1–7. [DOI] [PubMed] [Google Scholar]


