Abstract
Spiders at southern latitudes commonly produce multiple clutches, but this has not been observed at high latitudes where activity seasons are much shorter. Yet the timing of snowmelt is advancing in the Arctic, which may allow some species to produce an additional clutch. To determine if this is already happening, we used specimens of the wolf spider Pardosa glacialis caught by pitfall traps from the long-term (1996–2014) monitoring programme at Zackenberg, NE Greenland. We dissected individual egg sacs and counted the number of eggs and partially developed juveniles, and measured carapace width of the mothers. Upon the discovery of a bimodal frequency distribution of clutch sizes, as is typical for wolf spiders at lower latitudes producing a second clutch, we assigned egg sacs to being a first or second clutch depending on clutch size. We tested whether the median capture date differed among first and second clutches, whether clutch size was correlated to female size, and whether the proportion of second clutches produced within a season was related to climate. We found that assigned second clutches appeared significantly later in the season than first clutches. In years with earlier snowmelt, first clutches occurred earlier and the proportion of second clutches produced was larger. Likely, females produce their first clutch earlier in those years which allow them time to produce another clutch. Clutch size for first clutches was correlated to female size, while this was not the case for second clutches. Our results provide the first evidence for Arctic invertebrates producing additional clutches in response to warming. This could be a common but overlooked phenomenon due to the challenges associated with long-term collection of life-history data in the Arctic. Moreover, given that wolf spiders are a widely distributed, important tundra predator, we may expect to see population and food web consequences of their increased reproductive rates.
Keywords: arthropods, climate change, life-history variation, phenology, reproduction
1. Introduction
Shifts in phenology, i.e. the timing of biological events, are the most widely reported biological response to climate change [1–3]. The demographic consequences of climate-induced phenological variation are typically studied in the context of phenological mismatch, where the mistiming of resource availability and resource demand lead to reduced growth, survival, or reproduction [4–6]. Some studies have examined how the number of generations per year may increase in response to a warmer climate [7–9], but little is known about how extended growing seasons may affect total reproductive output [10,11]. Yet warmer conditions can extend the time available for reproduction, which may result in phenological shifts that could also affect demography and population dynamics [5]. Due to the low temperatures and time limitations of short growing seasons in northern environments, invertebrate organisms that produce multiple clutches in temperate ecosystems can typically only produce one clutch at higher latitudes [12,13]. Arctic temperatures are currently rising at twice the global average and climate projections indicate that the Arctic will continue to warm at a higher rate than the rest of the globe, which will lead to longer growing seasons [14,15]. These warmer conditions and associated extended growing seasons may result in higher reproductive rates among Arctic species that are opportunistically capable of producing additional clutches.
Arctic arthropods are expected to be particularly affected by climate, and changes in their phenology have been widely documented [16–18]. Wolf spiders (family: Lycosidae) are abundant on the Arctic tundra [19–23] and they play an important role as top predators in the ecosystem [24–26]. They are also very responsive to environmental change [27,28]. In the North, their life cycle typically takes 2 or more years [29,30], while in the temperate zone, wolf spiders have annual life cycles [31]. Adult spiders die after completing reproduction, but female wolf spiders at lower latitudes typically produce more than one clutch of eggs over their lifetimes [32,33]. Female wolf spiders weave their eggs into an egg sac; each egg sac is regarded as a single clutch. Subsequent egg clutches are produced approximately one month after the first clutch, and they differ from first clutches in that there are typically fewer eggs produced per clutch [32]. In the Arctic, due to the time constraints of extremely short growing seasons, female wolf spiders have always been assumed to produce only one clutch [34]. However, the opportunity to produce a second clutch could confer a big fitness advantage, which may be possible with the warmer temperatures and extended growing seasons that are currently being brought on by climate change [35]. The characteristics associated with the production of second clutches in temperate regions suggest that if longer growing seasons enable female wolf spiders in the Arctic to produce second clutches, these clutches would likely occur later in the summer, contain fewer eggs per clutch and occur more frequently in years with early snowmelt or higher temperatures.
Longer growing seasons may also increase reproductive rates indirectly through changes in female body size. Fecundity is typically positively associated with body size in invertebrates [36], including among spiders, whereby larger females produce larger clutches [37,38]. Body size––and hence fecundity––also vary according to growing season length in these organisms. For example, previous studies from multiple Arctic locations have found that wolf spider body size and fecundity decrease with rising elevation, a proxy for shortening growing season length [39,40]. Whether larger females are also more likely to produce second clutches is unknown. We would expect body size of females producing a second clutch to be larger if only the biggest individuals are able to produce two clutches, or alternatively, females should be of similar size if the ability to produce two clutches mainly depends on the length of the growing season.
Here, we use long-term (1996–2014) data on clutch size variation in the only wolf spider species (Pardosa glacialis, Thorell 1872) known from the study area at Zackenberg, NE Greenland, to examine if climate change is enabling the spiders to produce an additional clutch through a lengthening of the growing season. Previous work has found that body sizes of wolf spiders at this high-Arctic site are larger in years with earlier snowmelt [41]. Climate change at Zackenberg is resulting in warmer summer temperatures and earlier start of the growing season [42]. We argue that the combination of three patterns would indicate that second clutches are produced in a population: (i) a bimodal frequency distribution of clutch sizes which would allow for an assignment of clutches to either first or second clutches. (ii) Females with egg sacs assigned to first clutches should be caught earlier than those females with egg sacs assigned to second clutches. (iii) Females producing clutches assigned as first clutches should not be bigger than females carrying egg sacs that are assigned as second clutches. Using the long-term dataset, we examine the likelihood of whether second clutches are being produced by female wolf spiders. We make the following predictions in light of climate change: (i) a proportion of all egg sacs are from second clutches, (ii) this proportion varies across years at the site and (iii) the proportion of second clutches is related to timing of snowmelt date.
2. Material and methods
(a). Field and laboratory work
Female wolf spiders carry their egg sac attached to their spinnerets. Pitfall trap samples collected across the breeding season can therefore be used to assess the prevalence of potential second clutches. We used samples of wolf spiders that were collected from pitfall traps in five different plots at Zackenberg, NE Greenland (74°28′N, 20°34′W, 35–50 m.a.s.l.) as part of the Greenland Ecosystem Monitoring programme. The plots are placed in three different habitats with one plot in a fen and two plots each in mesic heath and arid heath habitats. All plots are located within a study area of about 1 km2 [43]. Pitfall traps were emptied weekly during June, July, and August, although trapping continued into September in a few years. In order to standardize trapping effort, we omitted samples (totalling 17 egg sacs) collected before 1 June and after 27 August. Only one species of wolf spider (P. glacialis) has been collected from the study area over this 18-year period [27], so all egg sacs are assumed to belong to this species. The species has a generation time of 2 years at the site [41]. All egg sacs from years 1996–2014 (no available data for 2010) were opened. We counted the number of eggs or partially developed juveniles. Egg sacs sometimes detach from the mother during trapping. If the egg sac was still attached to the mother, we measured the width of her carapace to the nearest 0.01 mm using a reticle fitted to a stereo microscope. Otherwise, the mother could only be identified if there was only one female in the sample. A total of 1069 egg sacs were collected across the 18 years of sampling. The mother could be confidently identified in 280 of these cases. We calculated the date of snowmelt across plots as the average date at which 50% of the snow had disappeared from each plot during spring snowmelt, measured as the day of the year after 1 January.
(b). Data analysis
We found a clear bimodal frequency distribution of the sizes of clutches and could therefore assign each clutch to a first or second clutch depending on clutch size (figure 1). We tested if there was a significant difference in the mean capture dates between egg sacs that were assigned to first or second clutches using a one-way ANOVA. We then tested whether the proportion of second clutches produced across years varied among habitat types. Likewise, we tested whether this proportion exhibited a trend across the study period or was related to the date of snowmelt using general linear models with a Gaussian error distribution. We also tested if the mean date of trapping first clutches was related to timing of snowmelt. Finally, for the subset of data for which the mother could be identified, we tested if clutch size was related to body size of the mother separately for first and second clutches. For this subset, we also tested if mothers that were assigned to first or second clutches differed in body size using a general linear model. All analyses were conducted in R (R Core Team 2020).
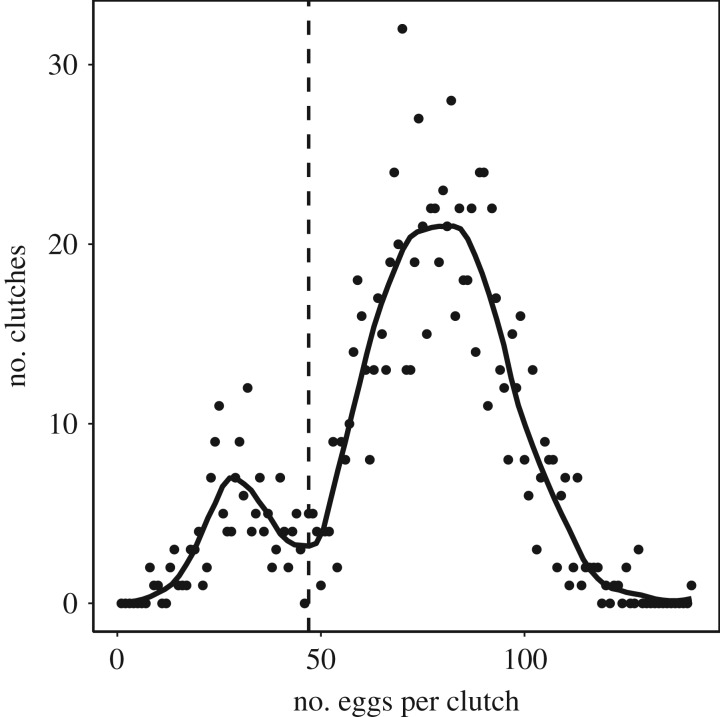
Figure 1.
Frequency distribution of clutch sizes in the wolf spider Pardosa glacialis across 1069 egg sacs collected in pitfall traps during 1996–2014 at Zackenberg, NE Greenland. The solid line represents a locally weighted smoothing with a span parameter = 0.2 and identifies a local minimum at a clutch size of 47 eggs used to separate first and second clutches, as indicated by the vertical hatched line.
3. Results
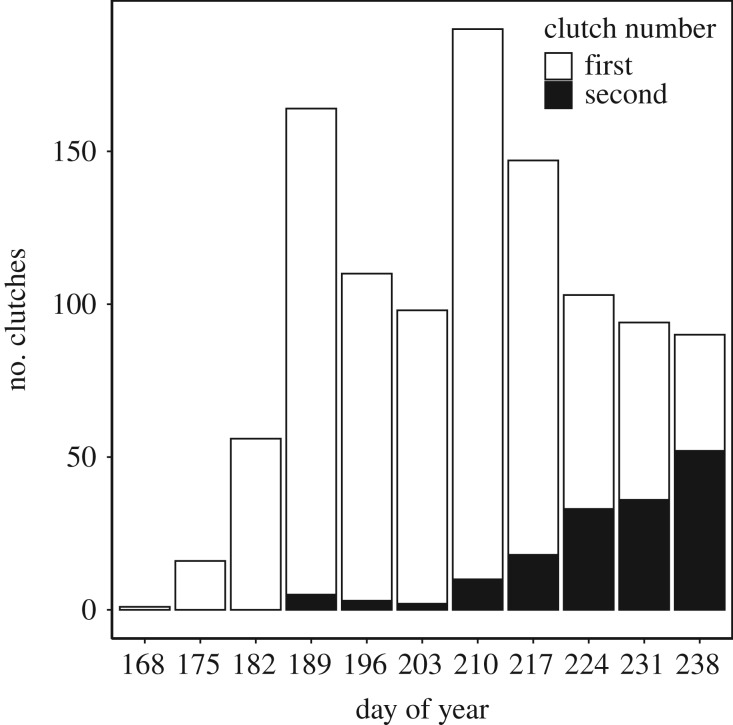
Clutch sizes exhibited a distinct bimodal distribution with a split between peaks of clutch size at 47 eggs (figure 1). Among the total of 1069 egg sacs, we assigned egg sacs with more than 47 eggs to first clutches (n = 910) and considered clutches with 47 eggs or less to be second clutches (n = 159). Clutches with 47 eggs or less were caught significantly later in the season than larger clutches (difference = 20.7 ± 1.3 days, F1,1067 = 253.6, p < 0.001; figure 2). Of the 17 omitted egg sacs collected after 27 August, 15 (88%) had 47 eggs or less and, therefore, would have been assigned to second clutches. Across all years, the proportion of second clutches varied among habitats and was highest in the wet fen (arid heath = 0.113, mesic heath = 0.128, and wet fen = 0.543).
Figure 2.
Seasonal variation in total egg sac collection across the study period (grouped by 7 day periods). Dates are presented as day of year since 1 January. The white section of each bar indicates clutches with more than 47 eggs (first clutches) and the black sections are for those clutches with 47 or less eggs (second clutches).
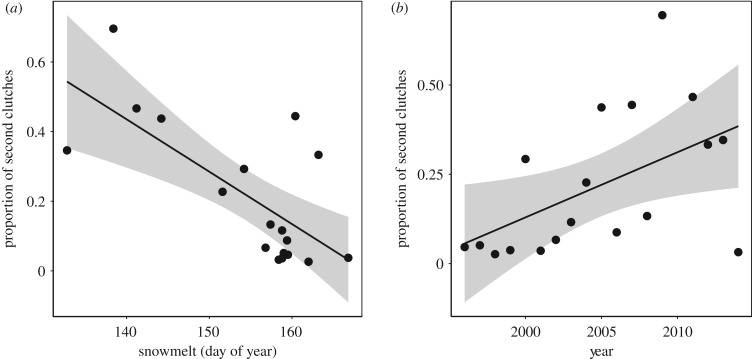
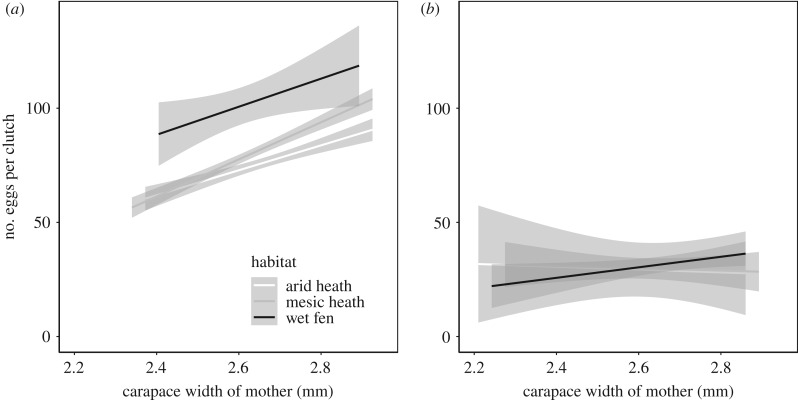
Snowmelt advanced significantly during the study period (slope = −0.79 ± 0.36, n = 18, p = 0.043). We found a negative relationship between the proportion of second clutches and date of snowmelt (slope = −0.015 ± 0.0038, n = 18, p = 0.0012; figure 3a) and sampling year (slope = 0.0182 ± 0.0075, n = 18, p = 0.028; figure 3b). We also found earlier mean capture date of first clutches in years with earlier snowmelt (slope = 0.90 ± 0.20, n = 18, p = 0.0032). Clutch size of first clutches was largest in wet habitats, intermediate in mesic habitats and smallest in arid habitats (arid versus wet = 28.40 ± 3.29, t = 8.65, p < 0.001; arid versus mesic = 5.66 ± 1.56, t = 3.63, p = 0.00035) and was positively related to carapace width of the mother (estimate = 68.48 ± 5.15, t = 13.29, p < 0.0001; figure 4a). The size of second clutches was independent of habitats (arid versus mesic = 0.60 ± 3.93, t = 0.15, p = 0.88; arid versus wet = 2.45 ± 4.10, t = 0.60, p = 0.56) and unrelated to carapace width of mothers (estimate = 3.10 ± 8.2, t = 0.38, p = 0.71; figure 4b). There was no significant difference in body size between mothers of first and second clutches (difference = −0.0039 ± 0.025, F1,278 = 0.024, p = 0.88).
Figure 3.
The proportion of second clutches out of total number of clutches in a given year regressed against (a) average date of snowmelt (day of year since January 1) across the study plots (slope = −0.0150 ± 0.0038, t16 = −3.949, p = 0.0012) and (b) sampling year (slope = 0.0182 ± 0.0075, t16 = 2.418, p = 0.028).
Figure 4.
Relationship between clutch size and body size of the mother for each habitat type (wet, mesic and arid) for (a) first and (b) second clutches. The relationships are significant for first clutches, but not for second clutches (see text for details). Body size of the mother was estimated as width of the carapace (in mm).
4. Discussion
Over the last two decades, the climate at Zackenberg has changed significantly, including snowmelt occurring progressively earlier and temperatures becoming warmer. These changes have had rapid measurable impacts on the biology of species in the area [41,44]. In addition to previously documented changes, we have detected strong evidence of climate change induced shifts to multivoltinism in a dominant spider species. Our results provide the first evidence of an Arctic spider species being able to produce two clutches. This is demonstrated by a striking bimodal frequency distribution of clutch sizes in the wolf spider P. glacialis, which is indicative of second clutches in temperate regions [32,45]. Moreover, clutches with fewer eggs (47 eggs or less) were produced significantly later in the season than larger clutches. We are able to rule out the possibility that this was driven by inter-annual variation in egg sac phenology, because second clutches were laid later in the season in all years. Together, these findings provide support for the hypothesis that P. glacialis is able to produce a second clutch at our high-Arctic study site in NE Greenland. We were further able to demonstrate that as spring snowmelt becomes earlier, a greater proportion of P. glacialis females are able to produce a second clutch and that the production of second clutches is increasing over time. In fact, the proportion of second clutches increased from zero to greater than 50% as snowmelt became progressively earlier over the study period, suggesting that the ability to produce second clutches is a common yet overlooked phenomenon in northern ecosystems.
It is known that larger female wolf spiders produce more offspring [46] and our results confirm this for first clutches. However, we found no indication that larger females are more likely to produce a second clutch than smaller females or that larger females produce larger second clutches. These findings suggest that the production of second clutches in association with earlier snowmelt is not due to females obtaining more resources and attaining larger body sizes. Rather, earlier snowmelt enables females to produce their first clutch earlier and gives them enough time to produce a second clutch before the season ends. We note that earlier snowmelt is also leading to larger female body size in the same spider species [41]. It is possible that climate change is altering the reproductive strategy of P. glacialis, allowing females to produce second clutches for the first time. However, given that Arctic wolf spiders have been shown to respond rapidly to environmental conditions and given the broad distribution of individual species [34], it seems plausible that this species is already adapted to opportunistically reproduce a second time when conditions allow. Although our study was limited to a single site, Zackenberg is at the northern edge of the range of P. glacialis and one of the most climatically extreme locations inhabited by this species. Thus, it is likely that P. glacialis and possibly other wolf spider species are also already producing second clutches at lower Arctic and boreal latitudes. Similar studies at other sites and for other invertebrate species are needed to unravel the ubiquity of climate-induced shifts in voltinism and the potential ecological consequences.
We found a difference in the mean date of capture of first and second clutches of about 20 days. This period is less than previous reports of a 30-day interval between the production of first and second clutches in temperate wolf spiders [32], but it may be related to the long daily hours of incident solar radiation at high latitudes. The growing season at Zackenberg is extremely short and P. glacialis could be adapted to rapidly respond to favourable conditions by producing second clutches whenever the opportunity allows. It is possible that some of the clutches that were assigned as second clutches could have been from females who lost their first egg sacs (e.g. due to predation from birds). In such cases, the production of a second, smaller clutch would happen earlier in the season and would reduce our estimate of a time difference. Still, the combination of evidence presented here strongly suggest that P. glacialis are indeed producing second clutches in years with earlier snowmelt at our high-Arctic site.
Increased reproductive output can increase population size if other factors such as competition or predation are not limiting population growth. Yet, we have not observed significant changes in population size of P. glacialis at this site during the study period [27]. Thus, it is unclear whether increased reproductive rates associated with the production of second clutches will have population-level consequences for this species in the future. For instance, it is unknown whether there are differences in viability between eggs from first clutches versus those from second clutches. Recent evidence from our field site suggests that P. glacialis is not food limited [47], nor are its populations affected by parasitism as in other Arctic wolf spiders [48,49]. However, wolf spiders are density-dependent cannibals (e.g. [50–52]), and a lack of observed population growth could be a result of increased intraspecific competition with rising reproductive rates [53]. As P. glacialis is the largest invertebrate predator at our site, further studies should address whether there are implications of higher reproductive rates among the spiders for prey populations. If prey populations do not respond in a similar way to earlier snowmelt, they might be negatively affected by an increasing predation pressure as top-down forces appears to be the main control mechanism of tundra food webs [26,54,55]. Smaller arthropod predators, such as other spiders, might also suffer from the increase in abundance of P. glacialis due to the limited differentiation in diet between predators in the high Arctic [27,53,56]. Studies of the effects on population dynamics, cascading effects in food webs and repercussions on ecosystem function continue to establish wolf spiders as key model organisms for the study of Arctic climate change effects.
Supplementary Material
Acknowledgements
Access to spider specimens and climate data from the Greenland Ecosystem Monitoring programme is greatly appreciated. Spider specimens are curated by the Natural History Museum, Aarhus, Denmark.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.79cnp5hs9 [57].
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Arctic Research Centre, Aarhus University, Denmark. Articles and presentations written by Canadian Government civil servants in the course of their duties are covered by Crown copyright.
References
- 1.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 2.Høye TT, Post E, Meltofte H, Schmidt NM, Forchhammer MC. 2007. Rapid advancement of spring in the high Arctic. Curr. Biol. 17, R449–R451. ( 10.1016/j.cub.2007.04.047) [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Sys. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 4.Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569. ( 10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. 2010. The effects of phenological mismatches on demography. Phil. Trans. R. Soc. B 365, 3177–3186. ( 10.1098/rstb.2010.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramula S, Johansson J, Linden A, Jonzen N. 2015. Linking phenological shifts to demographic change. Clim. Res. 63, 135–144. ( 10.3354/cr01289) [DOI] [Google Scholar]
- 7.Jönsson AM, Appelberg G, Harding S, Bärring L. 2009. Spatio-temporal impact of climate change on the activity and voltinism of the spruce bark beetle, Ips typographus. Glob. Change Biol. 15, 486–499. ( 10.1111/j.1365-2486.2008.01742.x) [DOI] [Google Scholar]
- 8.Mitton JB, Ferrenberg S.M. 2012. Mountain pine beetle develops an unprecedented summer generation in response to climate warming. Am. Nat. 179, E163-E171. ( 10.1086/665007) [DOI] [PubMed] [Google Scholar]
- 9.Altermatt F. 2010. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. B Biol. Sci. 277, 1281–1287. ( 10.1098/rspb.2009.1910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Møller AP, Flensted-Jensen E, Klarborg K, Mardal W, Nielsen J.T. 2010. Climate change affects the duration of the reproductive season in birds. J. Anim. Ecol. 79, 777–784. ( 10.1111/j.1365-2656.2010.01677.x) [DOI] [PubMed] [Google Scholar]
- 11.Hovel RA, Carlson SM, Quinn T.P. 2017. Climate change alters the reproductive phenology and investment of a lacustrine fish, the three-spine stickleback. Glob. Change Biol. 23, 2308–2320. ( 10.1111/gcb.13531) [DOI] [PubMed] [Google Scholar]
- 12.Baba YG, Walters RJ, Miyashita T. 2013. Complex latitudinal variation in the morphology of the kleptoparasitic spider Argyrodes kumadai associated with host use and climatic conditions. Popul. Ecol. 55, 43–51. ( 10.1007/s10144-012-0334-5) [DOI] [Google Scholar]
- 13.Bayram A. 2000. A study of egg production in three species of wolf spiders (Araneae, Lycosidae), Pardosa amentata, P. palustris, and P. pullata in the field. Israel J. Ecol. Evol. 46, 297–303. ( 10.1560/0AM1-NTRX-V05V-3JBD) [DOI] [Google Scholar]
- 14.IPCC. 2014. Climate change 2014: Impacts, adaptation, and vulnerability. Part B: Regional Aspects. In Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change, 688 p Cambridge, UK; New York, NY, USA: Cambridge University Press. [Google Scholar]
- 15.AMAP. 2017. Snow, water, ice and permafrost in the Arctic (SWIPA) 2017. (p. xiv + 269 pp.
- 16.Høye TT, Forchhammer M.C. 2008. Phenology of high-Arctic arthropods: effects of climate on spatial, seasonal and inter-annual variation. Adv. Ecol. Res. 40, 299–324. ( 10.1016/S0065-2504(07)00013-X) [DOI] [Google Scholar]
- 17.Høye TT, Eskildsen A, Hansen RR, Bowden JJ, Schmidt NM, Kissling WD. 2014. Phenology of high-Arctic butterflies and their floral resources: species-specific responses to climate change. Curr. Zool. 60, 243–251. ( 10.1093/czoolo/60.2.243) [DOI] [Google Scholar]
- 18.Tulp I, Schekkerman H. 2008. Has prey availability for Arctic birds advanced with climate change? Hindcasting the abundance of tundra arthropods using weather and seasonal variation. Arctic 61, 48–60. ( 10.14430/arctic6) [DOI] [Google Scholar]
- 19.Bolduc E, et al. 2013. Terrestrial arthropod abundance and phenology in the Canadian Arctic: modelling resource availability for Arctic-nesting insectivorous birds. Can. Entomol. 145, 155–170. ( 10.4039/tce.2013.4) [DOI] [Google Scholar]
- 20.Hansen RR, Hansen OLP, Bowden JJ, Treier UA, Normand S, Høye TT. 2016. Meter scale variation in shrub dominance and soil moisture structure Arctic arthropod communities. Peerj 4, e2224 ( 10.7717/peerj.2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asmus A, Koltz A, McLaren J, Shaver GR, Gough L. 2018. Long-term nutrient addition alters arthropod community composition but does not increase total biomass or abundance. Oikos 127, 460–471. ( 10.1111/oik.04398) [DOI] [Google Scholar]
- 22.Rich ME, Gough L, Boelman N.T. 2013. Arctic arthropod assemblages in habitats of differing shrub dominance. Ecography 36, 994–1003. ( 10.1111/j.1600-0587.2012.00078.x) [DOI] [Google Scholar]
- 23.Høye TT, Bowden JJ, Hansen O.LP, Hansen RR, Henriksen TN, Niebuhr A, Skytte M.G. 2018. Elevation modulates how Arctic arthropod communities are structured along local environmental gradients. Polar Biol. 41, 1555–1565. ( 10.1007/s00300-017-2204-2) [DOI] [Google Scholar]
- 24.Kuusk A.-K, Ekbom B. 2012. Feeding habits of lycosid spiders in field habitats. J. Pest Sci. 85, 253–260. ( 10.1007/s10340-012-0431-4) [DOI] [Google Scholar]
- 25.Lawrence KL, Wise D.H. 2000. Spider predation on forest-floor Collembola and evidence for indirect effects on decomposition. Pedobiologia – Int. J. Soil Biol. 44, 33–39. ( 10.1078/S0031-4056(04)70026-8) [DOI] [Google Scholar]
- 26.Koltz AM, Asmus A, Gough L, Pressler Y, Moore J.C. 2018. The detritus-based microbial-invertebrate food web contributes disproportionately to carbon and nitrogen cycling in the Arctic. Polar Biol. 41, 1531–1545. ( 10.1007/s00300-017-2201-5) [DOI] [Google Scholar]
- 27.Bowden JJ, Hansen OLP, Olsen K, Schmidt NM, Høye TT. 2018. Drivers of inter-annual variation and long-term change in high-Arctic spider species abundances. Polar Biol. 41, 1635–1649. ( 10.1007/s00300-018-2351-0) [DOI] [Google Scholar]
- 28.Asmus AL, Chmura HE, Høye TT, Krause JS, Sweet SK, Perez JH, Boelman NT, Wingfield JC, Gough L. 2018. Shrub shading moderates the effects of weather on arthropod activity in Arctic tundra. Ecol. Entomol. 43, 647–655. ( 10.1111/een.12644) [DOI] [Google Scholar]
- 29.Pickavance JR. 2001. Life-cycles of four species of Pardosa (Araneae, Lycosidae) from the island of Newfoundland, Canada. J. Arachnol. 29, 367–377. ( 10.1636/0161-8202(2001)029[0367:LCOFSO[2.0.CO;2) [DOI] [Google Scholar]
- 30.Buddle CM. 2000. Life history of Pardosa moesta and Pardosa mackenziana (Araneae, Lycosidae) in central Alberta, Canada. J. Arachnol. 28, 319–328. ( 10.1636/0161-8202(2000)028[0319:LHOPMA]2.0.CO;2) [DOI] [Google Scholar]
- 31.Zoltán R, Balázs K, Ferenc S. 2017. Effect of weather conditions on cohort splitting in a wolf spider species. J. Arachnol. 45, 444–447. ( 10.1636/JoA-S-17-008.1) [DOI] [Google Scholar]
- 32.Brown CA, Sanford BM, Swerdon RR. 2003. Clutch size and offspring size in the wolf spider Pirata sedentarius (Araneae, Lycosidae). J. Arachnol. 31, 285–296. ( 10.1636/m01-62) [DOI] [Google Scholar]
- 33.Hein N, Feilhauer H, Löffler J, Finch O-D. 2015. Elevational variation of reproductive traits in five Pardosa (Lycosidae) species. Arct. Antarct. Alp. Res. 47, 473–479. ( 10.1657/aaar0013-111) [DOI] [Google Scholar]
- 34.Dondale CD, Redner JH. 1990. The insects and arachnids of Canada. Part 17. The wolf spiders, nurseryweb spiders, and lynx spiders of Canada and Alaska. Araneae: Lycosidae, Pisauridae, and Oxyopidae. Ottawa, Ontario: Agriculture Canada. [Google Scholar]
- 35.Bowden JJ, Buddle CM. 2012. Life history of tundra-dwelling wolf spiders (Araneae: Lycosidae) from the Yukon Territory, Canada. Can. J. Zool. Revue Canadienne De Zoologie 90, 714–721. ( 10.1139/Z2012-038) [DOI] [Google Scholar]
- 36.Blanckenhorn WU. 2000. The evolution of body size: what keeps organisms small? Q. Rev. Biol. 75, 385–407. ( 10.1086/393620) [DOI] [PubMed] [Google Scholar]
- 37.Reed DH, Nicholas AC. 2008. Spatial and temporal variation in a suite of life-history traits in two species of wolf spider. Ecol. Entomol. 33, 488–496. ( 10.1111/j.1365-2311.2008.00994.x) [DOI] [Google Scholar]
- 38.Fritz RS, Morse DH. 1985. Reproductive success and foraging of the crab spider Misumena vatia. Oecologia 65, 194–200. ( 10.1007/BF00379217) [DOI] [PubMed] [Google Scholar]
- 39.Bowden JJ, Høye TT, Buddle C.M. 2013. Fecundity and sexual size dimorphism of wolf spiders (Araneae: Lycosidae) along an elevational gradient in the Arctic. Polar Biol. 36, 831–836. ( 10.1007/s00300-013-1308-6) [DOI] [Google Scholar]
- 40.Høye TT, Hammel J.U. 2010. Climate change and altitudinal variation in sexual size dimorphism of Arctic wolf spiders. Clim. Res. 41, 259–265. ( 10.3354/cr00855) [DOI] [Google Scholar]
- 41.Høye TT, Hammel JU, Fuchs T, Toft S. 2009. Climate change and sexual size dimorphism in an Arctic spider. Biol. Lett. 5, 542–544. ( 10.1098/rsbl.2009.0169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt NM, Reneerkens J, Christensen JH, Olesen M, Roslin T. 2019. An ecosystem-wide reproductive failure with more snow in the Arctic. PLoS Biol. 17, e3000392 ( 10.1371/journal.pbio.3000392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt NM, Hansen LH, Hansen J, Berg TB, Meltofte H. 2019. BioBasis manual: conceptual design and sampling procedures of the biological monitoring programme within Zackenberg basic, 22nd edn Aarhus, Denmark: Aarhus University, Department of Bioscience. [Google Scholar]
- 44.Bowden JJ, Eskildsen A, Hansen RR, Olsen K, Kurle CM, Høye T.T. 2015. High-Arctic butterflies become smaller with rising temperatures. Biol. Lett. 11, 20150574 ( 10.1098/rsbl.2015.0574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendrickx F, Maelfait J-P. 2003. Life cycle, reproductive patterns and their year-to-year variation in a field population of the wolf spider Pirata piraticus (Araneae, Lycosidae). J. Arachnol. 31, 331–339. ( 10.1636/m01-98) [DOI] [Google Scholar]
- 46.Simpson M.R. 1993. Reproduction in two species of arctic arachnids, Pardosa glacialis and Alopecosa hirtipes. Can. J. Zool. 71, 451–457. ( 10.1139/z93-065) [DOI] [Google Scholar]
- 47.Visakorpi K, Wirta HK, Ek M, Schmidt NM, Roslin T. 2015. No detectable trophic cascade in a high-Arctic arthropod food web. Basic Appl. Ecol. 16, 652–660. ( 10.1016/j.baae.2015.06.003) [DOI] [Google Scholar]
- 48.Bowden JJ, Buddle CM. 2012. Egg sac parasitism of Arctic wolf spiders (Araneae: Lycosidae) from northwestern North America. J. Arachnol. 40, 348–350. ( 10.1636/P11-50.1) [DOI] [Google Scholar]
- 49.Koltz AM, Culler LE, Bowden JJ, Post E, Høye TT. 2019. Dominant Arctic predator is free of major parasitoid at northern edge of its range. Front. Ecol. Evol. 7, 250 ( 10.3389/fevo.2019.00250) [DOI] [Google Scholar]
- 50.Buddle CM, Walker SE, Rypstra AL. 2003. Cannibalism and density-dependent mortality in the wolf spider Pardosa milvina (Araneae: Lycosidae). Can. J. Zool. 81, 1293–1297. ( 10.1139/z03-124) [DOI] [Google Scholar]
- 51.Wise DH. 2006. Cannibalism, food limitation, intraspecific competition and the regulation of spider populations. Annu. Rev. Entomol. 51, 441–465. ( 10.1146/annurev.ento.51.110104.150947) [DOI] [PubMed] [Google Scholar]
- 52.Wagner JD, Wise DH. 1996. Cannibalism regulates densities of young wolf spiders: evidence from field and laboratory experiments. Ecology 77, 639–652. ( 10.2307/2265637) [DOI] [Google Scholar]
- 53.Koltz AM, Wright JP. 2020. Impacts of female body size on cannibalism and juvenile abundance in a dominant arctic spider. J. Anim. Ecol. ( 10.1111/1365-2656.13230 [DOI] [PubMed] [Google Scholar]
- 54.Legagneux P, et al. 2012. Disentangling trophic relationships in a high Arctic tundra ecosystem through food web modeling. Ecology 93, 1707–1716. ( 10.1890/11-1973.1) [DOI] [PubMed] [Google Scholar]
- 55.Gauthier G, Berteaux D, Bêty J, Tarroux A, Therrien J-F, McKinnon L, Legagneux P, Cadieux M-C. 2011. The tundra food web of Bylot Island in a changing climate and the role of exchanges between ecosystems. Ecoscience 18, 223–235. ( 10.2980/18-3-3453) [DOI] [Google Scholar]
- 56.Wirta HK, Weingartner E, Hamback PA, Roslin T. 2015. Extensive niche overlap among the dominant arthropod predators of the high Arctic. Basic Appl. Ecol. 16, 86–92. ( 10.1016/j.baae.2014.11.003) [DOI] [Google Scholar]
- 57.Høye TT, Kresse J-C, Koltz AM, Bowden JJ. 2020. Data from: Earlier springs enable high-Arctic wolf spiders to produce a second clutch. Dryad Digital Repository ( 10.5061/dryad.79cnp5hs9) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Høye TT, Kresse J-C, Koltz AM, Bowden JJ. 2020. Data from: Earlier springs enable high-Arctic wolf spiders to produce a second clutch. Dryad Digital Repository ( 10.5061/dryad.79cnp5hs9) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.79cnp5hs9 [57].