Abstract
Anthropogenic underwater noise may negatively affect marine animals. Yet, while fishes are highly sensitive to sounds, effects of acoustic disturbances on fishes have not been extensively studied at the population level. In this study, we use a size-structured model based on energy budgets to analyse potential population-level effects of anthropogenic noise on Atlantic cod (Gadus morhua). Using the model framework, we assess the impact of four possible effect pathways of disturbance on the cod population growth rate. Through increased stress, changes in foraging and movement behaviour, and effects on the auditory system, anthropogenic noise can lead to (i) increased energy expenditure, (ii) reduced food intake, (iii) increased mortality, and (iv) reduced reproductive output. Our results show that population growth rates are particularly sensitive to changes in energy expenditure and food intake because they indirectly affect the age of maturation, survival and fecundity. Sub-lethal effects of sound exposure may thus affect populations of cod and fishes with similar life histories more than lethal effects of sound exposure. Moreover, anthropogenic noise may negatively affect populations when causing persistent increases of energy expenditure or decreases of food intake. Effects of specific acoustic pollutants on energy acquisition and expenditure should therefore be further investigated.
Keywords: sound exposure, PCoD, life history, anthropogenic noise
1. Introduction
Anthropogenic noise forms a potential threat to fishes [1,2] because fishes rely on advanced hearing and sound production systems for orientation and communication [3]. Although the extent varies geographically, ambient noise levels have increased considerably over the past 40 years [4,5]. This increase has been related to an increase of anthropogenic activities of which the most important are probably shipping and seismic surveys (explorations for oil and gas) [5]. Also, anthropogenic activities such as drilling (oil and gas), operation of wind farms, pile driving (wind farm construction), the use of sonar (fisheries and navy) and underwater explosions produce sounds underwater. Because low-frequency sounds spread easily underwater and attenuate slowly over large distances [6,7], acoustic disturbances can lead to moderately elevated sound levels over large areas. Exposure to loud sounds, such as produced during pile driving, may cause serious (lethal) injuries in animals that are close by [8]. More often, sound exposure leads to non-lethal effects [9]. In experimental studies, anthropogenic noise has been found to increase stress, reduce foraging, reduce sound perception and increase movement in fishes [9].
The non-lethal effects of sound exposure on fishes seem subtle, but small changes in behaviour can lead to significant reductions in growth and reproduction [10,11]. Non-lethal effects of acoustic disturbance can be assessed using the ‘population consequences of disturbance approach' (PCoD) framework, which was originally developed for marine mammals [11,12]. The PCoD framework translates changes in physiology or behaviour into changes in vital rates (e.g. reproduction, mortality and growth) to estimate population-level effects. Population-level effects form the basis of many current policy decisions regarding disturbance mitigation and nature conservation, such as, for example, the Birds and Habitats Directives of the European Union (Council directives 92/43/EEC [13] and 2009/147/EC [14]). However, there is currently no assessment method to estimate population-level effects of acoustic disturbances on fishes.
In this study, we use a model to evaluate the population-level consequences of changes in individual-level processes that might result from lethal and non-lethal effects of sound exposure for Atlantic cod (Gadus morhua). The size-structured life-history model for cod is based on individual energy budgets. The advantage of using such a mechanistic model is that effects of changes in food intake or energy expenditure are, through both direct and indirect effects, translated to changes in the vital rates. This type of model is considered suitable for estimating population-level effects of non-lethal disturbances [11,15]. Using the model, we explore the sensitivity of the cod population growth rate to changes in four different processes that can be affected by sound disturbance. The population growth rate is a relevant metric for population consequences of disturbances because it indicates when disturbance leads to negative population growth [16].
The effect of sound exposure on fishes is not thoroughly understood and quantitative data on the relationship between sound exposure and vital rates is unavailable. However, a number of effect pathways have been suggested (electronic supplementary material, table S1), including increased stress, changes in foraging and movement behaviour and effects on the auditory system. These effects may lead to changes in energy expenditure, food intake, mortality and reproduction (electronic supplementary material, table S1). We use the size-structured life-history model introduced above, to examine the relative importance of these four potential effect pathways. This work lays the foundation for an assessment framework for anthropogenic noise effects on Atlantic cod populations. As understanding of the effects of acoustic disturbance on cod develops further, the model can be used to study population consequences of specific anthropogenic sources of noise pollution. The current analysis shows which mechanisms potentially lead to the largest population-level effects. The outcomes give an indication of how acoustic disturbances may affect cod most and provide guidance for future experimental and empirical research.
2. Model description
(a). Population model framework
To analyse the effect of acoustic disturbances on fishes, we conduct a demographic analysis of a size-structured life-history model of cod. The life-history model is based on the model previously described by van Leeuwen et al. [17]. Our model is adjusted to use a constant, size-dependent feeding level representing individual-level food availability. We do not consider starvation conditions; we assume a feeding level which is sufficiently high to cover the metabolic rate for fishes of all body sizes. The energy budget is affected by two of the disturbance pathways that we test. As soon as the net-energy drops below zero at any point in the life history before maturation occurs, the model calculations stop. Without maturation, the population growth rate is undefined as reproduction does not take place. In other respects, we follow the model structure previously described by van Leeuwen et al. [17]. Here, we describe the model in general terms and the functions related to the implementation of acoustic disturbance. Additional details are provided in the electronic supplementary material.
From the moment an individual starts feeding actively, the model continuously tracks its age and body size. The model uses size-dependent functions for energy uptake, storage and expenditure. Energy uptake depends on the feeding level, which is defined as the food uptake rate as a fraction of the maximum feeding rate given an individual's body size. The feeding level is assumed to be size-dependent but constant in time. Reproduction is modelled as a discrete process occurring once per year. Following the demographic analysis method described in de Roos [18], we calculated population growth rates for exponentially growing populations based on Lotka's integral equation. Using this analysis method and the cod life-history model, we tested the sensitivity of the population growth rate to changes in energy expenditure, food intake, mortality and reproductive output. Because fish populations are generally spread out over large areas, we expect that a given acoustic disturbance often only affects part of the population. Therefore, we tested how the disturbance of a fraction of the population, rather than the entire population, affects the population growth rate.
(b). Accounting for acoustic disturbance
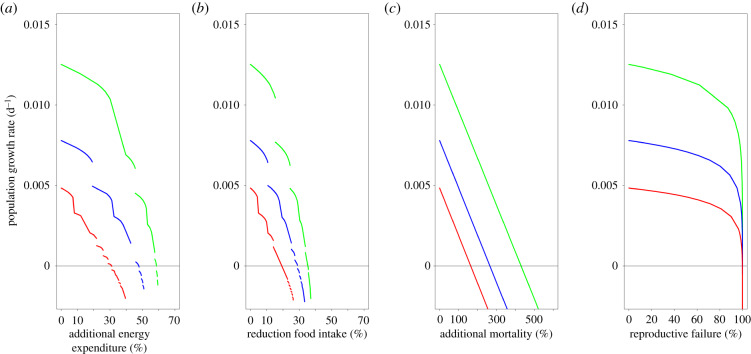
There is no quantitative empirical information available regarding sound exposure levels of cod in the field or the effects of sound exposure on cod. An overview of experimental studies with fishes shows that anthropogenic noise may lead to increased stress, changes in foraging and movement behaviour, and effects on the auditory system (electronic supplementary material, table S1). The (combined) effects of anthropogenic noise may lead to increased energy expenditure, reduced food intake, an increased mortality rate and a reduced reproductive output (electronic supplementary material, table S1). We therefore assessed the potential negative effects of sound exposure on cod by analysing the consequences of relative changes in its energy expenditure, food intake, mortality rate and reproductive output on its population growth. Owing to a lack of detailed information, the effects of sound exposure are assumed continuous through time and independent of age or size. As we have no quantitative information regarding the values of the disturbance parameters described below, we tested the effect of a range of values (figure 2).
Figure 2.
The population growth rate for three feeding levels (high, green; intermediate, blue; low, red) as a function of (a) additional energy expenditure, (b) reduction of the food intake, (c) additional mortality, and (d) reproductive failure owing to acoustic noise disturbance.
The food ingestion rate I(l) depends on length l. It is defined as the ratio between the feeding level F(l) and the time the individual needs to digest a unit mass of food G(l) (the inverse 1/G(l) equals an individual's maximum feeding rate):
Food ingestion decreases proportionally with a sound exposure foraging effect parameter ψI. Reduced food intake as a result of sound exposure is thus defined as a proportional reduction of the standard food intake.
Ingested food is assimilated to energy, with efficiency σ. The energy is first used to cover the metabolic maintenance requirements. The net-energy N(l, w) thus equals: N(l, w) = σ I(l) − (1 + ψT) T(w). The standard energy expenditure for metabolic maintenance T(w) depends on the total body weight w. The term ψTT(w) represents the increase in energy expenditure owing to acoustic disturbance. These costs increase proportionally with the sound exposure energy expenditure effect parameter ψT relative to the standard energy expenditure.
Each individual suffers from background mortality μ0, size-dependent background mortality Ds and fisheries mortality Dv. These result in the following equation for the per capita mortality rate: D(l) = (1 + ψD)μ0 + Ds(l) + Dv(l). The term ψD μ0, background mortality multiplied by the acoustic disturbance mortality effect parameter ψD, represents the increase in mortality owing to acoustic disturbance.
For mature individuals with sufficient energy storage (see the electronic supplementary material), spawning occurs at the end of each year n at day , at time points :
Here, and , respectively, represent the time points just prior to and following reproduction. The number of offspring B that the individual produces depends on the mass of the gonads yg prior to spawning, the mass at the size of birth m(lb) and the gonad-to-offspring conversion efficiency σr. The number of offspring produced decreases proportionally with the acoustic disturbance reproductive failure effect parameter ψB. To calculate the expected cumulative lifetime reproductive output R0, the number of offspring is multiplied by the survival probability s of the individual and added to the offspring the individual has produced so far. After spawning the gonadal mass is depleted, while all other variables are unchanged.
(c). Analytical method
Individual life histories were modelled with a mix of continuous time ordinary differential equations (ODEs) and discrete time recurrence relations (see the electronic supplementary material). The computation of the population growth rate follows the approach presented by de Roos [18]. This method finds the population growth rate by calculating the value of r that satisfies the equation:
This is equivalent to the discrete time Euler–Lotka equation for computation of the population growth rate, r. As in the discrete time Euler–Lotka equation, the summed quantity L(An, r) discounts the expected offspring produced at every age with the growth rate-dependent factor . The expected cumulative lifetime reproductive output R0(Ai) represents reproduction up to and including reproduction occurring at age Ai, which depends on the survival probability up to age Ai. The increase in lifetime reproductive output R0 from age Ai−1 to age Ai is computed by integration of the continuous time ODE system for life-history processes and application of recurrence relations for discrete events related to reproduction (see the electronic supplementary material). The maximum age An is defined as the moment at which the survival probability of the individual is lower than 10−9.
When a fraction ps of the population experiences a disturbance, the population growth rate r is equal to the value for which the dominant eigenvalue of the following matrix is 1 (see [18], for the theoretical background):
Stressed individuals, which experience a disturbance, produce an expected number Ls(An, r) of offspring during their lives. The analogous quantity for unstressed individuals is given by Lns(An, r). Of these newly produced offspring, a fraction ps will experience a disturbance, while a fraction (1 − ps) will not. The resultant population growth rate r of a partly stressed population hence satisfies the condition:
We used the R package deSolve [19] to solve the system of ODEs and recurrence relations. The population growth rate calculations were executed using a C-based, open source software package that solves generic systems of nonlinear equations (https://bitbucket.org/amderoos/findcurve). We made the model implementation files publicly available online (doi:10.5281/zenodo.3779843).
(d). Parameterization of the model
Parameters and their values are listed in the electronic supplementary material, table S2; details regarding parameter derivation are described in the electronic supplementary material. The parameter values used by van Leeuwen et al. [17] are based on Atlantic cod in the Baltic Sea. We adjusted length at maturation, adult condition target and size-dependent functions for the maintenance rate, digestion time and fisheries retention (electronic supplementary material, figure S1) on the basis of available literature data on Atlantic cod in the North Sea. Otherwise, parameter values are as given in van Leeuwen et al. [17].
The feeding level F(l) is assumed constant in time, but body size dependent (electronic supplementary material, figure S1). The high feeding level function corresponds to a situation with unlimited food (electronic supplementary material). Under these conditions, growth depends only on the parameters of maximum feeding and energy expenditure. These were derived from experimental data from the literature (electronic supplementary material). We chose the shape and parameters of an intermediate and a low feeding level function to match observed growth patterns of Atlantic cod in the North Sea (figure 1). Together with the high feeding level function, the intermediate and low feeding level functions cover the range of observed growth patterns of Atlantic cod in the North Sea (electronic supplementary material, figure S1, figure 1).
Figure 1.
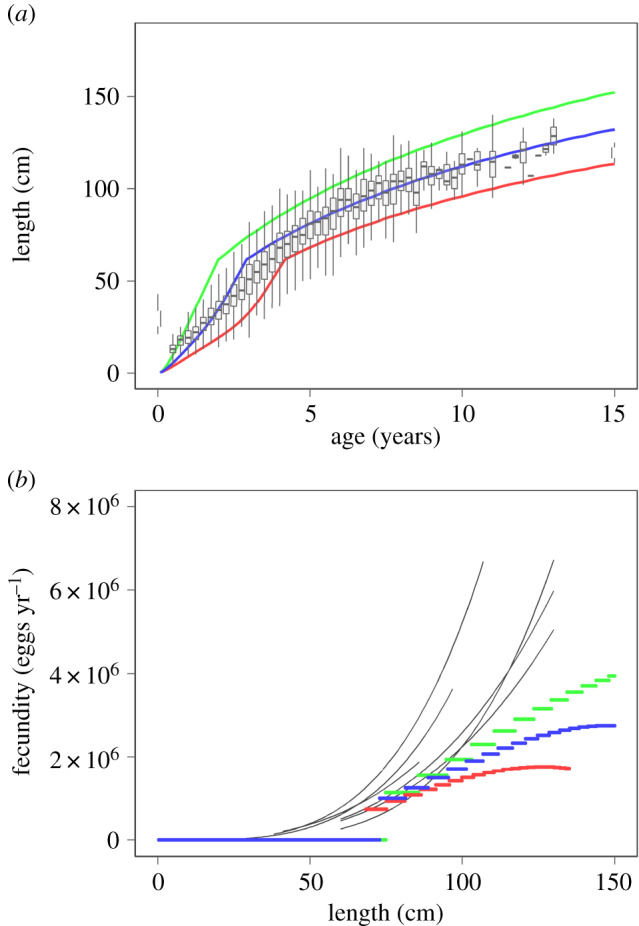
Comparison between field data (boxes and whiskers in upper panel, thin black lines in lower panel) and model output for three different feeding level functions (high, green; intermediate, blue; low, red) of (a) length-at-age and (b) annual fecundity as a function of body size. Length-at-age data is based on cod IBTS data between 1970 and 2018 [20], the ages were adjusted for the quarter in which the survey was conducted. Fecundity–length relationships are based on data of Atlantic cod in the North Sea for several years, compiled by Lambert et al. [21].
(e). Data of Atlantic cod
We used lengths at age from North Sea International Bottom Trawl Survey (IBTS) data for Atlantic cod between 1970 and 2018 (figure 1, [20]). We adjusted the ages for the quarter of the year in which the survey took place (quarter 1, no adjustment; quarter 2, +0.25 year; quarter 3, +0.5 year; quarter 4, +0.75 year). Fecundity–length relationships are based on field data of Atlantic cod in the North Sea in several different years (figure 1, [21]).
3. Results
For unlimited food (high feeding level), we compared model output to length-at-age and fecundity–length data for Atlantic cod in the North Sea. The model growth curve for the high feeding level corresponds well to the high end of the length-at-age data range (figure 1). This indicates that maximum growth in the model is similar to that in field observations. Fecundity in the model is similar to field observations for small-sized cod but deviates for large-sized cod (figure 1).
Without acoustic disturbance, the population growth rate is estimated to be 0.0125 for high, 0.0072 for intermediate and 0.0048 for low feeding levels (figure 2). The population growth rates thus predict undisturbed populations to grow for all three feeding levels. The population growth rates are negatively affected through all sound exposure effect pathways. They are more strongly affected by increased energy expenditure and a lower food intake than by additional mortality and lower reproductive output (figure 2). For the highest feeding level, the population growth rate becomes negative with an approximately 60% increase in energy expenditure or an approximately 35% reduction of the food intake. This switch occurs at approximately 450% additional mortality and an approximately reduction of the reproductive output (figure 2). For intermediate and low feeding levels, the population growth rate is lower overall. As a result, it becomes negative already at lower disturbance levels (figure 2). For example, for the low feeding level, a negative population growth rate already occurs at an approximately 20% reduction in food intake (figure 2b).
These results are based on a situation where the entire population is affected equally. We also test the effect of the proportion of the population that is disturbed (figure 3). Increasing the proportion affected decreases the population growth rate. The shape of this relationship depends on the strength of the disturbance. For a weak disturbance, for example, a 10% increase of the energy expenditure, the population growth rate shows a slow decrease with the proportion affected (figure 3). For strong disturbances, the population growth rate initially decreases slowly. When 50% or more of the population is affected, it decreases more rapidly. The shape of the relationship between the population growth rate and proportion affected is independent of the sound exposure effect pathway (energy expenditure, food intake, mortality or reproductive failure, results not shown).
Figure 3.
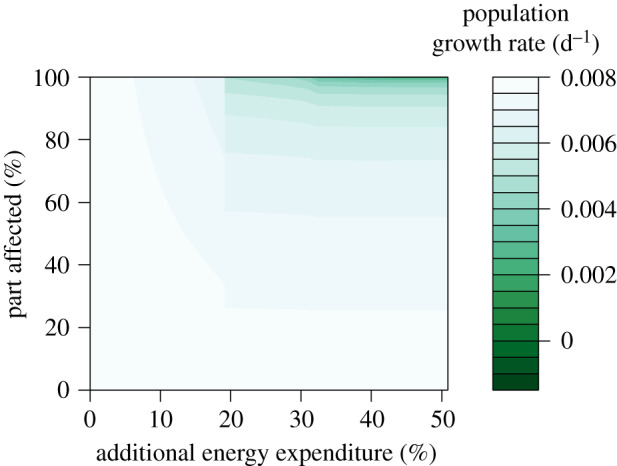
The population growth rate as a function of the proportion of the population that experiences additional energy expenditure, for an intermediate feeding level. (Online version in colour.)
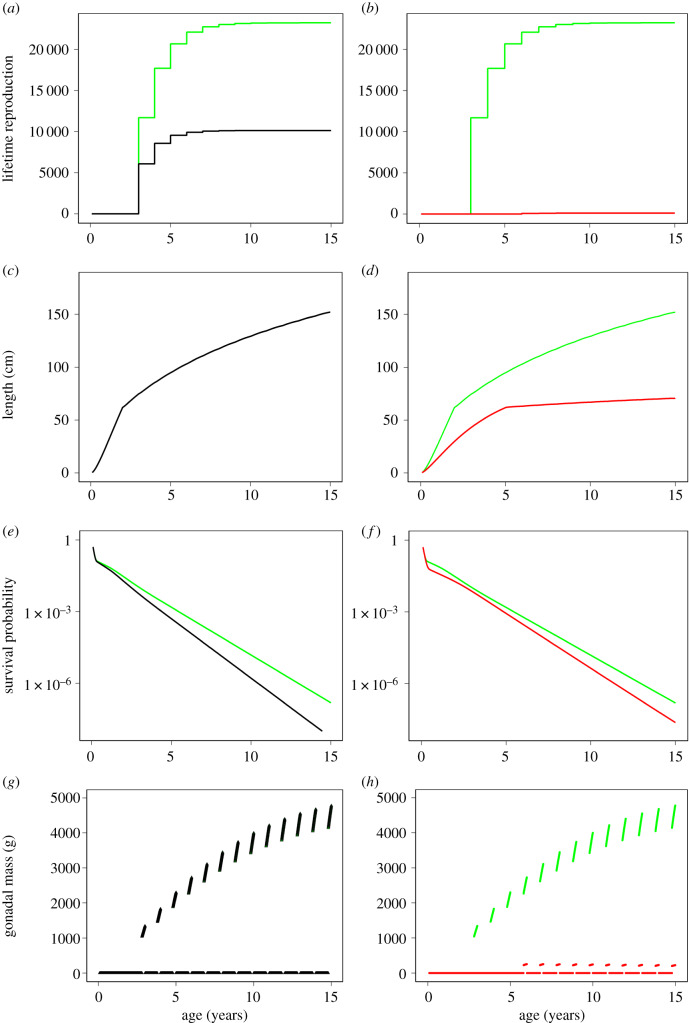
The individual-level life-history trajectories can be used to explain the different effects of the sound exposure effect pathways on the population growth rates (figure 4). A reduction of the population growth rate results from a decrease of the cumulative lifetime reproductive output. The cumulative lifetime reproductive output is more strongly affected by a 30% decrease of the food intake than by 30% additional mortality (figure 4a,b). It depends on age at maturation, survival and the annual reproductive output. Somatic growth is inhibited by a lower food intake but unaffected by additional mortality (figure 4c,d, note that the black line lies on top of the green line). As a result, maturation is delayed from years 2 to 5 for individuals with a lower food intake (figure 4c,d). Survival is reduced by both a lower food intake and additional mortality (figure 4e,f). For the lower food intake, individuals grow more slowly and are subject for longer to high mortality in the smallest size range (electronic supplementary material, figure S1D). Finally, the energy in the gonads, and thus the annual reproductive output, is reduced by a lower food intake but unaffected by mortality (figure 4g,h). In summary, changes in food intake directly affect the individual growth curve and indirectly affect the age at maturation, the survival up to maturation and the annual reproductive output. On the other hand, mortality and reproductive failure directly reduce, respectively, survival and the annual reproductive output, while both have no further indirect effects. The effect of increased energy expenditure is similar to a reduction in food intake: both lead to a reduction of the net-energy availability and affect the individual growth curve.
Figure 4.
Comparison of the individual life history with no disturbance (green) versus additional mortality (30%, black; a,c,e,g) and no disturbance versus reduced food intake (30%, red; b,d,f,h), for a high feeding level. Plotted are the cumulative lifetime reproductive output (a,b), length (c,d), survival probability (e,f), and investment in reproduction (gonadal mass; g,h) as a function of age.
4. Discussion
Our study uses a size-structured life-history model to evaluate population-level consequences of changes in individual-level processes that might result from noise pollution for Atlantic cod. The model framework incorporates energetics and, with the exception of the fecundity of large cod, matches patterns of maximum growth and reproductive output observed for cod in the field. Based on experimental studies with fishes, anthropogenic noise may directly lead to higher energy expenditure, lower food intake, higher mortality and lower reproductive output (electronic supplementary material, table S1). Of these four possible effect pathways, a higher energy expenditure and a lower food intake have a strong effect on the population growth rate in particular. This is because indirect effects lead to an increased age at maturation, a decreased survival up to maturation and a decreased annual reproductive output. The population growth rate decreases most rapidly in response to disturbances that affect at least 50% of the population.
In this study, we test the relative importance of gradual changes in four processes that could be affected by acoustic disturbance. We have chosen this approach because there is still insufficient empirical information available to relate sound exposure explicitly to changes in life-history parameters. The actual importance of each of the pathways is, of course, determined by how strongly each of them is affected by sound exposure. For example, despite the fact that the population growth rate is more sensitive to changes in food intake, a large increase in mortality per-unit-disturbance may cause a stronger effect on the population growth rate than a small decrease in food intake per-unit-disturbance. When dose–response relationships that estimate effects of sound exposure for cod become available, the modelling approach we have developed can be used to estimate the effects of sound exposure on cod populations. Our results suggest that the strongest population-level effects will, through effects on energetics, stem from the sub-lethal effects of sound exposure on individuals.
(a). Empirical sound exposure studies
In our model, the population growth rate is most sensitive to sound exposure effects through increased energy expenditure and a lower food intake. The energy expenditure and food intake of fishes are probably affected by anthropogenic noise through stress and changes in foraging and movement behaviour (electronic supplementary material, table S1). Stress increases the metabolic rate [22]. Foraging success would be affected by sound exposure when it distracts fishes from or masks acoustic stimuli of prey [23,24]. Alternatively, foraging may be affected by sound exposure indirectly through shifts in behaviour [23] or lower appetite owing to stress [25,26]. Changes in movement behaviour in response to anthropogenic noise include changes such as higher activity and swimming speed as well as partial disintegration of schools [27–29], which all cost energy [30,31].
At the same time, the population growth rate is relatively insensitive to direct additional mortality and reduced reproductive output. At the lowest feeding level, the population growth rate becomes negative only when mortality reaches approximately 250% compared to natural mortality. Fish mortality after sound exposure has mostly been studied for pile driving (e.g. [8,32,33]). It is generally thought that mortal injuries after sound exposure occur in relatively few individuals, situated close to the sound source. Mortality after sound exposure might also occur further away from the source, through additional predation mortality owing to masking [34]. For example, predation risk was found to increase for Ambon damselfish (Pomacentrus amboinensis) exposed to boat noise [35]. However, in a recent meta-analysis of sound experiments with fishes, predation mortality showed no significant relation with anthropogenic noise [9]. Reproductive output may also be directly affected by sound exposure, as the mating success of cod depends on auditory cues [36]. However, our results show that the population growth rate is only significantly reduced by a strong decrease of the reproductive output.
The high sound exposure levels needed for direct mortality are likely to occur only in limited areas directly around loud sound sources. Because sound attenuates over large distances underwater, low to moderate sound exposure levels will be experienced by many individuals during sound disturbances. These scale differences imply that the sub-lethal effects of sound exposure are likely to occur in a larger part of the population than lethal effects. The most influential sound exposure effect pathways at the population level could thus also be the pathways that occur on a larger scale at the individual level.
In summary, empirical support exists for the effect of sound exposure on fishes through all of the four pathways that we investigated. Our understanding is far from complete [37], also because different fish species react differently to anthropogenic noise [38]. While the effect of sound exposure on Atlantic cod specifically has received little attention, available studies of cod indicate that sound exposure may affect foraging activity and movement [39], cortisol levels [40] and larval growth [41]. A more exact quantification of the effects of sound exposure on cod is needed to allow assessments of the impact of noise pollution on cod populations.
(b). Theoretical sound exposure studies
Previous theoretical studies applied a bioenergetics approach to study population consequences of sound exposure for several species of marine mammals (e.g. [15,42–44]). Our study is, to our knowledge, the first to develop such methodology for a species of fish. A similar approach was used by Hin et al. [15] to study the effect of sound disturbance on the population growth rate of pilot whales (Globicephala melas). Together with the work described here, this illustrates the usefulness of our methodology; an energy budget model continuously tracks the effect of sound exposure on growth, reproduction and survival throughout the life history of an individual. Subsequently, it expresses the significance of these effects on the population level in the form of changes in the cumulative lifetime reproductive output and population growth rate. The approach appears to be generally applicable across different taxa.
(c). Future model improvements
Our model contains size-dependent functions for feeding and energy expenditure that are parameterized on the basis of empirical data. Our model predictions match maximum growth observations of Atlantic cod quite well. Yet, like many other theoretical models [45], our model underestimates the fecundity of large fishes. This is either owing to an underestimation of the feeding rate, or, an overestimation of the energetic or reproduction costs for these large-sized individuals. As a consequence, our model may underestimate the population growth rate of cod and the sensitivity of the population growth rate to lower food intake and increased energy expenditure. A lower food intake and increased energy expenditure reduce early-stage survival and thus the occurrence of large-sized individuals.
Our model could be further refined by incorporating temporal variation, in terms of life-history stages, seasonality and sound exposure. Life history is likely to modulate the effects of sound exposure, because cod undergo morphological, diet and habitat changes over their lifetime. If the effects of sound exposure or sound exposure levels change between life stages, this could affect our results but it is impossible to say how. Seasonal variation in sound exposure can be important when the food availability displays seasonal variation and sound exposure decreases food intake. For example, for pilot whales, sound exposure is expected to have a stronger effect during a period with low food availability [15]. Furthermore, sound exposure may affect species that cod depend on as a food source [46,47]. The effect of changes in food availability can be assessed by changing the feeding level function in the current framework. Finally, the model assumes processes to be density independent. A more complex, density-dependent model framework, which is available for cod, includes multiple food sources and feedbacks between the food sources and the cod population [17]. However, this level of model complexity is unsuited for a first exploration of potential effects with unknown magnitude.
(d). Perspectives for future studies
During spawning, cod aggregate in specific areas [48] and male cod produce mating grunts during courting [36]. Sound exposure of cod during the spawning period could thus potentially result in failure of reproduction for part of the population. It is often thought that reproduction is the most sensitive part of cod life history [49]. At the same time, our analysis shows that, for cod, reproductive failure per se does not have a strong effect at the population level. Our work highlights that subtle effects of sound exposure on fishes, e.g. on their behaviour and physiology, most easily reduce population growth rates. This finding has important ramifications for future experimental and empirical work, as well as for management aimed at mitigating effects of sound exposure. This work calls for elucidation of the relationship between sound exposure and individual-level effects for cod and other fish species. Only then, can our model framework be used to properly assess the effects of marine underwater noise disturbance.
Supplementary Material
Acknowledgements
This study is supported by the E&P Sound and Marine Life Joint Industry Programme (JIP), which is a collaboration among oil and gas industry companies to gain insights relevant to the sustainable exploration for and exploitation of natural resources at sea. There were no publication restrictions, the investigators operated scientifically independent from this industrial partner and the co-authors take full responsibility for the content of the paper. Linda McPhee Consulting provided writing support.
Data accessibility
This code for recreation of figures 2 and 3 of the manuscript can be found at the Zenodo Repository (doi:10.5281/zenodo.3779843).
Authors' contributions
F.H.S. contributed to the concept of the study and the design of the model, conducted the model analysis, analysed the data for the parameterization of the model, interpreted the results and wrote the manuscript. T.v.K. contributed to the concept of the study, provided feedback on the results and interpretation and participated in the writing process. H.S. contributed to the concept of the study, provided feedback on the results and interpretation and participated in the writing process. A.M.d.R. conceived the concept of the study, developed the method for the model analysis, provided feedback on the results and interpretation and participated in the writing process.
Competing interests
We declare we have no competing interests.
Funding
This study is supported by the E&P Sound and Marine Life Joint Industry Programme (JIP).
References
- 1.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427. ( 10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Shannon G, et al. 2016. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 91, 982–1005. ( 10.1111/brv.12207) [DOI] [PubMed] [Google Scholar]
- 3.Fay R. 2009. Soundscapes and the sense of hearing of fishes. Integr. Zool. 4, 26–32. ( 10.1111/j.1749-4877.2008.00132.x) [DOI] [PubMed] [Google Scholar]
- 4.McDonald MA, Hildebrand JA, Wiggins SM. 2006. Increases in deep ocean ambient noise in the northeast Pacific west of San Nicolas Island, California. J. Acoust. Soc. Am. 120, 711–718. ( 10.1121/1.2216565) [DOI] [PubMed] [Google Scholar]
- 5.Hildebrand JA. 2009. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 395, 5–20. ( 10.3354/meps08353) [DOI] [Google Scholar]
- 6.Urick R. 1975. Principles of underwater sound. New York, NY: Tata McGraw-Hill Education. [Google Scholar]
- 7.Sertlek HÖ, Slabbekoorn H, ten Cate C, Ainslie MA. 2019. Source specific sound mapping: spatial, temporal and spectral distribution of sound in the Dutch North Sea. Environ. Pollut. 247, 1143–1157. ( 10.1016/j.envpol.2019.01.119) [DOI] [PubMed] [Google Scholar]
- 8.Halvorsen MB, Casper BM, Matthews F, Carlson TJ, Popper AN. 2012. Effects of exposure to pile-driving sounds on the lake sturgeon, Nile tilapia and hogchoker. Proc. R. Soc. B 279, 4705–4714. ( 10.1098/rspb.2012.1544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox K, Brennan LP, Gerwing TG, Dudas SE, Juanes F. 2018. Sound the alarm: a meta-analysis on the effect of aquatic noise on fish behavior and physiology. Glob. Change Biol. 24, 1–12. ( 10.1111/gcb.14106) [DOI] [PubMed] [Google Scholar]
- 10.Fraser DF, Gilliam JF. 1992. Nonlethal impacts of predator invasion: facultative suppression of growth and reproduction. Ecology 73, 959–970. ( 10.2307/1940172) [DOI] [Google Scholar]
- 11.Pirotta E, et al. 2018. Understanding the population consequences of disturbance. Ecol. Evol. 8, 9934–9946. ( 10.1002/ece3.4458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Research Council. 2005. Marine mammal populations and ocean noise: determining when noise causes biologically significant effects. Washington, DC: National Academies Press. [Google Scholar]
- 13.The European Parliament and the Council of the European Union. 1992. Council Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union L206, 7–50. [Google Scholar]
- 14.The European Parliament and the Council of the European Union. 2009. Council Directive 2009/147/EC on the conservation of wild birds. Off. J. Eur. Union L20, 7–25. [Google Scholar]
- 15.Hin V, Harwood J, de Roos AM, Roos AM.. 2019. Bio-energetic modeling of medium-sized cetaceans shows high sensitivity to disturbance in seasons of low resource supply. Ecol. Appl. 29, e01903 ( 10.1002/eap.1903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caswell H. 2001. Matrix population models, 2nd edn Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 17.van Leeuwen A, Huss M, Gårdmark A, Casini M, Vitale F, Hjelm J, Persson L, de Roos AM.. 2013. Predators with multiple ontogenetic niche shifts have limited potential for population growth and top-down control of their prey. Am. Nat. 182, 53–66. ( 10.1086/670614) [DOI] [PubMed] [Google Scholar]
- 18.de Roos AM. 2008. Demographic analysis of continuous-time life-history models. Ecol. Lett. 11, 1–15. ( 10.1111/j.1461-0248.2007.01121.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soetaert K, Petzoldt T, Setzer RW. 2010. Solving differential equations in R: package deSolve. J. Stat. Softw. 33, 1–25. ( 10.18637/jss.v033.i09)20808728 [DOI] [Google Scholar]
- 20.ICES. 2018. ICES Database of Trawl Surveys (DATRAS). See https://www.ices.dk/data/data-portals/Pages/DATRAS.aspx.
- 21.Lambert Y, Kjesbu OS, Kraus G, Marteinsdottir G. 2005. How variable is the fecundity within and between cod stocks? ICES CM 2005/Q:13:1–19 ( 10.11250/100759) [DOI]
- 22.Barton BA, Schreck CB. 1987. Metabolic cost of acute physical stress in juvenile steelhead. Trans. Am. Fish. Soc. 116, 257–263. () [DOI] [Google Scholar]
- 23.Voellmy IK, Purser J, Flynn D, Kennedy P, Simpson SD, Radford AN. 2014. Acoustic noise reduces foraging success in two sympatric fish species via different mechanisms. Anim. Behav. 89, 191–198. ( 10.1016/j.anbehav.2013.12.029) [DOI] [Google Scholar]
- 24.Purser J, Radford AN. 2011. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS ONE 6, e17478 ( 10.1371/journal.pone.0017478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernier NJ. 2006. The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen. Comp. Endocrinol. 146, 45–55. ( 10.1016/j.ygcen.2005.11.016) [DOI] [PubMed] [Google Scholar]
- 26.Madison BN, Tavakoli S, Kramer S, Bernier NJ. 2015. Chronic cortisol and the regulation of food intake and the endocrine growth axis in rainbow trout. J. Endocrinol. 226, 103–119. ( 10.1530/JOE-15-0186) [DOI] [PubMed] [Google Scholar]
- 27.Sarà G, et al. 2007. Effect of boat noise on the behaviour of bluefin tuna Thunnus thynnus in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 331, 243–253. ( 10.3354/meps331243) [DOI] [Google Scholar]
- 28.Andersson MH, Dock-Akerman E, Ubral-Hedenberg R, Ohman MC. 2007. Swimming behavior of roach (Rutilus rutilus) and three-spined stickleback (Gasterosteus aculeatus) in response to wind power noise and single-tone frequencies. AMBIO J. Hum. Environ. 36, 636–638. ( 10.1579/0044-7447(2007)36[636:sborrr]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 29.Herbert-Read JE, Kremer L, Bruintjes R, Radford AN, Ioannou CC. 2017. Anthropogenic noise pollution from pile-driving disrupts the structure and dynamics of fish shoals. Proc. R. Soc. B 284, 20171627 ( 10.1098/rspb.2017.1627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marras S, Killen SS, Lindström J, McKenzie DJ, Steffensen JF, Domenici P. 2015. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 69, 19–226. ( 10.1007/s00265-014-1834-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb PW. 1971. The swimming energetics of trout II. Oxygen consumption and swimming efficiency. J. Exp. Biol. 55, 521–540. [DOI] [PubMed] [Google Scholar]
- 32.Bolle LJ, et al. 2012. Common sole larvae survive high levels of pile-driving sound in controlled exposure experiments. PLoS ONE 7, e33052 ( 10.1371/journal.pone.0033052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolle LJ, de Jong CAF, Bierman SM, Beek PJGV, Wessels PW, Blom E, Damme CJGV, Winter HV, Dekeling RPA. 2016. Effect of pile-driving sounds on the survival of larval fish. In The effects of noise on aquatic life II. Advances in experimental medicine and biology, vol. 875 (eds A Popper, A Hawkins), pp. 839–846. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 34.Clark CW, Ellison WT, Southall BL, Hatch L, Van Parijs SM, Frankel A, Ponirakis D.. 2009. Acoustic masking in marine ecosystems: intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 395, 201–222. ( 10.3354/meps08402) [DOI] [Google Scholar]
- 35.Simpson SD, Chivers DP, Nedelec SL, Meekan MG, Ferrari MCO, McCormick MI. 2018. School is out on noisy reefs: the effect of boat noise on predator learning and survival of juvenile coral reef fishes. Proc. R. Soc. B 285, 20180033 ( 10.1098/rspb.2018.0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe S, Hutchings JA. 2008. A link between sound producing musculature and mating success in Atlantic cod. J. Fish Biol. 72, 500–511. ( 10.1111/j.1095-8649.2007.01713.x) [DOI] [Google Scholar]
- 37.Carroll AG, Przeslawski R, Duncan A, Gunning M, Bruce B. 2017. A critical review of the potential impacts of marine seismic surveys on fish & invertebrates. Mar. Pollut. Bull. 114, 9–24. ( 10.1016/j.marpolbul.2016.11.038) [DOI] [PubMed] [Google Scholar]
- 38.Kastelein RA, Van Der Heul S, Verboom W, De Haan D, Reijnders P.. 2008. Acoustic dose-response effects in marine fish. Bioacoustics 17, 201–202. ( 10.1080/09524622.2008.9753816) [DOI] [Google Scholar]
- 39.Engås A, Løkkeborg S, Ona E, Soldal AV. 1996. Effects of seismic shooting on local abundance and catch rates of cod (Gadus morhua) and haddock (Melanogrammus aeglefinus). Can. J. Fish. Aquatic Sci. 2249, 2238–2249. ( 10.1139/f96-177) [DOI] [Google Scholar]
- 40.Sierra-Flores R, Atack T, Migaud H, Davie A. 2015. Stress response to anthropogenic noise in Atlantic cod Gadus morhua L. Aquac. Eng. 67, 67–76. ( 10.1016/j.aquaeng.2015.06.003) [DOI] [Google Scholar]
- 41.Nedelec SL, Simpson SD, Morley EL, Nedelec B, Radford AN. 2015. Impacts of regular and random noise on the behaviour, growth and development of larval Atlantic cod (Gadus morhua). Proc. R. Soc. B 282, 20151943 ( 10.1098/rspb.2015.1943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pirotta E, et al. 2019. Anthropogenic disturbance in a changing environment: modelling lifetime reproductive success to predict the consequences of multiple stressors on a migratory population. Oikos 128, 1340–1357. ( 10.1111/oik.06146) [DOI] [Google Scholar]
- 43.Farmer NA, Baker K, Zeddies DG, Denes SL, Noren DP, Garrison LP, Machernis A, Fougères EM, Zykov M. 2018. Population consequences of disturbance by offshore oil and gas activity for endangered sperm whales (Physeter macrocephalus). Biol. Conserv. 227, 189–204. ( 10.1016/j.biocon.2018.09.006) [DOI] [Google Scholar]
- 44.McHuron EA, Schwarz LK, Costa DP, Mangel M. 2018. A state-dependent model for assessing the population consequences of disturbance on income-breeding mammals. Ecol. Model. 385, 133–144. ( 10.1016/j.ecolmodel.2018.07.016) [DOI] [Google Scholar]
- 45.Barneche DR, Robertson DR, White CR, Marshall DJ. 2018. Fish reproductive-energy output increases disproportionately with body size. Science 360, 642–645. ( 10.1126/science.aao6868) [DOI] [PubMed] [Google Scholar]
- 46.Morley EL, Jones G, Radford AN. 2013. The importance of invertebrates when considering the impacts of anthropogenic noise. Proc. R. Soc. B 281, 20132683 ( 10.1098/rspb.2013.2683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCauley RD, Day RD, Swadling KM, Fitzgibbon Q, Watson R, Semmens J. 2017. Widely used marine seismic survey air gun operations negatively impact zooplankton. Nat . Ecol. Evol. 1, 0195 ( 10.1038/s41559-017-0195) [DOI] [PubMed] [Google Scholar]
- 48.González-Irusta JM, Wright PJ. 2016. Spawning grounds of Atlantic cod (Gadus morhua) in the North Sea. ICES J. Mar. Sci. 73, 304–315. ( 10.1093/icesjms/fsv180) [DOI] [Google Scholar]
- 49.Stelzenmüller V, Ellis JR, Rogers SI. 2010. Towards a spatially explicit risk assessment for marine management: assessing the vulnerability of fish to aggregate extraction. Biol. Conserv. 143, 230–238. ( 10.1016/j.biocon.2009.10.007) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This code for recreation of figures 2 and 3 of the manuscript can be found at the Zenodo Repository (doi:10.5281/zenodo.3779843).