Abstract
Glucocorticoids (GCs) are metabolic hormones that promote catabolic processes, which release stored energy and support high metabolic demands such as during prolonged flights of migrating birds. Dietary antioxidants (e.g. anthocyanins) support metabolism by quenching excess reactive oxygen species produced during aerobic metabolism and also by activating specific metabolic pathways. For example, similar to GCs' function, anthocyanins promote the release of stored energy, although the extent of complementarity between GCs and dietary antioxidants is not well known. If anthocyanins complement GCs functions, birds consuming anthocyanin-rich food can be expected to limit the secretion of GCs when coping with a metabolically challenging activity, avoiding the exposure to potential hormonal detrimental effects. We tested this hypothesis in European starlings (Sturnus vulgaris) flying in a wind tunnel. We compared levels of corticosterone, the main avian GC, immediately after a sustained flight and at rest for birds that were fed diets with or without an anthocyanin supplement. As predicted, we found (i) higher corticosterone after flight than at rest in both diet groups and (ii) anthocyanin-supplemented birds had less elevated corticosterone after flight than unsupplemented control birds. This provides novel evidence that dietary antioxidants attenuate the activation of the HPA axis (i.e. increased secretion of corticosterone) during long-duration flight.
Keywords: corticosterone, flight, exercise, antioxidants, anthocyanin, HPA axis
1. Introduction
Long-duration flights performed by migratory birds during their seasonal travels between reproductive and wintering grounds are a major energetic challenge supported by multiple physiological changes [1–3]. Glucocorticoids (GCs)––metabolic hormones secreted by the hypothalamus–pituitary–adrenals (HPA) axis to cope with several kinds of stressors [4,5]––are elevated during bird migration [6–17], in both long- and short-distance migrants [7,18,19], as well as when flight costs are experimentally increased [20,21]. However, the increase of GCs secretion is not free of long-term costs. GCs can mediate energetic trade-offs by favouring immediate survival over other processes imposing phenotypic damage and behavioural disruption [4,22–24]. GCs promote catabolism of proteins and lipids [5,25,26] that then facilitates the utilization of energy to satisfy, for example, the substantial metabolic needs of birds during prolonged flight [9,17,27–29]. However, the uncontrolled breakdown of structural proteins from key tissues like pectoral muscle can potentially affect flight performance [5,30,31], suggesting that a fine-tuned secretion of these hormones is needed.
Endurance flapping flight of birds is an extraordinary physical performance carried out at a high metabolic rate that can expose birds to the negative effects of oxidative stress [32–34]. In exercising individuals, oxidative stress can be defined as a physiological condition in which high concentrations of reactive oxygen species (ROS) produced during aerobic metabolism are not completely buffered by the antioxidant defenses [35,36]. Such high levels of ROS can damage DNA and other vital macromolecules, like fatty acids and proteins, and impair cell and tissue functionality [35]. Due to their signalling activity, ROS can also regulate metabolism by, for example, uncoupling the use of respiratory oxygen from the production of mitochondrial energy (ATP) [37–40]. Consequently, if ROS can impair metabolic performance, a functional antioxidant barrier is expected to improve intense physical exercise [41,42].
Antioxidants can be endogenously produced as well as acquired with the diet, and the collective antioxidant system can directly enhance aerobic metabolism as well as control oxidative stress [35,41,43]. Although dietary antioxidants can reduce oxidative damage in diverse species and contexts, and in relation to flight performance [44–46], their benefit in mitigating oxidative stress is not fully accepted [47,48]. Antioxidants such as vitamin E are important modulators of lipid and glucose homeostasis [49], and vitamin C increases gene expression of enzymes involved in the synthesis of catecholamines [50], which are able to enhance gluconeogenesis and aerobic glycolysis [51]. Among dietary antioxidants, anthocyanins––hydrophilic dark purple pigments that are common in vegetables and fruits consumed by wild songbirds [32,46]––have raised particular medical interest because of their roles in controlling glucose and lipid metabolism [52]. Specifically, anthocyanins promote the gene expression of PGC-1α (peroxisome γ-coactivator 1-α) [52], a master regulator of the biogenesis and functioning of mitochondria [53]. Similar to GCs, anthocyanins can also promote catabolic functions and provide energetic substrates for respiration due to increased phosphorylation of AMPK (adeno-mono-phosphate activated kinase) [52,54–56]. The multitude of benefits of a robust antioxidant system may complement that of GCs especially during situations of high energy demand (e.g. long-duration flapping flight of migratory birds). However, the effect of anthocyanins on GC dynamics has not yet been investigated.
Multiple studies suggest a link between exposure to elevated levels of oxidative stress and GCs, which are thought to enhance the production of ROS and to downregulate the availability of antioxidants [31,57], although evidence of a pro-oxidant effect of naturally secreted GCs is weak for birds. The main detrimental physiological effect of GCs is the overconsumption of stored resources, often observed in wild birds when body fat is depleted, a condition that triggers the breakdown of structural elements, like muscular proteins [5,30,58–60]. If anthocyanins can improve the availability of metabolic energy, either by neutralizing ROS or directly controlling the efficiency of metabolism, individuals consuming food rich in anthocyanins are expected to inhibit the secretion of other signalling molecules with the same function, but with less target specificity, like GCs. In this study, we explored the effects of anthocyanins on the regulation of the HPA axis during endurance flight by exposing wild caught, hand-raised European starlings (Sturnus vulgaris) to isocaloric diets either enriched with or devoid of anthocyanins. We then assessed how dietary antioxidants affected baseline (i.e. sampled within 3 min) plasma corticosterone levels––the main GCs in birds––measured immediately after a long-duration flight and at rest, 48 h after the endurance flight. We tested the hypothesis that the complementary functions of corticosterone and anthocyanins will cause the expected increase in corticosterone during flight to be reduced by the consumption of anthocyanins.
2. Material and methods
(a). Starling colony
Female hatchlings of European starling (Sturnus vulgaris) were collected from nest boxes in spring (late April to early May) 2015 and spring 2016 from a native colony in Upper Bavaria, South Germany (47°58′ N, 11°13'142 E) and brought into the animal care facility of the Max Planck Institute for Ornithology (MPIO), Seewiesen, Germany. Hatchlings were hand-raised like in previous experiments [61]. At independency, young starlings (ca 35 days old) were moved to outdoor aviaries and maintained on a standard diet of insect powder, lettuce, fresh and dried fruits and meal worms.
(b). Experimental diets
Starting in early September 2016, birds were randomly assigned to be fed either an unsupplemented, control diet (without added anthocyanins, n = 29) or a diet supplemented with anthocyanin powder (n = 25) at a concentration of 119 mg per kg of wet food, an ecologically relevant amount of anthocyanin [62] that is equivalent to the amount a bird ingests when eating about 17 berries per day based on a daily food intake of 35 g per day. Further details on dietary and housing conditions are available in the electronic supplementary material.
(c). Experimental procedure and wind tunnel flight training
During autumn (September–December) 2016 and spring (February–April) 2017, we flew 33 (16 anthocyanin and 17 control birds) and 21 (nine anthocyanin and 12 control birds) starlings, respectively, in a recirculating wind tunnel at the MPIO under controlled conditions (15°C, 70% humidity, 12 m s−1 wind speed). Birds in each flight-training cohort were housed in aviaries (3 × 4 × 2.5 m high) that were adjacent to the wind tunnel and enclosed by nylon-mesh walls. This set-up allowed us to release birds from their aviaries and guide them to fly directly into the air stream without additional handling of birds. For each bird, we recorded actual time spent flying.
On the day before the longest duration flight on day 15 and just before lights out, we removed food from the flight cages. On day 15, and 105 min after lights on (06.30 until 30 Oct, 05.30 until 26 March, 06.30 thereafter; daily light schedule in the electronic supplementary material), birds were released from their aviaries, guided into the wind tunnel airstream and allowed to fly for as long as they could up to a maximum of 6 h. Birds that refused to fly or repeatedly perched on the ground or the net were dropped from the experiment (n = 18). Immediately (less than 3 min) after completing their longest flight, we collected two 160 µl blood samples into heparinized capillary tubes from the brachial vein after puncture with a 17 G needle and measured body mass, fat score and flight muscle score [63]––this time point we refer to hereafter as ‘after flight’. Since birds finished their endurance flight at different times of the day and since corticosterone can show circadian fluctuation, we checked and found no effect of time on corticosterone values (F1,40 = 1.24, p = 0.47). Birds were then allowed to rest in the wind tunnel aviaries until the next day at approximately 19.00 h when birds were transferred to the laboratory for overnight fasting and measurements of resting metabolic rate (data not covered in this paper). Food restriction both during flight and rest allowed us to control for the effect of food on the GC levels. On day 17 and 90 min after lights on, we immediately (less than 3 min) took a 1000 µl blood sample from the brachial vein after puncture with a 17 G needle and measured again body mass, fat score and flight muscle mass score––this time point we refer to hereafter as ‘at rest’. Within 10 min of sampling, all blood samples were centrifuged at 214g for 5 min to separate plasma from the red blood cells. Plasma was stored at −80°C until laboratory analyses. We assume that plasma corticosterone levels measured for each bird during this ‘at rest’ time point on day 17 is representative of baseline levels for birds during early morning on other days after flight training. This sampling approach for ‘at rest’ birds allowed us to avoid undue disturbance to the birds prior to embarking on their longest duration flight on day 15, and sample all birds soon after their 15 days of flight training yet after recovery from their longest flight.
(d). Circulating levels of corticosterone
Plasma corticosterone concentrations after flight and at rest were determined using an enzyme immunoassay kit (cat. no. K014-H1; Corticosterone ELISA Kit, Arbor Assays) following a double diethyl ether extraction of a 10 µl plasma sample. The inter-plate coefficient of variation (CV) was calculated as the average concentrations of the four controls (for both high and low concentrations) of the six plates and was 8.73 ± 0.41%. The intra-plate CV was calculated as the average CV of the concentrations of all the unknown samples run on six plates and was 4.36 ± 0.24% (further details in electronic supplementary material).
(e). Statistical analysis
We obtained data for 54 experimental birds, sampled twice (after flight and at rest). Since body mass and flight muscle mass score had one missing value each and corticosterone had 16 missing values (no blood available), the sample size has been specified for each model. We assessed the effect of flight time and dietary treatment on circulating corticosterone, body mass, fat score and flight muscle mass score by running a linear mixed model for each response variable with diet (anthocyanin or control), time point (after flight or rest) and their interaction as fixed factors. To account for any effect of body condition on flight performance and on corticosterone, we also included flight muscle mass score as a covariate. We used flight muscle mass score among the other variables describing body condition because it is functionally related to flight performance and because adding other variables would have created a collinearity problem (correlations between flight muscle mass score and body condition variables during flight: body mass, r = 0.67, p < 0.0001, fat score, r = 0.47, p = 0.0003; during rest: body mass, r = 0.68, p < 0.0001, fat score, r = 0.55, p < 0.0001). Since the experiment was run both in autumn and spring, the effect of ‘season’ was also included as a fixed effect. Each model had ‘individual’ as a random factor to account for the repeated measure structure of the study. The model for corticosterone had a second random factor represented by the plate number of the assay (table 1). Because birds differed in the duration of flight performance, the effect of flight duration on circulating levels of corticosterone was assessed also at the individual level by considering only the time point ‘flight’ in a model with diet, duration of flight, interaction of diet and duration of flight, and season as fixed effects. The same analysis has been run with flight muscle mass score. Assay number was included as a random factor (table 2). In addition, between-group differences in flight duration were tested in a regression model with diet as a fixed factor.
Table 1.
Models describing the effect of dietary antioxidants (diet[A] = anthocyanin) versus controls, reference group) on different response variables measured immediately after flight (time[F]) and at rest (reference group) and during autumn (season[autumn]) and spring (reference group) (figures 1b, 2a–c). Asterisks indicate the significance level of random factors as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
| variable (n/ R2Adj) | fixed effect |
random effects |
|||||
|---|---|---|---|---|---|---|---|
| estimate (s.e.) | d.f. | F | p | factor | % of total variance | var. comp. | |
| cort (92/0.64) | |||||||
| time [F] | 0.61 (0.08) | 47.67 | 65.04 | <0.0001 | ID | 11.98 | 0.08 [−0.10,0.26] |
| diet[A] | −0.20 (0.09) | 44.48 | 5.04 | 0.029 | assay | 15.86 | 0.11 [−0.09,0.31] |
| time[F]*diet[A] | −0.20 (0.07) | 48.01 | 6.77 | 0.012 | residual | 72.16 | 0.49 [0.33,0.79] |
| muscle mass | −0.21 (0.08) | 70.54 | 6.25 | 0.015 | |||
| season[autumn] | 0.04 (0.13) | 26.22 | 0.097 | 0.76 | |||
| body mass (107/0.96) | |||||||
| time [F] | −0.13 (0.03) | 51.12 | 24.56 | <0.0001 | ID | 92.08 | 0.86 [0.51,1.21]*** |
| diet[A] | −0.005 (0.13) | 51.04 | 0.001 | 0.97 | residual | 7.92 | 0.07 [0.05,0.11] |
| time[F]*Diet[A] | −0.02 (0.03) | 51.12 | 0.82 | 0.37 | |||
| season[autumn] | −0.23 (0.13) | 51.00 | 3.07 | 0.09 | |||
| fat score (108/0.53) | |||||||
| time [F] | −0.30 (0.08) | 52.00 | 14.06 | <0.001 | ID | 33.87 | 0.36 [0.05,0.66]* |
| diet[A] | −0.02 (0.11) | 51.00 | 0.04 | 0.85 | residual | 66.13 | 0.70 [0.49,1.07] |
| time[F]*diet[A] | −0.03 (0.08) | 52.00 | 0.10 | 0.75 | |||
| season[autumn] | −0.05 (0.12) | 51.00 | 0.16 | 0.69 | |||
| muscle mass (107/0.74) | |||||||
| time [F] | −0.08 (0.06) | 51.71 | 1.66 | 0.20 | ID | 58.32 | 0.62 [0.29,0.96]** |
| diet[A] | −0.05 (0.13) | 51.28 | 0.14 | 0.71 | residual | 40.68 | 0.43 [0.30,0.66] |
| time[F]*diet[A] | 0.006 (0.06) | 51.71 | 0.009 | 0.93 | |||
| season[autumn] | 0.02 (0.13) | 51.11 | 0.02 | 0.90 | |||
Table 2.
Models describing the effect of dietary antioxidants (diet[A] = anthocyanin versus controls, reference group) on circulating levels of corticosterone in relation to the time spent flying and flight muscle mass score and during autumn (season[autumn]) and spring (reference group) (only time point ‘after flight’ was considered).
| variable (n/ R2Adj) | fixed effect |
random effects |
|||||
|---|---|---|---|---|---|---|---|
| estimate (s.e.) | d.f. | F | p | factor | % of total variance | var. comp. | |
| cort (45/0.37) | |||||||
| flight duration | 0.05 (0.17) | 36.30 | 0.08 | 0.77 | assay | 26.88 | 0.33 [−0.25,0.91] |
| diet[A] | −0.39 (0.15) | 37.04 | 6.85 | 0.013 | residual | 73.12 | 0.90 [0.59,1.53] |
| flight duration*diet[A] | −0.36 (0.18) | 37.96 | 4.19 | 0.045 | |||
| season[autumn] | 0.02 (0.23) | 23.99 | 0.0004 | 0.95 | |||
| cort (45/0.23) | |||||||
| muscle mass | 0.36 (0.15) | 40 | 5.89 | 0.02 | |||
| diet[A] | −0.38 (0.16) | 40 | 5.83 | 0.02 | |||
| muscle mass*diet[A] | −0.06 (0.15) | 40 | 0.15 | 0.70 | |||
| season[autumn] | −0.05 (0.17) | 40 | 0.10 | 0.70 | |||
All analyses were performed using JMP 15 (SAS Institute Inc., Cary, NC, USA), which calculates the significance level (p-values) for each fixed effect using F-statistics. In line with this approach, we analysed post-hoc between-group differences in least squared means with the Student's t-test. We did not adjust the results of our models by applying a Bonferroni correction, in accordance with Moran et al. [64] and Nakagawa [65]. For all models we used z-score normalized variables (original mean values reported in electronic supplementary material, table S1). We checked whether variables met the assumptions of homogeneity of variance and normal distribution by visually analysing the graphical distributions of fitted values versus their residuals. The presence of outliers was excluded using the quantile range method. All data are given as least squares means ±95% confidence intervals.
3. Results
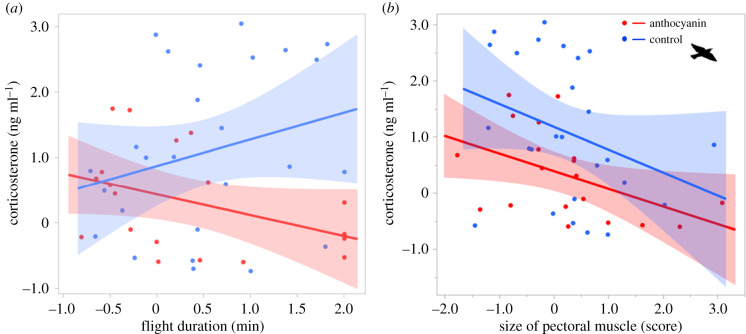
Corticosterone levels were higher after flight compared to at rest (table 1 and figure 1), although birds fed with anthocyanins had lower levels of corticosterone than control birds after flight (significant effect of diet × time, table 1 and figure 1). No difference in hormone levels was observed at rest (figure 1), indicating that the positive effect of antioxidants on corticosterone only occurred when birds were metabolically challenged. In addition, corticosterone was positively associated with flight duration in control birds and negatively associated in anthocyanin-fed birds (significant effect of diet × flight duration, table 2 and figure 2a), even though the duration of flight did not differ between the two dietary treatments (F1,52.00 = 0.13, p = 0.72, n = 54). In addition, birds that had smaller pectoral muscles showed higher levels of corticosterone irrespectively of the diet (table 1 and figure 2b). Corticosterone levels did not change between autumn and spring seasons (table 1; original values reported in electronic supplementary material, table S1).
Figure 1.
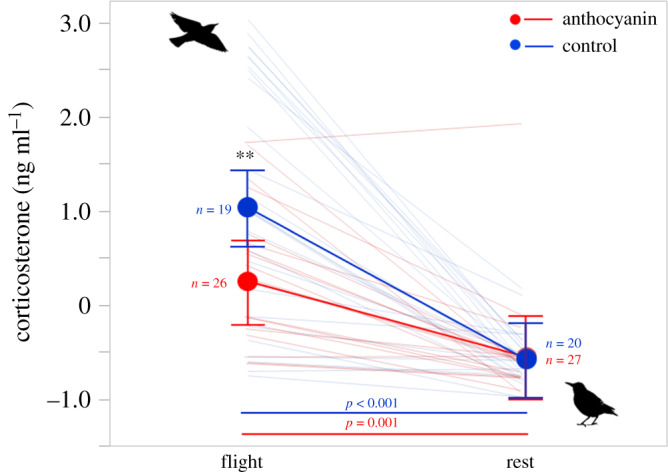
Circulating levels of baseline corticosterone (taken within 3 min from capture in spring and autumn) after flight (‘flight’ in x-axis) and 48 h after flight (‘rest’ in x-axis). Dots (red for anthocyanin treatment) represent least squares means; bars are 95% confidence intervals. Asterisks represent within-time/between-treatment post-hoc results (**=p < 0.01); coloured lines and related p-values represent between-time/within treatment comparisons. Sample size refers to total observations per each group and time point. All variables were z-scored but units of measures were reported for clarity. See electronic supplementary material, table S1 for means of raw data.
Figure 2.
Relationship between circulating corticosterone and (a) actual amount of time spent flying (only time point ‘after flight’ considered) and (b) mass of pectoral muscle. Data for spring and autumn have been combined. Red colour for individual dots, regression line and shaded confidence region represent anthocyanin birds; blue colour for controls. All variables were z-scored but units of measures were reported for clarity. See electronic supplementary material, table S1 for means of raw data.
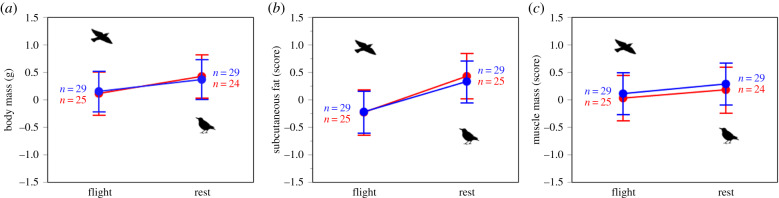
Diet did not affect body mass, fat score or flight muscle mass score (no significant effect of diet × time, table 1 and figure 3a–c), suggesting that the effect of anthocyanins in influencing corticosterone levels was not mediated by a change in body condition. Body mass and fat score, but not flight muscle mass score, were higher at rest than after flight, irrespective of diet (significant effect of time, table 1 and figure 3a–c). None of these variables changed with season (table 1).
Figure 3.
Body mass (a), fat score (b) and flight muscle mass score (c) measured immediately after flight (‘flight’ in x-axis) and 48 h after flight (‘rest’ in x-axis). Data for spring and autumn have been combined. Dots (red for anthocyanin treatment) represent least squares means; bars are 95% confidence intervals. All variables were z-scored but units of measures were reported for clarity. See electronic supplementary material, table S1 for means of raw data. Sample size refers to total observations per each group and time point.
4. Discussion
Corticosterone was higher after flight than at rest in both treatment groups and, in accordance to our prediction, this increase in corticosterone after flight was reduced in anthocyanin-supplemented birds compared to unsupplemented controls. The different degree of HPA axis activation was not related to differences in flight performance in that birds of the two dietary groups flew for the same amount of time. This provides the first evidence to date that dietary antioxidants affect the degree of activation of the HPA axis (i.e. secretion of corticosterone) during long-duration flight and suggests that satisfying the energy needs for long-duration flights involves the functional coordination of key hormones and the antioxidant system.
During sustained flights in the wind tunnel, as well as during migratory flights in nature, birds cannot rest, eat or drink. To meet the energy and nutrient demands imposed by flapping flight, birds must rely entirely on stored energy and metabolic water, a shift to catabolic metabolism that occurs quickly within the first hour of flight [66,67]. Corticosterone can orchestrate the required metabolic shift and promote the release of energetic substrates needed for the production of ATP [9]. Dietary supplementation with anthocyanins affected the extent to which this hormone was upregulated after flight but not when at rest (figure 1), suggesting that the action of these natural antioxidants was most effective when birds were metabolically challenged. We propose that this effect of dietary anthocyanins on the regulation of corticosterone was due to the metabolic function of anthocyanins in supporting metabolism in a general way, i.e. by controlling ROS [37,68,69] see introduction), or more specifically, by upregulating catabolic metabolism needed to sustain flapping flight.
Anthocyanins can directly modulate specific metabolic pathways including the activation of AMPK, an enzyme that is phosphorylated when levels of ATP are low and need to be replenished during catabolism [55]. AMPK is directly activated by low levels of ATP, but also by other signals, including exogenous ones [55]. Activation of AMPK by anthocyanins promotes the catabolism of fat and inhibits lipogenesis (targeting liposomal enzymes FAS, acetyl-coenzyme A carboxylase and SCD-1; [52] and by promoting the uptake of fatty acids into mitochondria and the β-oxidation pathway [55]). Anthocyanin-activated AMPK can also trigger mitochondrial biogenesis (through PCG1-α; [70]), increase the oxidation of both glucose and fatty acids, and so boost endurance exercise [55,71]. Among the catabolic events mediated by AMPK is the enhanced glucose uptake observed during muscle contraction, a function similar to insulin. However, insulin promotes glucose uptake even at rest, whereas we found that anthocyanins differentially affected corticosterone levels only after flight, when they could make a difference in supporting metabolism, whereas we did not find such an effect when birds were at rest.
Contrary to anthocyanins, corticosterone is less specific in targeting substrates, causing the breakdown of structural proteins, a catabolic pathway often observed in migrating wild birds [7,9,10,13,27,59,63,72]. It seems that birds are able to control to some extent the activation of the HPA axis to avoid excessive protein catabolism. For example, at time of spring migration, wild caught red knots (Calidris canutus) increased corticosterone only in association with body mass gain, an adjustment that allowed them to activate catabolism only when proteins were protected by the availability of fat stores [9]. This stepwise action of corticosterone in selecting substrates has been well observed in birds [59].
Dietary supplementation with antioxidants did not affect total flight duration; however, the association between corticosterone and duration of flight measured for each bird was positive in unsupplemented controls and negative in anthocyanin-supplemented birds (figure 2a). This suggests that the action of corticosterone and anthocyanins was complementary in the sense that dietary anthocyanins were already promoting catabolic metabolism and this reduced the need to upregulate hormones that promote further catabolism while also reducing any potential detrimental effects of high circulating corticosterone [5,30,58]. Anthocyanins could also have complemented the action of GCs by quenching the increase in ROS produced by the increase in GCs and metabolic rate during flight; increased ROS can lower the efficiency of mitochondria in producing ATP, which is needed to support the work of flight muscles. The design of our study allowed us to specifically narrow the perspective on the effect of flight on circulating corticosterone while controlling for the major confounding factors that can stimulate the activation of the HPA axis, like temperature, rainfall or food availability [4,5]. There is only one other published study that measured baseline corticosterone in wild caught birds immediately after endurance flight in a wind tunnel [67] although the effect of dietary antioxidants was not considered. Similar to our findings, red knots Calidris canatus showed higher levels of baseline corticosterone after 2 h of flight although this increase was not statistically significant [67] perhaps because of lack of statistical power to reject the null hypothesis. The same effect of dietary antioxidants on the secretion of corticosterone was observed in both autumn and spring seasons suggesting that the higher levels of corticosterone shown in other migratory species during spring [5] are most likely to be due to environmental conditions and seasonal migratory patterns [13,73]. Our birds were treated with anthocyanins in both seasons, an element that could have contributed to the lack of seasonal effect of diet on the HPA functioning. Availability of natural food rich in anthocyanins can be an important resource for migrating birds [74,75], but these could be more limited during specific migration stages or routes. The levels of corticosterone observed in this study after the endurance flight were relatively high (62.18 ng ml−1, electronic supplementary material, table S1), but lower than the stress-induced levels observed in wild females of the same species (e.g. 80.05 ng ml−1; [76]). Further studies are needed to determine how the corticosterone levels reported here for starlings performing endurance flights in a wind tunnel are comparable to those of free-ranging birds.
5. Conclusion
Migratory birds are athletes performing challenging physical activity in environmental conditions that are often unpredictable and that can push them very close to their physiological limits [77]. The upregulation of GCs is generally beneficial and promotes survival when coping with challenging conditions, by activating a range of physiological adjustments that support the energetic needs of migration. However, prolonged exposures to high levels of these hormones, especially when close to physiological limits, can be detrimental [24,72,78]. We showed that anthocyanins––water soluble antioxidants that are actively selected by foraging birds [46,62,74], especially during migration [74]––are able to interact with the HPA axis, controlling the excessive secretion of GCs. We propose that the interaction between GCs and anthocyanins occurred at the metabolic level, because it was observed only when the birds had been flying and not when at rest, and because corticosterone was not positively correlated with flight duration in birds receiving anthocyanins (but it was in controls). The evidence that anthocyanins can support the metabolic function of GCs, and potentially attenuate the endocrine stress response, is novel and opens the road to future studies able to uncover how specific dietary components facilitate successful bird migration in a fast-changing planet.
Supplementary Material
Acknowledgements
We are grateful to Katherine Carbeck for her help with blood sampling, to Susan Forrest for raising the starlings and the Max Planck Institute for Ornithology animal care staff for handling much of the animal husbandry responsibilities.
Ethics
All the experimental protocols were approved by the University of Rhode Island IACUC (protocol no. AN08-02-014) and the Government of Upper Bavaria, Germany (AZ 55.2-1-54-2532-216-2014).
Data accessibility
The datasets supporting this article are available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.j9kd51c8q [79].
Authors' contributions
S.C. formulated the study hypothesis, carried out laboratory analyses, performed statistical analyses and drafted the manuscript. S.R.W., U.B. and B.J.P. designed the experiment. K.J.D.M., L.T., B.J.P., A.B., M.D., E.T.S. and U.B. conducted the experiment. All authors edited and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The experiment was supported by a grant from the National Science Foundation to S.R.M. and B.J.P. (IOS-1354187); by OPUS grant from National Science Foundation (NCN), Poland, to U.B. (UMO-2015/19/B/NZ8/ 01394) and DS grant from the Institute of Environmental Sciences, Jagiellonian University to U.B. (DS/WBINOZ/INOS/757). S.C. was supported by the Evolutionary Physiology Research Group of the Max Planck Institute for Ornithology.
References
- 1.Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. ( 10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 2.Lennox RJ, Chapman JM, Souliere CM, Tudorach C, Wikelski M, Metcalfe J, Cooke J. 2016. Conservation physiology of animal migrations. Conserv. Physiol. 4, cov72 ( 10.1093/conphys/cov072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wikelski M, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser HG. 2003. Costs of migration in free-flying songbirds. Nature 423, 2003 ( 10.1038/423704a) [DOI] [PubMed] [Google Scholar]
- 4.Hau M, Casagrande S, Ouyang JQ, Baugh AT. 2016. Glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. In Advances in the study of behavior (eds Naguib M, Mitani JC, Simmons LW, Barrett L, Healy S, Zuk M), pp. 41–115. New York, NY: Academic Press. [Google Scholar]
- 5.Romero LM, Wingfield J. 2016. Tempests, poxes, predators, and people. New York, NY: Oxford University Press. [Google Scholar]
- 6.Mizrahi DS, Holberton RL, Gauthreaux SA. 2001. Patterns of corticosterone secretion in migrating semipalmated sandpipers at a major spring stopover site. Auk 118, 79–91. ( 10.2307/4089759) [DOI] [Google Scholar]
- 7.Landys-Ciannelli MM, Ramenofsky M, Piersma T, Jukema J, Wingfield JC. 2002. Baseline and stress-induced plasma corticosterone during long-distance migration in the bar-tailed godwit, Limosa lapponica. Physiol. Biochem. Zool. 75, 101–110. ( 10.1086/338285) [DOI] [PubMed] [Google Scholar]
- 8.Landys MM, Ramenofsky M, Guglielmo CG, Wingfield JC. 2004. The low-affinity glucocorticoid receptor regulates feeding and lipid breakdown in the migratory Gambel's white-crowned sparrow Zonotrichia leucophrys gambelii. J. Exp. Biol. 207, 143–154. ( 10.1242/jeb.00734) [DOI] [PubMed] [Google Scholar]
- 9.Landys MM, Piersma T, Ramenofsky M, Wingfield JC. 2004. Role of the low-affinity glucocorticoid receptor in the regulation of behavior and energy metabolism in the migratory red knot Calidris canutus islandica. Physiol. Biochem. Zool. 77, 658–668. ( 10.1086/420942) [DOI] [PubMed] [Google Scholar]
- 10.Falsone K, Jenni-eiermann S, Jenni L. 2009. Corticosterone in migrating songbirds during endurance flight. Horm. Behav. 56, 548–556. ( 10.1016/j.yhbeh.2009.09.009) [DOI] [PubMed] [Google Scholar]
- 11.Loshchagina J, Tsvey A, Naidenko S. 2018. Baseline and stress-induced corticosterone levels are higher during spring than autumn migration in European robins. Horm. Behav. 98, 96–102. ( 10.1016/j.yhbeh.2017.12.013) [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Singh D, Malik S, Gupta NJ, Rani S, Kumar V. 2018. Difference in control between spring and autumn migration in birds: insight from seasonal changes in hypothalamic gene expression in captive buntings. Proc. R. Soc. B 285, 20181531 ( 10.1098/rspb.2018.1531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelius JM, Boswell T, Jenni-eiermann S, Breuner CW, Ramenofsky M. 2013. Contributions of endocrinology to the migration life history of birds. Gen. Comp. Endocrinol. 190, 47–60. ( 10.1016/j.ygcen.2013.03.027) [DOI] [PubMed] [Google Scholar]
- 14.Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149. ( 10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 15.Eikenaar C, Bairlein F, Stöwe M, Jenni-Eiermann S. 2014. Corticosterone, food intake and refueling in a long-distance migrant. Horm. Behav. 65, 480–487. ( 10.1016/j.yhbeh.2014.03.015) [DOI] [PubMed] [Google Scholar]
- 16.Eikenaar C, Mu F, Leutgeb C, Hessler S, Lebus K, Taylor PD, Schmaljohann H. 2017. Corticosterone and timing of migratory departure in a songbird. Proc. R. Soc. B 284, 20162300 ( 10.1098/rspb.2016.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eikenaar C, Müller F, Rüppel G, Stöwe M. 2018. Endocrine regulation of migratory departure from stopover: evidence from a longitudinal migratory restlessness study on northern wheatears. Horm. Behav. 99, 9–13. ( 10.1016/j.yhbeh.2018.01.008) [DOI] [PubMed] [Google Scholar]
- 18.Gwinner E, Zeman M, Schwabl-Benzinger I, Jenni-Eiermann S, Jenni L, Schwabl H. 1992. Corticosterone levels of passerine birds during migratory flight. Naturwissenschaften 79, 276–278. ( 10.1007/BF01175396) [DOI] [Google Scholar]
- 19.Nilsson ALK, Sandell MI. 2009. Stress hormone dynamics: an adaptation to migration? Biol. Lett. 5, 480–483. ( 10.1098/rsbl.2009.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivers JW, Newberry GN, Schwarz CJ, Ardia DR. 2017. Success despite the stress: violet-green swallows increase glucocorticoids and maintain reproductive output despite experimental increases in flight costs. Funct. Ecol. 31, 235–244. ( 10.1111/1365-2435.12719) [DOI] [Google Scholar]
- 21.Casagrande S, Hau M. 2018. Enzymatic antioxidants but not baseline glucocorticoids mediate the reproduction–survival trade-off in a wild bird. Proc. R. Soc. B 285, 20182141 ( 10.1098/rspb.2018.2141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 23.Vitousek MN, Taff CC, Hallinger KK, Zimmer C, Winkler DW. 2018. Hormones and fitness: evidence for trade-offs in glucocorticoid regulation across contexts. Front. Ecol. Evol. 6, 1–14. ( 10.3389/fevo.2018.00042) [DOI] [Google Scholar]
- 24.McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. ( 10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 25.Romero LM. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255. ( 10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 26.Kraus-Friedmann N. 2017. Hormonal regulation of hepatic gluconeogenesis. Physiol. Rev. 64, 170–259. ( 10.1152/physrev.1984.64.1.170) [DOI] [PubMed] [Google Scholar]
- 27.Pradhan DS, Van Ness R, Jalabert C, Hamden JE, Austin SH, Soma KK, Ramenofsky M, Schlinger BA. 2019. Phenotypic flexibility of glucocorticoid signaling in skeletal muscles of a songbird preparing to migrate. Horm. Behav. 116, 104586 ( 10.1016/j.yhbeh.2019.104586) [DOI] [PubMed] [Google Scholar]
- 28.Ramenofsky M, Campion AW, Pe JH, Krause JS, Nemeth Z. 2017. Behavioral and physiological traits of migrant and resident white-crowned sparrows: a common garden approach. J. Exp. Biol. 220, 1330–1340. ( 10.1242/jeb.148171) [DOI] [PubMed] [Google Scholar]
- 29.Angelier F, Holberton RL, Marra PP. 2009. Does stress response predict return rate in a migratory bird species? A study of American redstarts and their non-breeding habitat. Proc. R. Soc. B 276, 3545–3551. ( 10.1098/rspb.2009.0868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crespi EJ, Williams TD, Jessop TS, Delehanty B. 2013. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct. Ecol. 27, 93–106. ( 10.1111/1365-2435.12009) [DOI] [Google Scholar]
- 31.Majer AD, et al. 2019. Is there an oxidative cost of acute stress? Characterization, implication of glucocorticoids and modulation by prior stress experience. Proc. R. Soc. B 286, 20191698 ( 10.1098/rspb.2019.1698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McWilliams SR, Guglielmo C, Pierce B, Klaassen M. 2004. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J. Avian Biol. 35, 377–393. ( 10.1111/j.0908-8857.2004.03378.x) [DOI] [Google Scholar]
- 33.Costantini D, Dell'ariccia G, Lipp H-P. 2008. Long flights and age affect oxidative status of homing pigeons (Columba livia). J. Exp. Biol. 211, 377–381. ( 10.1242/jeb.012856) [DOI] [PubMed] [Google Scholar]
- 34.Jenni-Eiermann S, Jenni L, Smith S, Costantini D. 2014. Oxidative stress in endurance flight: an unconsidered factor in bird migration. PLoS ONE 9, e97650 ( 10.1371/journal.pone.0097650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliwell B, Gutteridge JMC. 2007. Free radicals in biology and medicine. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Seifried HE, Anderson DE, Fisher EI, Milner JA. 2007. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 18, 567–579. ( 10.1016/j.jnutbio.2006.10.007) [DOI] [PubMed] [Google Scholar]
- 37.Mailloux RJ, Harper ME. 2012. Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol. Metab. 23, 451–458. ( 10.1016/j.tem.2012.04.004) [DOI] [PubMed] [Google Scholar]
- 38.Roussel D, Salin K, Dumet A, Romestaing C, Rey B, Voituron Y. 2015. Oxidative phosphorylation efficiency, proton conductance and reactive oxygen species production of liver mitochondria correlates with body mass in frogs. J. Exp. Biol. 218, 3222–3228. ( 10.1242/jeb.126086) [DOI] [PubMed] [Google Scholar]
- 39.Monternier P, Marmillot V, Rouanet J, Roussel D. 2014. Mitochondrial phenotypic flexibility enhances energy savings during winter fast in king penguin chicks. J. Exp. Biol. 217, 2691–2697. ( 10.1242/jeb.104505) [DOI] [PubMed] [Google Scholar]
- 40.Brand MD. 2000. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 35, 811–820. ( 10.1016/S0531-5565(00)00135-2) [DOI] [PubMed] [Google Scholar]
- 41.Steinbacher P, Eckl P. 2015. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 5, 356–377. ( 10.3390/biom5020356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powers SK, Jackson MJ. 2008. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 88, 1243–1276. ( 10.1152/physrev.00031.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finkel T, Holbrook NJ. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. ( 10.1038/35041687) [DOI] [PubMed] [Google Scholar]
- 44.Skrip MM, McWilliams SR. 2016. Oxidative balance in birds: an atoms-to-organisms-to-ecology primer for ornithologists. J. F. Ornithol. 87, 1–20. ( 10.1111/jofo.12135) [DOI] [Google Scholar]
- 45.Catoni C, Peters A, Martin Schaefer H. 2008. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim. Behav. 76, 1107–1119. ( 10.1016/j.anbehav.2008.05.027) [DOI] [Google Scholar]
- 46.Schaefer HM, McGraw K, Catoni C. 2008. Birds use fruit colour as honest signal of dietary antioxidant rewards. Funct. Ecol. 22, 303–310. ( 10.1111/j.1365-2435.2007.01363.x) [DOI] [Google Scholar]
- 47.Speakman JR, et al. 2015. Oxidative stress and life histories: unresolved issues and current needs. Ecol. Evol. 5, 5745–5757. ( 10.1002/ece3.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selman C, Mclaren JS, Collins AR, Duthie GG, Speakman JR, Speakman JR. 2013. Deleterious consequences of antioxidant supplementation on lifespan in a wild-derived mammal. Biol. Lett. 9, 20130432 ( 10.1098/rsbl.2013.0432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He W, Xu Y, Ren X, Xiang D, Lei K, Zhang C, Liu D. 2019. Vitamin E ameliorates lipid metabolism in mice with nonalcoholic fatty liver disease via Nrf2/CES1 signaling pathway. Dig. Dis. Sci. 64, 3182–3191. ( 10.1007/s10620-019-05657-9) [DOI] [PubMed] [Google Scholar]
- 50.Figueroa-Méndez R, Rivas-Arancibia S. 2015. Vitamin C in health and disease: its role in the metabolism of cells and redox state in the brain. Front. Physiol. 6, 1–11. ( 10.3389/fphys.2015.00397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, Radermacher P, Calzia E. 2007. Glucose metabolism and catecholamines. Crit. Care Med. 35, S508–S518. ( 10.1097/01.CCM.0000278047.06965.20) [DOI] [PubMed] [Google Scholar]
- 52.Piovezana GJ, Buttow RT, da Silveira SM, Isaura AL, Duarte MH, de Oliveira Barbosa RC. 2019. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: a systematic review. Nutrition 66, 192–202. ( 10.1016/j.nut.2019.05.005) [DOI] [PubMed] [Google Scholar]
- 53.Scarpulla RC, Vega RB, Kelly DP. 2012. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 23, 459–466. ( 10.1016/j.tem.2012.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nader N, Ng SSM, Lambrou GI, Pervanidou P, Wang Y, Chrousos GP, Kino T. 2010. Adenosine 5′-monophosphate-activated protein kinase regulates metabolic actions of glucocorticoids by phosphorylating the glucocorticoid receptor through p38 mitogen-activated protein kinase. Mol. Endocrinol. 24, 1748–1764. ( 10.1210/me.2010-0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardie DG, Ross FA, Hawley SA. 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262. ( 10.1038/nrm3311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. 2003. Nutrient-gene interactions dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 133, 2125–2130. ( 10.1093/jn/133.7.2125) [DOI] [PubMed] [Google Scholar]
- 57.Costantini D, Marasco V, Møller AP. 2011. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 181, 447–456. ( 10.1007/s00360-011-0566-2) [DOI] [PubMed] [Google Scholar]
- 58.Zaytsoff SJM, Brown CLJ, Montina T, Metz GAS, Abbott DW, Uwiera RRE, Inglis GD. 2019. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 9, 1–13. ( 10.1038/s41598-019-52267-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenni L, Jenni-Eiermann S, Spina F, Schwabl H. 2000. Regulation of protein breakdown and adrenocortical response to stress in birds during migratory flight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1182–R1189. ( 10.1152/ajpregu.2000.278.5.R1182) [DOI] [PubMed] [Google Scholar]
- 60.Schwabl H, Bairlein F, Gwinner E. 1991. Basal and stress-induced corticosterone levels of garden warblers, Sylvia borin, during migration. J. Comp. Physiol. Part A. 161, 576–580. ( 10.1007/BF00260747) [DOI] [Google Scholar]
- 61.Carbeck KM, et al. 2018. Environmental cues and dietary antioxidants affect breeding behavior and testosterone of male European starlings (Sturnus vulgaris). Horm. Behav. 103, 36–44. ( 10.1016/j.yhbeh.2018.05.020) [DOI] [PubMed] [Google Scholar]
- 62.Catoni C, Schaefer HM, Peters A. 2008. Fruit for health: the effect of flavonoids on humoral immune response and food selection in a frugivorous bird. Funct. Ecol. 22, 649–654. ( 10.1111/j.1365-2435.2008.01400.x) [DOI] [Google Scholar]
- 63.Bauchinger U, Biebach H. 2001. Differential catabolism of muscle protein in garden warblers (Sylvia borin): flight and leg muscle act as a protein source during long-distance migration. J. Comp. Physiol. - B Biochem. Syst. Environ. Physiol. 171, 293–301. ( 10.1007/s003600100176) [DOI] [PubMed] [Google Scholar]
- 64.Moran MD. 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 2, 403–405. ( 10.1034/j.1600-0706.2003.12010.x) [DOI] [Google Scholar]
- 65.Nakagawa S. 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 15, 1044–1045. ( 10.1093/beheco/arh107) [DOI] [Google Scholar]
- 66.Jenni-Eiermann S, Jenni L. 1991. Metabolic responses to flight and fasting in night-migrating passerines. J. Comp. Physiol. B 161, 465–474. ( 10.1007/BF00257901) [DOI] [Google Scholar]
- 67.Jenni-Eiermann S, Hasselquist D, Lindström Å, Koolhaas A, Piersma T. 2009. Are birds stressed during long-term flights? A wind-tunnel study on circulating corticosterone in the red knot. Gen. Comp. Endocrinol. 164, 101–106. ( 10.1016/j.ygcen.2009.05.014) [DOI] [PubMed] [Google Scholar]
- 68.Brown JCL, Chung DJ, Belgrave KR, Staples JF. 2012. Mitochondrial metabolic suppression and reactive oxygen species production in liver and skeletal muscle of hibernating thirteen-lined ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R15–R28. ( 10.1152/ajpregu.00230.2011) [DOI] [PubMed] [Google Scholar]
- 69.Spinelli JB, Haigis MC. 2018. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20, 745–754. ( 10.1038/s41556-018-0124-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jäer S, Handschin C, St-Pierre J, Spiegelman BM. 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl Acad. Sci. USA 104, 12 017–12 022. ( 10.1073/pnas.0705070104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Narkar VA, et al. 2008. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415. ( 10.1016/j.cell.2008.06.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Integr. Comp. Biol. 38, 191–206. ( 10.1093/icb/38.1.191) [DOI] [Google Scholar]
- 73.Dawson A, King VM, Bentley GE, Ball GF. 2001. Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16, 365–380. ( 10.1177/074873001129002079) [DOI] [PubMed] [Google Scholar]
- 74.Bolser JA, Alan RR, Smith AD, Li L, Seeram NP, McWilliams SR. 2013. Birds select fruits with more anthocyanins and phenolic compounds during autumn migration. Wilson J. Ornithol. 125, 97–108. ( 10.2307/41932839) [DOI] [Google Scholar]
- 75.Cooper-Mullin C, McWilliams SR. 2016. The role of the antioxidant system during intense endurance exercise: lessons from migrating birds. J. Exp. Biol. 219, 3684–3695. ( 10.1242/jeb.123992) [DOI] [PubMed] [Google Scholar]
- 76.Love OP, Chin EH, Wynne-Edwards KE, Williams TD. 2005. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 166, 751–766. ( 10.1086/497440) [DOI] [PubMed] [Google Scholar]
- 77.Klaassen M, Hoye BJ, Nolet BA, Buttemer WA. 2012. Ecophysiology of avian migration in the face of current global hazards. Phil. Trans. R. Soc. B Biol. Sci. 367, 1719–1732. ( 10.1098/rstb.2012.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wingfield JC. 2013. Ecological processes and the ecology of stress: the impacts of abiotic environmental factors. Funct. Ecol. 27, 37–44. ( 10.1111/1365-2435.12039) [DOI] [Google Scholar]
- 79.Casagrande S, DeMoranville KJ, Trost L, Pierce B, Bryła A, Dzialo M, Sadowska ET, Bauchinger U, McWilliams SR. 2020. Data from: Dietary antioxidants attenuate the endocrine stress response during long-duration flight of a migratory bird. Dryad Digital Repository. ( 10.5061/dryad.j9kd51c8q) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Casagrande S, DeMoranville KJ, Trost L, Pierce B, Bryła A, Dzialo M, Sadowska ET, Bauchinger U, McWilliams SR. 2020. Data from: Dietary antioxidants attenuate the endocrine stress response during long-duration flight of a migratory bird. Dryad Digital Repository. ( 10.5061/dryad.j9kd51c8q) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.j9kd51c8q [79].