Abstract
Flight is a unique adaptation at the core of many behaviours in most bird species, whether it be foraging, migration or breeding. Birds have developed a wide diversity of flight modes (e.g. flapping, gliding, soaring, hovering) which involves very specialized behaviours. A key issue when studying flight behaviours is to understand how they develop through all the ontogenetic stages of birds, from the embryo to the flying adult. This question typically involves classical debates on animal behaviour about the importance of maturation and experience. Here, we review the literature available on the development of flight behaviours in birds. First, we focus on the early period when young birds are not yet capable of flight. We discuss examples and show how endogenous processes (e.g. wing flapping in the nest, flight development timing) and environmental factors (e.g. maternal stress, nutritional stress) can influence the development of flight behaviours. Then, we review several examples showing the different processes involved in the development of flight in flight-capable juveniles (e.g. practice, trial and error learning, social learning). Despite the lack of experimental studies investigating this specific question at different developmental stages, we show that several patterns can be identified, and we anticipate that the development of new tracking techniques will allow us to study this question more thoroughly in more bird species.
Keywords: juvenile, maturation, experience, foraging flight, altricial–precocial spectrum
1. Introduction
Flight is a unique adaptation which has allowed some taxonomic groups to undergo dramatic adaptive radiations. The three main groups using flight are insects, the most diverse and numerous class of animals (greater than 1 million species described; [1]), birds (approx. 11 000 species; [2]) and bats which comprise 25% of mammal species (approx. 1300 species; [3]). Birds, particularly, are a group whose evolution has been largely influenced by flight. Their anatomy, physiology and behaviour are adapted to this complex mode of locomotion [4]. Flight is a very efficient way to transport a unit of mass over a unit of distance [5]. Using flight, birds are able to forage on extensive areas, they can migrate over long distances and they were able to colonize all terrestrial habitats on Earth including high elevations, polar regions and distant islands. Birds are able to use various flight modes, from passive flight (i.e. without wingstrokes) to active flight (i.e. flapping). Passive flight includes gliding, where the bird trades height to maintain forward speed, and soaring, where the bird uses wind and aerological gradients to maintain or gain height (slope soaring [6]; thermal soaring [7]; dynamic soaring [8]). Active flight includes level flapping flight, ascending flapping flight such as performed after take-off [9], and hovering [10]. Active flight requires high power output, i.e. high energy expenditure per unit of time [5]. Some flight modes are called intermittent flight [11,12] and imply an alternation of flapping and passive flight, with extended (flap-gliding flight) or folded wings (flap-bounding flight).
Flight behaviours are extremely diversified in birds, within and among species, and it is legitimate to wonder how these complex behaviours develop within an individual bird. A spontaneous question would be: are flight behaviours innate in birds, or is learning necessary? The role of nature versus nurture has been a classic debate when investigating the development of behaviour [13]. Schneirla [14] stated that the distinction between the ‘unlearned’ and the ‘learned’ was unrealistic, and instead proposed that all behaviours are developed under the combined influence of two concepts: maturation and experience. Here, Schneirla defines ‘maturation’ as ‘the contributions of tissue growth and differentiation’ and ‘experience’ as ‘the contributions of stimulation from the developmental medium’. These two processes are not additive but inseparably coalescent, and considering one or the other in isolation would be equivalent to studying the effect of just the length or the width of a rectangle on its area [15]. Moreover, when studying locomotor behaviours such as flight, it is useful to further subdivide experience into learning and practice. Learning is defined as an irreversible change in response to particular stimuli, excluding ontogenetic processes such as maturation, injury and ageing [16]. Besides, practice is defined as an aspect of ontogeny in which repeated movements accelerate the development of behaviour [16]. Behaviours developing through practice improve as they are performed and do not simply improve with time. Unlike learning, practice depends upon experience but not upon specific consequences of the behaviour.
Based on Schneirla's point of view, modern theories on the development of behaviour, like probabilistic epigenesis [17], emphasize the reciprocity of influences within and between levels of an organism's developmental manifold (genetic activity, neural activity, behaviour and the physical, social and cultural influences of the external environment). Thus, all behaviour is influenced to some extent by the animal's genetic make-up and, at the same time, by the environmental conditions that exist during development. The extent to which the different influences determine the outcome varies greatly from species to species, and from activity to activity within a species [16]. Hence, when studying the development of flight behaviours in birds, a—reformulated—central question is to determine whether maturation or experience is more important.
Flight behaviours develop in juvenile birds, and this life stage is crucial in the population dynamics of most birds: many species suffer high juvenile mortality through predation and starvation [18–21]. Therefore, selection on juvenile anatomy and behaviour may be very intense and have important consequences for the adult phenotype [22–25]. Consequently, determining the importance of maturational and environmental factors in the development of various flight behaviours may give new insights into selective pressures acting on juvenile birds. Birds show a great diversity of developmental strategies from altricial birds, which hatch naked and stay in the nest, to precocial birds, which hatch covered with down and rapidly leave the nest [25]. Given the high diversity of life-history traits combinations in birds, understanding the development of flight behaviours in various species may enable a consistent picture to be drawn across a number of bird groups.
Here, we aim to review how different flight behaviours develop through the ontogenetic stages of birds, from embryo to adult. Studies on the growth of flight organs per se (e.g. limb skeleton, muscles, feathers), without any explicit link with behaviour, are out of the scope of this literature review. Moreover, questions regarding orientation and navigation, especially in the context of migratory behaviours, constitute an extensive field of research and are also out of the scope of this review.
When studying a specific behaviour, it may be useful to refer to Tinbergen's four questions [26], allowing us to delineate logically complementary ways of understanding this behaviour: causation, survival value, ontogeny and evolution. Our review will mainly focus on the ontogeny of flight behaviours, but relevant aspects of causation (e.g. internal determinism) and consequences of flight behaviours on survival (e.g. energy expenditure, escape from predators) will also be discussed.
2. Terminology
When studying the development of birds, it is common to come across some terms such as ‘chicks’, ‘young’, ‘fledglings’, sometimes used interchangeably without being defined, which does not facilitate the understanding of the developmental processes. The developmental stages used in this review will be defined below.
First, the altricial–precocial spectrum is an important concept in bird development (see [25]). Altricial species typically hatch with their eyes closed, absent or sparse down, almost immobile, very dependent on their parents and do not leave the nest until they approach adult size and are able to fly. Precocial species typically hatch with their eyes open, already covered with down, are able to walk and/or swim and rapidly leave the nest, long before being adult-sized and capable of flight. The altricial–precocial spectrum is a continuum, many categories can be defined within this spectrum (see electronic supplementary material, SA and [25]), and each species present a different mix of developmental features (e.g. [27]).
In order to study behavioural development across this spectrum, it is important to use words applicable to all flight-capable bird species. When studying flight behaviour, the pre-fledging and post-fledging periods might represent a convenient abstraction. The term ‘fledging’ is often used to indicate the moment when a bird becomes capable of flight [28]. However, the ‘post-fledging period’, or ‘post-fledging dependence period’ [29], is commonly defined as the time between fledging and family break-up [18,19]. This definition would thus not be applicable to species without parental care after fledging, which are found among precocial (e.g. megapodes; [30]) but also altricial birds (e.g. swifts; [31]).
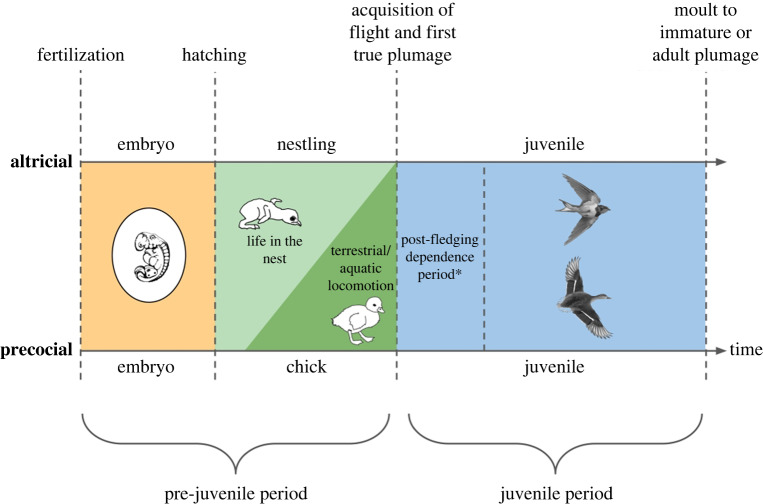
In this review, we choose to base the terminology on the word ‘juvenile’, defined by Howell [32] as a bird in juvenile plumage. This is the first plumage of ‘true’ or vaned (non-downy) feathers, often the plumage in which a bird takes flight. This period thus extends from the acquisition of the first true plumage to the subsequent moult. It lasts several weeks for some passerines, to several months for large birds like raptors. This definition is objectively based on anatomy and applicable to all species, altricial and precocial. Consequently, we introduce the term ‘pre-juvenile period’ to describe the period extending from the embryo to the acquisition of the first true plumage. The pre-juvenile period thus includes life in the nest (‘nestlings’ of altricial species) or an early life in the nest followed by a period of terrestrial and/or aquatic locomotion before fledging (‘chicks’ of precocial species). The correspondence and relationships between these terms are detailed in figure 1. With these two distinct periods: pre-juvenile period and juvenile period, the ontogeny of flight-capable birds can be conveniently classified in order to understand better the timing of different influences on the development of flight behaviour.
Figure 1.
Summary of the altricial–precocial developmental spectrum in birds, and of the terminology used in this review. Orange, life in the egg; light green, life in the nest, outside the egg; dark green, terrestrial/aquatic life outside the nest; blue, aerial life. *The post-fledging dependence period does not exist in all species. (Online version in colour.)
3. Pre-juvenile development of flight behaviours
The pre-juvenile period, extending from the embryo to the acquisition of the first true plumage allowing flight, is likely to be a sensitive period where maturational and experiential effects play a crucial role in shaping flight behaviours.
(a). Wing flapping before flight
Wing flapping of young birds before they can fly has been observed in many bird species [31,33–36], and consists of repeated wing movements mimicking active flight, often performed in the nest. The role of this early behaviour has been tested in several experiments.
An early experiment was carried out by Spalding [37], who reared a group of barn swallows (Hirundo rustica) in a space so small that they could not fully extend their wings. The birds were released at the age when swallows normally fly, and they flew at the first opportunity. Similarly, Grohmann [38] reared pigeons (Columba livia) in narrow tubes, and they flew normally when released. Krischke [39] also observed that pigeons raised in a narrow box developed the basic motor patterns of flight, but juvenile pigeons whose movements had been hindered showed differences in manoeuvrability compared to unhindered ones. In precocial species, few studies exist on these questions. Provine [40] found that domestic chicks (Gallus gallus domesticus) whose wings were immobilized by bandages from hatching until 13 days old could achieve rates of wing flapping similar to adults and normal flight distances. In a more dramatic demonstration of maturation of wing flapping without practice, Provine [41] also found that domestic chicks whose wings were amputated at hatching flapped wing prostheses at normal rates. Neither postnatal practice nor sensory and trophic feedback from the wings could contribute to the development of flapping behaviour in these wingless chicks. However, these prostheses were ‘soda straws’, likely to offer less air resistance and inertia than normal wings. Moreover, an uncontrolled factor in all these experiments is the spontaneous motility of embryo's wings in the egg, which was observed, for example, in the domestic chick [42], and could potentially play a role in muscle and joint development [43].
These different pieces of evidence tend to indicate that the development of flight behaviours does not heavily depend on post-hatching wing flapping before flight. This early flapping behaviour could simply be due to the premature expression of an instinctive urge. Against this hypothesis, in common swift (Apus apus) nestlings, Lack [31] showed that wing movements were not just incipient flying movements. Indeed, nestlings perform ‘press-ups’, the wings partly extended and pressed down on the floor while the body is raised above the ground. According to Lack, these specialized movements could have been specially evolved. Their function could be to exercise muscles [31], but more recently, Wright et al. [44] hypothesized that these ‘press-ups’ could allow swifts to assess their body mass relative to their wing area in order to adjust food intake and thus wing loading at fledging. Consequently, in altricial species, even if wing flapping before flight may have originated through premature development of flight behaviours, the persistence and specialization of this behaviour in some species suggests that it has some adaptive value for nestlings. It should be noted that common swift fledglings fly continuously immediately after leaving the nest (i.e. without resting on a nearby branch), a particular case that might have put higher pressure on functional flapping practice in the nest.
A few studies focusing on developing pre-juveniles [45,46] or pre-migratory adults [46,47] analysed the relationship between wing exercise and fine metabolic and anatomic changes, shedding light on overlooked processes, like an increase in muscular enzyme activity during development [45], or an increase in fatty acid binding protein concentration before long distance migratory flights [46].
(b). Environmental influences
Assessing the effects of pre-juvenile environment on flight behaviours is only possible in experimental studies analysing the complete development of individuals from embryonic development to after fledging. Several studies have attempted to modify pre-hatching or post-hatching environmental factors and to measure various parameters relevant to flight behaviour.
(i). Pre-hatching
Few studies have investigated the effect of pre-hatching environment on the development of flight in birds, but significant findings were made. Coslovsky & Richner [48] tested for maternal effects on great tits (Parus major) by exposing females before and during ovulation to stuffed models and sounds of a predator. Offspring of exposed mothers were then raised by foster parents subjected to no treatment in order to separate pre- and post-hatching environmental effects. They found that nestlings of predator-stressed mothers were smaller than those of control mothers but showed higher growth rates of the wings. First-year recruits from the predator treatment thus had longer wings at maturity. The authors hypothesized that this effect may be a consequence of higher circulating stress hormone levels in mothers, which would result in eggs enriched in these hormones. Indeed, Love & Williams [49] found that female European starlings (Sturnus vulgaris) of one group hatching from corticosterone-injected eggs had longer wings than controls. In the case of great tits, the accelerated wing growth could just be compensatory growth occurring at the cost of body mass, or it could be adaptive since longer wings and lower weight may modify flight behaviour and facilitate predator evasion and dispersal. However, no behavioural experiment was carried out to demonstrate that flight behaviour could be modified by these anatomic differences. In another experiment manipulating pre-hatching environment, Chin et al. [50] directly measured behavioural traits by measuring flight performance of juvenile European starlings. They found that juveniles exposed to increased corticosterone in their egg performed better during flight performance trials (measured through mechanical energy output during the birds' first flights). They also exhibited lower wing loading and heavier and more mature flight muscles. These two experiments suggest that pre-hatching stress might trigger some adapted responses in offspring, modifying flight behaviour to be able to cope with a stressful environment.
(ii). Post-hatching
More studies investigated the effect of post-hatching environment in birds, and several have found negative consequences of developmental stress for flight performance. These are examples of a ‘silver spoon’ effect [51] where individuals born in favourable conditions develop improved phenotypes later in life, and those born in adverse conditions develop disadvantaged phenotypes. Here, developmental stress is defined with a broad sense, including nutritional stress due to low feeding rates or low-quality food. For example, O'Hagan et al. [52] cross-fostered starlings to nests where they were either slightly larger (advantaged treatment) or slightly smaller (disadvantaged treatment) than the other nestlings. The treatment had no effect on growth, but it affected performance in escape flights a year later. Disadvantaged birds faced a steeper trade-off between take-off speed and take-off angle: they had to sacrifice more take-off speed for every degree of take-off angle gained.
In another experimental study on European starlings, Verspoor et al. [53] manipulated maternal care by clipping wing and tail feathers in some mothers, which consequently decreased their provisioning rate. Although the manipulation decreased body mass and structural size (tarsus, wing length) in daughters, only the flight performance (speed and mechanical energy output) of sons was negatively affected. Males are the larger sex in starlings and size is important in male competition, which suggests that there could be a trade-off between flight performance and body size, favouring the latter in males growing in a poor environment.
Several other studies have shown an effect of post-hatching nutritional conditions on flight behaviour. Criscuolo et al. [54] have shown that female zebra finch (Taeniopygia guttata) nestlings experiencing a switch from low- to high-quality food recovered in body size, but showed a steeper decline in escape flight performance over the breeding period. This suggests that diet-induced rapid recovery of body size can carry locomotory costs in later life. This may relate to the need for breeding females to use proteins from their flight muscles to produce eggs, with a consequent impact on flying ability [55,56]. Similarly, Miller [57] showed an effect of rearing conditions on the take-off speed of mourning doves (Zenaida macroura). An increased brood size during the nestling stage had delayed effects on flight performance: juvenile doves were able to minimize the effects of nutritional stress on take-off speed at early ages, when escape ability from predators is especially important. However, at the age of 90 days, birds from enlarged broods were slower at take-off. Thus, this early manipulation of nutrition had long-term effects on the flight performance of these birds.
(iii). Pre-juvenile environment: synthesis
Overall, the different experimental manipulations of pre-juvenile environment seem to draw a consistent picture. The two studies modifying the pre-hatching environment reported positive effects of environmental stress on flight performance, while all studies modifying the post-hatching environment reported negative effects. Obviously, the nature of the stressors was different (predator and corticosterone exposure for pre-hatching, nutritional stress for post-hatching), but these findings still suggest a consistent pattern. The pre-hatching experiments would be examples of adaptive developmental plasticity, where an early stressor triggers an evolved anticipatory response to adverse situations later in life, and thus improves some traits [58]. However, post-hatching studies are more consistent with the ‘silver spoon’ effect where developmental stress imposes constraints on adult phenotypic quality [51]. In several pre- and post-hatching studies, the existence of developmental trade-offs is highlighted. The development of flight performance (through the maturation of muscles or wing length) may occur at the cost of body mass [48], or reciprocally [53,54], and early flight performance may be privileged over later flight performance [57]. Such trade-offs are more apparent in stressful environments and show which selective pressures act on the development of flight in different bird species, revealing sex-specific effects in some species [53].
In addition to wing flapping before flight and environmental factors, the timing of flight development is also influenced by species-specific growth dynamics strategies, in both precocial and altricial birds (see electronic supplementary material, SB and SC).
4. Juvenile development of flight behaviours
Once birds acquire their first true plumage and become capable of flight, they have to go through further developmental steps in order to develop a mature flight apparatus (see electronic supplementary material, SD) and to master all the flight behaviours of their species.
(a). Development of flight modes
Flapping flight seems to be the first flight mode to develop in many species, and flapping is often practised before flying, as previously discussed. When other flight modes are required, their acquisition is, most of the time, gradual. In many raptors, a transition from flapping to gliding was reported in juveniles. For example, in juvenile red kites (Milvus milvus), Bustamante [29] observed an increase in time spent flying and a gradual transition from flapping to gliding and soaring. Similar transitions are widespread in many juvenile diurnal raptors (e.g. [34,59,60]). In griffon vultures (Gyps fulvus), juveniles are as efficient as adults in selecting favourable thermals for soaring, presumably because they forage in mixed-age groups [61]. However, adults have a better capacity to sharp-turn within thermals, and thus juveniles exhibit a higher proportion of flapping flight, and higher energy expenditure. A gradual change in flight mode was also observed in other bird groups. In brown boobies (Sula leucogaster), juveniles gradually increase the proportion of time spent gliding during flight, as well as flight speed, trip duration and distance [62,63]. Overall, these observations indicate that gliding and soaring efficiently may require additional skills compared to flapping and that it takes time for juvenile birds to develop these techniques. While flapping may develop mainly through maturational processes and practice, the importance of learning may be greater in flight modes which require taking advantage of environmental phenomena (thermal uplift, wind).
Contrarily to this general tendency, complex flight modes in some pelagic seabirds seem to develop more rapidly, and are sometimes fully developed at the first flight. Recently, Corbeau et al. [64] reported that juvenile great frigatebirds (Fregata minor) gradually increase the proportion of time spent at sea, flight speed and travelled distance during a four to eight month-long dependence period. However, juvenile frigatebirds rapidly equal or even surpass gliding and soaring performance of adults (within a few days or immediately at the first flight), and do not expend more energy than adults using these flight modes. Similarly, juvenile wandering albatrosses (Diomeda exulans) were reported to increase travelled distance during development, but they are almost as effective as adults in their use of tail and side winds immediately after fledging [65]. These examples show that, in the case of some seabird species having to travel long distances rapidly after fledging and with fewer opportunities to train over land, even more complex flight modes may be well developed immediately after fledging, and more innate components are exhibited.
(b). Development of migratory flight
Migration is a large-scale movement which often requires specific flight skills, and mastering flight techniques to save energy during these long flights is important for the survival of juvenile birds. In several species, juveniles were reported to migrate less efficiently than adults. In white storks (Ciconia ciconia), Rotics et al. [66] reported that juveniles used less soaring flight and more flapping flight than adults during migration, which resulted in greater energy expenditures, and this may be one of the major factors explaining the lower survival rate of juvenile storks during migration. However, juveniles showed an improvement in flight efficiency during migration (decreasing flapping/gliding time ratio and consequently decreasing flight energy expenditure), suggesting that they learnt to use thermals more efficiently. Similarly, juvenile golden eagles (Aquila chrysaetos) were reported to migrate less efficiently than adults in autumn, with juveniles using more slope soaring and less thermal soaring than adults [6]. Age differences were also observed in passerines, as in savannah sparrows (Passerculus sandwichensis) where it was found that juveniles departed under wind conditions that were less supportive, resulting in a longer time to complete the same flight routes as adults [67]. In fact, in long-lived species where the migratory flight involves highly complex techniques, flight behaviour may continue to develop well beyond the juvenile period, as was shown in black kites (Milvus migrans) where the ability to cope with wind drift and to exploit tail winds during migration improves until about 7 years old [68].
(c). Development of foraging flight
In many bird species, flight is an important component of foraging behaviours. Predators, especially, may use complex flight techniques whose development is more likely to involve learning.
(i). Development of foraging flight with no apparent learning
In some species, juveniles do not appear to learn foraging flight techniques. For example, in red kites, Bustamante [29] observed that juveniles do not follow their parents to hunting areas and do not appear to practise hunting techniques during the post-fledging dependence period. The same phenomenon was observed in black kites [59], and Bustamante [29] suggested that the absence of a gradual development of hunting techniques can be related to the generalist feeding habits of the genus Milvus: habitual preys (carrion, insects and young animals) do not require very specialized capture techniques or manipulation, and a progressive development before independence may not be necessary. Similarly, Bustamante & Negro [69] observed that juvenile lesser kestrels (Falco naumanni) do not appear to learn or practise hunting skills during the post-fledging dependence period, which could be related to their diet almost exclusively composed of abundant and easily caught insects. However, in the previous examples, it remains unclear if juveniles' foraging was as efficient as adults’ foraging immediately after independence or if they needed some later learning, for example, by trial and error.
(ii). Development of foraging flight while learning alone
In several passerine species, the development of foraging flight was observed, and trial and error learning seems to be the prevalent process. For example, Baker & Ferree [70] studied the foraging development of juvenile black phoebes (Sayornis nigricans), which pursue and catch insects in flight. The proportion of successful foraging attempts increased gradually to reach the same level as adults at the age of seven weeks. This increase is potentially due to trial and error learning, but the maturation of cognitive or visual system cannot be ruled out [71,72]. In northern wheatears (Oenanthe oenanthe), Moreno [73] also found a gradual development of foraging efficiency. However, foraging techniques that require flight (aerial hawking, perch-to-ground sallying) took longer to develop than ground-based foraging (ground-gleaning). Similar results were reported in spotted flycatchers (Muscicapa striata; [74]).
In many raptors, juveniles were observed to perform some predatory attempts on small animals during the post-fledging dependence period, while parents were temporarily away [34,35,60,75]. Such observations cannot rule out the existence of social learning, but at least indicate that some juvenile raptors are able to practise hunting techniques alone.
In all the previous examples where the flight skills of juveniles progressively increase, it is not possible to determine the importance of maturational processes relative to learning solely with observations. Experimental studies are needed to determine the relative importance of those different processes.
(iii). Development of foraging flight through social learning
The development of various foraging flight behaviours may involve interactions with conspecifics. Social learning typically involves a ‘demonstrator’, which performs a behaviour to be learnt by an ‘observer’ [76]. In this context, the demonstrator does not necessarily perform the behaviour for the observer's sake. Different forms of social learning of flight can be hypothesized in birds, mainly imitation and teaching.
Imitation occurs when an individual copies the behaviour of another individual in order to obtain the same consequences [77]. This type of social learning could possibly exist in several raptor species which require complex flight behaviours for hunting. Varland et al. [78] reported social hunting in juvenile American kestrels (Falco sparverius), which was defined as hunting activity by an individual occurring less than 3 m from one or more individuals that also hunt. The authors described this social foraging as imitative rather than cooperative because group members did not directly communicate and did not coordinate their movements. American kestrels foraged in groups composed of juveniles and adults often belonging to the same family, but sometimes unrelated. During this period, juveniles gradually increased their capture rate (captures per hour) and may have learnt hunting techniques by imitation. Similar interactions were observed in common kestrels (Falco tinnunculus; [79]), in Montagu's harriers (Circus pygargus; [80]) and in western marsh harriers (Circus aeruginosus; [81]). Moreover, it was shown in western marsh harriers that juveniles hunting in groups where adults were present performed more predatory attempts and had a higher hunting efficiency (more hunting sessions yielded at least one prey item) than juveniles hunting in juvenile-only groups [81].
Teaching is a specific type of social learning where a knowledgeable individual engages in some costly behaviour without immediate self-benefit, but which helps a naive individual to acquire some skill more rapidly [82]. In the context of foraging flight learning in birds, behaviours evoking teaching have been observed almost exclusively in raptors. The most widespread teaching behaviour in raptors is observed when adults release a live prey, often injured, near juveniles in order to allow them to practise hunting. For example, in Montagu's harriers, Kitowski [83] reported that adults dropped grasshoppers near juveniles and repeated the process until juveniles performed predatory flights and caught their prey on the ground. Similar teaching behaviours were reported in other raptor species (see [84]). A remarkable example was observed in peregrine falcons (Falco peregrinus), where adults can release a live bird in front of juveniles in order to encourage them to pursue it (Sherrod, 1983; cited in [84]). If the prey escapes, an adult may recapture it, carry it back and release it again.
A detailed example of teaching was described in ospreys (Pandion haliaetus) by Meinertzhagen [85], where adults dropped fish in the water in front of juveniles in order to motivate predatory flights, and these juveniles became able to dive and catch live fish within a few days. However, some hand-raised juvenile ospreys successfully caught fish within three weeks after release in the wild, in the absence of parental teaching [86], confirming that teaching would only accelerate the learning process in this case.
(iv). Development of foraging flight: synthesis
Observations of the development of foraging flight behaviour are scarce in smaller and less conspicuous birds, but a general pattern seems to emerge in raptors. Learning of foraging flight techniques (through trial and error, imitation or teaching) may be most important in agile and highly manoeuvrable raptors which capture elusive prey such as small mammals, reptiles, birds and fish [83]. By contrast, as noted by Kitowski [83], learning of flight foraging techniques seems less important in raptors whose habitual food is either widely available and easy to obtain (e.g. carrion) or highly abundant (e.g. lagomorphs or insects). Food generalist scavengers do not need highly specialized hunting techniques, because their foraging techniques are closer to gleaning. For raptors exhibiting teaching, the importance of this process is questionable, as predatory techniques were shown to develop even without teaching in some species. The fact that this kind of costly behaviour evolved would still indicate that it has some adaptive value, for example, by allowing juveniles to learn faster, thus increasing their survival chances.
5. Conclusion and perspectives
The development of flight behaviours in birds involves multiple processes at all ontogenetic stages. The pre-juvenile environment can influence the development of flight behaviours, either via a ‘silver spoon’ effect or via adaptive developmental plasticity. Wing flapping before flight could enhance initial performances and have additional functions in particular bird species. However, experimental studies modifying pre-juvenile environment vary in their measures of flight performance. Standardized comparisons are difficult and it is not yet possible to have a holistic vision of the effect of pre-juvenile conditions on the development of flight behaviours. Studies investigating possible interactions between the environment encountered during the pre- and post-hatching periods would also be needed.
In juveniles, flapping flight seems to be acquired mainly through practice and maturation while more complex flight modes like efficient gliding and soaring may require some learning. In species exhibiting highly complex foraging flight techniques, trial and error learning or social learning (through imitation and/or teaching) may be more important, especially when predation is involved.
The literature reveals that the juvenile flight of large conspicuous birds like raptors and seabirds was much more studied, and that considerably less data are available for smaller birds, probably due to difficulties to describe their flight behaviours in the wild. Similarly, precocial birds represent a small part of this literature compared to altricial birds, and the development of their flight behaviours is consequently less understood. Few experimental studies modifying the environmental conditions in which juvenile birds develop exist, and conclusions are essentially based on observations and correlations. In many observational studies, it is difficult to differentiate the effects of maturational processes from the effects of different types of learning. Moreover, improvements of flight skills beyond the juvenile period were documented [68], but few studies were able to monitor the flight behaviour of several individuals of known age over successive years and some long-term developmental patterns may have been overlooked. Besides, it is worth noting that some types of flight behaviours were, to our knowledge, not studied in the context of behavioural ontogeny, for example, flight courtship display. Early practice of courtship dances in immatures has been reported in several birds [87–90], and learning of a courtship display involving coordinated vocalisations and dance has been studied in Java sparrows (Lonchura oryzivor; [91]). Thus, learning of flight courtship display is probably present in some species, and would deserve further investigation. Another aspect rarely broached in the flight development literature is the inter-individual coordination necessary for some species during particular flocking flights [92,93], which could be hypothesized to require some learning.
Finally, it is worthwhile to note that, in some species, even adult birds may provide useful information about the development of flight, especially in species exhibiting simultaneous flight feather moult. This moult strategy, seen in waterbirds such as ducks [94], geese [95,96] or grebes [97–99], leaves the birds flightless for up to several months. Important anatomical and behavioural changes are observed during this period. For example, breast muscle atrophy has been observed during simultaneous moult, along with leg muscle hypertrophy in some species [94–98]. All these changes are reversed after moult, showing a temporary shift in locomotor strategy. Furthermore, flapping exercises were observed during the muscle rebuilding period [99]. Thus, adult birds have to regain flight through this moult process, making an interesting comparison to early-life onset of flight.
Overall, even if insightful tendencies seem to emerge, the available literature on the development of flight behaviours in birds is still too scarce to establish a comprehensive framework which would summarize the influence of different life-history traits on this locomotor ontogeny. In this perspective, comparative studies investigating the ontogeny of flight behaviours using standardized methods for different species showing contrasted life-history traits will be useful. Three-dimensional optical tracking tools have recently been developed in order to describe more accurately the flight behaviours of birds in the wild [100–102], and their use along an ontogenetic dimension might be a key step towards a better understanding of birds' flight development.
Supplementary Material
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Stork NE. 2018. How many species of insects and other terrestrial arthropods are there on earth? Annu. Rev. Entomol. 63, 31–45. ( 10.1146/annurev-ento-020117-043348) [DOI] [PubMed] [Google Scholar]
- 2.Gill F, Donsker D. 2019. IOC World Bird List (v9.2). (doi:10.14344/IOC.ML.9.2). [Google Scholar]
- 3.Burgin CJ, Colella JP, Kahn PL, Upham NS. 2018. How many species of mammals are there? J. Mammal. 99, 1–14. ( 10.1093/jmammal/gyx147) [DOI] [Google Scholar]
- 4.Podulka S, Rohrbaugh RW Jr, Bonney R (eds). 2004. Handbook of bird biology, 2nd edn Ithaca, NY: Cornell Lab of Ornithology in association with Princeton University Press. [Google Scholar]
- 5.Norberg UM. 1990. Vertebrate flight: mechanics, physiology, morphology, ecology and evolution. New York, NY: Springer. [Google Scholar]
- 6.Duerr AE, Miller TA, Lanzone M, Brandes D, Cooper J, O'Malley K, Maisonneuve C, Tremblay JA, Katzner T. 2015. Flight response of slope-soaring birds to seasonal variation in thermal generation. Funct. Ecol. 29, 779–790. ( 10.1111/1365-2435.12381) [DOI] [Google Scholar]
- 7.Cone CD., Jr 1962. Thermal soaring of birds. Am. Sci. 50, 180–209. [Google Scholar]
- 8.Sachs G, Traugott J, Nesterova AP, Dell'Omo G, Kümmeth F, Heidrich W, Vyssotski AL, Bonadonna F. 2012. Flying at no mechanical energy cost: disclosing the secret of wandering albatrosses. PLoS ONE 7, e41449 ( 10.1371/journal.pone.0041449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson SF. 1983. The flight mechanism of the pigeon Columbia livia during take-off. J. Zool. 200, 435–443. ( 10.1111/j.1469-7998.1983.tb02322.x) [DOI] [Google Scholar]
- 10.Warrick DR, Tobalske BW, Powers DR. 2005. Aerodynamics of the hovering hummingbird. Nature 435, 1094–1097. ( 10.1038/nature03647) [DOI] [PubMed] [Google Scholar]
- 11.Rayner JMV. 1985. Bounding and undulating flight in birds. J. Theor. Biol. 117, 47–77. ( 10.1016/S0022-5193(85)80164-8) [DOI] [Google Scholar]
- 12.Usherwood JR. 2016. Physiological, aerodynamic and geometric constraints of flapping account for bird gaits, and bounding and flap-gliding flight strategies. J. Theor. Biol. 408, 42–52. ( 10.1016/j.jtbi.2016.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barlow GW. 1991. Nature–nurture and the debates surrounding ethology and sociobiology. Am. Zool. 31, 286–296. ( 10.1093/icb/31.2.286) [DOI] [Google Scholar]
- 14.Schneirla TC. 1965. Aspects of stimulation and organization in approach/withdrawal processes underlying vertebrate behavioral development. In Advances in the study of behavior (eds Lehrman DS, Hinde RA, Shaw E), pp. 1–74. New York, NY: Academic Press. [Google Scholar]
- 15.Richard G. 1974. Les comportements instinctifs. Paris, France: Presses universitaires de France. [Google Scholar]
- 16.McFarland D. 2006. A dictionary of animal behaviour. Oxford, NY: Oxford University Press. [Google Scholar]
- 17.Gottlieb G. 2007. Probabilistic epigenesis. Dev. Sci. 10, 1–11. ( 10.1111/j.1467-7687.2007.00556.x) [DOI] [PubMed] [Google Scholar]
- 18.Naef-Daenzer B, Grüebler MU. 2016. Post-fledging survival of altricial birds: ecological determinants and adaptation. J. Field Ornithol. 87, 227–250. ( 10.1111/jofo.12157) [DOI] [Google Scholar]
- 19.Remeš V, Matysioková B. 2016. Survival to independence in relation to pre-fledging development and latitude in songbirds across the globe. J. Avian Biol. 47, 610–618. ( 10.1111/jav.00841) [DOI] [Google Scholar]
- 20.Wunderle JM. 1991. Age-specific foraging proficiency in birds. Curr. Ornithol. 8, 273–324. [Google Scholar]
- 21.Heers AM. 2016. New perspectives on the ontogeny and evolution of avian locomotion. Integr. Comp. Biol. 56, 428–441. ( 10.1093/icb/icw065) [DOI] [PubMed] [Google Scholar]
- 22.Carrier DR. 1996. Ontogenetic limits on locomotor performance. Physiol. Zool. 69, 467–488. ( 10.1086/physzool.69.3.30164211) [DOI] [Google Scholar]
- 23.Herrel A, Gibb AC. 2006. Ontogeny of performance in vertebrates. Physiol. Biochem. Zool. 79, 1–6. ( 10.1086/498196) [DOI] [PubMed] [Google Scholar]
- 24.Martin TE. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127. ( 10.2307/2937160) [DOI] [Google Scholar]
- 25.Starck JM, Ricklefs RE. 1998. Avian growth and development. Oxford, NY: Oxford University Press. [Google Scholar]
- 26.Tinbergen N. 1963. On aims and methods of ethology. Z. Tierpsychol. 20, 410–433. ( 10.1111/j.1439-0310.1963.tb01161.x) [DOI] [Google Scholar]
- 27.Dial TR, Carrier DR. 2012. Precocial hindlimbs and altricial forelimbs: partitioning ontogenetic strategies in mallards (Anas platyrhynchos). J. Exp. Biol. 215, 3703–3710. ( 10.1242/jeb.057380) [DOI] [PubMed] [Google Scholar]
- 28.Moreau RE. 1940. The recording of incubation and fledging period. Br. Birds 39, 66–70. [Google Scholar]
- 29.Bustamante J. 1993. Post-fledging dependence period and development of flight and hunting behaviour in the red kite Milvus milvus. Bird Study 40, 181–188. ( 10.1080/00063659309477181) [DOI] [Google Scholar]
- 30.Wesolowski T. 1994. On the origin of parental care and the early evolution of male and female parental roles in birds. Am. Nat. 143, 39–58. ( 10.1086/285595) [DOI] [Google Scholar]
- 31.Lack D. 1956. Swifts in a tower. London, UK: Chapman and Hall. [Google Scholar]
- 32.Howell SNG. 2010. Molt in north American birds. Boston, MA: Houghton Mifflin Harcourt. [Google Scholar]
- 33.Aumann T. 1988. Breeding behaviour of the brown goshawk Accipiter fasciatus. Aust. Bird Watcher 12, 258–267. [Google Scholar]
- 34.Debus SJS, Ley AJ, Tremont S, Tremont R. 1991. Breeding behaviour and diet of the Australian hobby Falco longipennis in Northern New South Wales. Aust. Bird Watcher 14, 123–137. [Google Scholar]
- 35.Delannoy CA, Cruz A. 1988. Breeding biology of the Puerto Rican sharp-shinned hawk (Accipiter striatus venator). Auk 105, 649–662. ( 10.1093/auk/105.4.649) [DOI] [Google Scholar]
- 36.Heers AM, Tobalske BW, Dial KP. 2011. Ontogeny of lift and drag production in ground birds. J. Exp. Biol. 214, 717–725. ( 10.1242/jeb.051177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spalding D. 1875. Instinct and acquisition. Nature 12, 507–508. ( 10.1038/012507a0) [DOI] [Google Scholar]
- 38.Grohmann J. 1939. Modifikation oder Funktionsreifung? Ein Beitrag zur Klärung der wechselseitigen Beziehungen zwischen Instinkthandlung und Erfahrung. Z. Tierpsychol. 2, 132–144. ( 10.1111/j.1439-0310.1939.tb01571.x) [DOI] [Google Scholar]
- 39.Krischke N. 1983. Beiträge Zur Ontogenese Der Flug- Und Manövrierfähigkeit Der Haustaube (Columba livia var. domestica). Behaviour 84, 265–286. ( 10.1163/156853983X00525) [DOI] [Google Scholar]
- 40.Provine RR. 1981. Development of wing-flapping and flight in normal and flap-deprived domestic chicks. Dev. Psychobiol. 14, 279–291. ( 10.1002/dev.420140317) [DOI] [PubMed] [Google Scholar]
- 41.Provine RR. 1979. ‘Wing-flapping’ develops in wingless chicks. Behav. Neural Biol. 27, 233–237. ( 10.1016/S0163-1047(79)91885-5) [DOI] [PubMed] [Google Scholar]
- 42.Hamburger V, Oppenheim R. 1967. Prehatching motility and hatching behavior in the chick. J. Exp. Zool. 166, 171–203. ( 10.1002/jez.1401660203) [DOI] [PubMed] [Google Scholar]
- 43.Drachman DB, Sokoloff L. 1966. The role of movement in embryonic joint development. Dev. Biol. 14, 401–420. ( 10.1016/0012-1606(66)90022-4) [DOI] [Google Scholar]
- 44.Wright J, Markman S, Denney SM. 2006. Facultative adjustment of pre-fledging mass loss by nestling swifts preparing for flight. Proc. R. Soc. B 273, 1895–1900. ( 10.1098/rspb.2006.3533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop CM, Butler PJ, Egginton S, el Haj AJ, Gabrielsen GW. 1995. Development of metabolic enzyme activity in locomotor and cardiac muscles of the migratory barnacle goose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 269, R64–R72. ( 10.1152/ajpregu.1995.269.1.R64) [DOI] [PubMed] [Google Scholar]
- 46.Pelsers MMAL, Butler PJ, Bishop CM, Glatz JFC. 1999. Fatty acid binding protein in heart and skeletal muscles of the migratory barnacle goose throughout development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 276, R637–R643. ( 10.1152/ajpregu.1999.276.3.R637) [DOI] [PubMed] [Google Scholar]
- 47.Portugal SJ, Green JA, White CR, Guillemette M, Butler PJ. 2012. Wild geese do not increase flight behaviour prior to migration. Biol. Lett. 8, 469–472. ( 10.1098/rsbl.2011.0975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coslovsky M, Richner H. 2011. Predation risk affects offspring growth via maternal effects. Funct. Ecol. 25, 878–888. ( 10.1111/j.1365-2435.2011.01834.x) [DOI] [Google Scholar]
- 49.Love OP, Williams TD. 2008. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 172, E135–E149. ( 10.1086/590959) [DOI] [PubMed] [Google Scholar]
- 50.Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, Burness G. 2009. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proc. R. Soc. B 276, 499–505. ( 10.1098/rspb.2008.1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Hagan D, Andrews CP, Bedford T, Bateson M, Nettle D. 2015. Early life disadvantage strengthens flight performance trade-offs in European starlings, Sturnus vulgaris. Anim. Behav. 102, 141–148. ( 10.1016/j.anbehav.2015.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verspoor JJ, Love OP, Rowland E, Chin EH, Williams TD. 2007. Sex-specific development of avian flight performance under experimentally altered rearing conditions. Behav. Ecol. 18, 967–973. ( 10.1093/beheco/arm089) [DOI] [Google Scholar]
- 54.Criscuolo F, Monaghan P, Proust A, Škorpilová J, Laurie J, Metcalfe NB. 2011. Costs of compensation: effect of early life conditions and reproduction on flight performance in zebra finches. Oecologia 167, 315–323. ( 10.1007/s00442-011-1986-0) [DOI] [PubMed] [Google Scholar]
- 55.Houston DC, Donnan D, Jones P, Hamilton I, Osborne D. 2008. Changes in the muscle condition of female zebra finches Poephila guttata during egg laying and the role of protein storage in bird skeletal muscle. Ibis 137, 322–328. ( 10.1111/j.1474-919X.1995.tb08028.x) [DOI] [Google Scholar]
- 56.Veasey JS, Houston DC, Metcalfe NB. 2000. Flight muscle atrophy and predation risk in breeding birds. Funct. Ecol. 14, 115–121. ( 10.1046/j.1365-2435.2000.00391.x) [DOI] [Google Scholar]
- 57.Miller DA. 2011. Immediate and delayed effects of poor developmental conditions on growth and flight ability of juvenile mourning doves Zenaida macroura. J. Avian Biol. 42, 151–158. ( 10.1111/j.1600-048X.2010.05124.x) [DOI] [Google Scholar]
- 58.Nettle D, Bateson M. 2015. Adaptive developmental plasticity: what is it, how can we recognize it and when can it evolve? Proc. R. Soc. B 282, 20151005 ( 10.1098/rspb.2015.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bustamante J, Hiraldo F. 1989. Post-fledging dependence period and maturation of flight skills in the black kite Milvus migrans. Bird Study 36, 199–204. ( 10.1080/00063658909477025) [DOI] [Google Scholar]
- 60.Walker DG. 1987. Observations on the post-fledging period of the golden eagle Aquila chrysaetos in England. Ibis 129, 92–96. ( 10.1111/j.1474-919X.1987.tb03163.x) [DOI] [Google Scholar]
- 61.Harel R, Horvitz N, Nathan R. 2016. Adult vultures outperform juveniles in challenging thermal soaring conditions. Sci. Rep. 6, 27865 ( 10.1038/srep27865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohno H, Yoda K. 2011. The development of activity ranges in juvenile brown boobies Sula leucogaster. Ibis 153, 611–615. ( 10.1111/j.1474-919X.2011.01128.x) [DOI] [Google Scholar]
- 63.Yoda K, Kohno H, Naito Y. 2004. Development of flight performance in the brown booby. Proc. R. Soc. Lond. B 271, 20030157 ( 10.1098/rsbl.2003.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corbeau A, Prudor A, Kato A, Weimerskirch H. 2019. Development of flight and foraging behaviour in a juvenile seabird with extreme soaring capacities. J. Anim. Ecol. 1365-2656.13121 ( 10.1111/1365-2656.13121) [DOI] [PubMed] [Google Scholar]
- 65.Riotte-Lambert L, Weimerskirch H. 2013. Do naive juvenile seabirds forage differently from adults? Proc. R. Soc. B 280, 20131434 ( 10.1098/rspb.2013.1434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rotics S, et al. 2016. The challenges of the first migration: movement and behaviour of juvenile vs. adult white storks with insights regarding juvenile mortality. J. Anim. Ecol. 85, 938–947. ( 10.1111/1365-2656.12525) [DOI] [PubMed] [Google Scholar]
- 67.Mitchell GW, Woodworth BK, Taylor PD, Norris DR. 2015. Automated telemetry reveals age specific differences in flight duration and speed are driven by wind conditions in a migratory songbird. Mov. Ecol. 3, 19 ( 10.1186/s40462-015-0046-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sergio F, Tanferna A, De Stephanis R, Jiménez LL, Blas J, Tavecchia G, Preatoni D, Hiraldo F.. 2014. Individual improvements and selective mortality shape lifelong migratory performance. Nature 515, 410–413. ( 10.1038/nature13696) [DOI] [PubMed] [Google Scholar]
- 69.Bustamante J, Negro JJ. 1994. The post-fledging dependence period of the lesser kestrel (Falco naumanni) in southwestern Spain. J. Raptor Res. 28, 158–163. [Google Scholar]
- 70.Baker J, Ferree ED. 2016. Foraging ontogeny in a suburban population of black phoebes (Sayornis nigricans). Wilson J. Ornithol. 128, 368 ( 10.1676/wils-128-02-368-377.1) [DOI] [Google Scholar]
- 71.Gall MD, Hough LD, Fernández-Juricic E. 2013. Age-related characteristics of foraging habitats and foraging behaviors in the black phoebe (Sayornis nigricans). Southwest. Nat. 58, 41–49. ( 10.1894/0038-4909-58.1.41) [DOI] [Google Scholar]
- 72.Marchetti K, Price T. 1989. Differences in the foraging of juvenile and adult birds: the importance of developmental constraints. Biol. Rev. 64, 51–70. ( 10.1111/j.1469-185X.1989.tb00638.x) [DOI] [Google Scholar]
- 73.Moreno J. 1984. Parental care of fledged young, division of labor, and the development of foraging techniques in the northern wheatear (Oenanthe oenanthe L.). Auk 101, 741–752. ( 10.2307/4086901) [DOI] [Google Scholar]
- 74.Davies NB. 1976. Parental care and the transition to independent feeding in the young spotted flycatcher (Muscicapa striata). Behaviour 59, 280–294. ( 10.1163/156853976X00415) [DOI] [Google Scholar]
- 75.Mueller HC, Mueller NS, Parker PG. 1981. Observation of a brood of sharp-shinned hawks in Ontario, with comments on the functions of sexual dimorphism. Wilson Bull. 93, 85–92. [Google Scholar]
- 76.Heyes CM. 1994. Social learning in animals: categories and mechanisms. Biol. Rev. 69, 207–231. ( 10.1111/j.1469-185X.1994.tb01506.x) [DOI] [PubMed] [Google Scholar]
- 77.Whiten A, Ham R. 1992. On the nature and evolution of imitation in the animal kingdom: reappraisal of a century of research. Adv. Study Behav. 21, 239–283. ( 10.1016/S0065-3454(08)60146-1) [DOI] [Google Scholar]
- 78.Varland DE, Klaas EE, Loughin TM. 1991. Development of foraging behavior in the American kestrel. J. Raptor Res. 25, 9–17. [Google Scholar]
- 79.Bustamante J. 1994. Behavior of colonial common kestrels (Falco tinnunculus) during the post-fledging dependence period in southwestern Spain. J. Raptor Res. 28, 79–83. [Google Scholar]
- 80.Kitowski I. 2003. Differences between social and non-social hunting of juvenile Montagu's harriers Circus pygargus in the post-fledging dependence period. Ornithologischer Anzeiger 42, 147–152. [Google Scholar]
- 81.Kitowski I. 2009. Social learning of hunting skills in juvenile marsh harriers Circus aeruginosus. J. Ethol. 27, 327–332. ( 10.1007/s10164-008-0123-y) [DOI] [Google Scholar]
- 82.Caro TM, Hauser MD. 1992. Is there teaching in nonhuman animals? Q. Rev. Biol. 67, 151–174. ( 10.1086/417553) [DOI] [PubMed] [Google Scholar]
- 83.Kitowski I. 2005. Play behaviour and active training of Montagu's harrier (Circus pygargus) offspring in the post-fledging period. J. Ethol. 23, 3–8. ( 10.1007/s10164-004-0120-8) [DOI] [Google Scholar]
- 84.Spofford WR, Amadon D. 1993. Live preys to young raptors—incidental or adaptive? J. Raptor Res. 27, 180–184. [Google Scholar]
- 85.Meinertzhagen R. 1954. The education of young ospreys. Ibis 96, 153–155. ( 10.1111/j.1474-919X.1954.tb04120.x) [DOI] [Google Scholar]
- 86.Schaadt CP, Rymon LM. 1982. Innate fishing behavior of osprey. Raptor Res. 16, 61–62. [Google Scholar]
- 87.Dinets V. 2013. Crane dances as play behaviour. Ibis 155, 424–425. ( 10.1111/ibi.12037) [DOI] [Google Scholar]
- 88.Frith CB, Cooper WT. 1996. Courtship display and mating of Victoria's riflebird Ptiloris victoriae with notes on the courtship displays of congeneric species. Emu—Austral Ornithol. 96, 102–113. ( 10.1071/MU9960102) [DOI] [Google Scholar]
- 89.Durães R. 2009. Lek structure and male display repertoire of blue-crowned manakins in eastern Ecuador. Condor 111, 453–461. ( 10.1525/cond.2009.080100) [DOI] [Google Scholar]
- 90.Collis K, Borgia G. 2010. The costs of male display and delayed plumage maturation in the satin bowerbird (Ptilonorhynchus violaceus). Ethology 94, 59–71. ( 10.1111/j.1439-0310.1993.tb00547.x) [DOI] [Google Scholar]
- 91.Soma M, Iwama M, Nakajima R, Endo R. 2019. Early-life lessons of the courtship dance in a dance-duetting songbird, the Java sparrow. R. Soc. Open Sci. 6, 190563 ( 10.1098/rsos.190563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis JM. 1980. The coordinated aerobatics of dunlin flocks. Anim. Behav. 28, 668–673. ( 10.1016/S0003-3472(80)80127-8) [DOI] [Google Scholar]
- 93.Yomosa M, Mizuguchi T, Vásárhelyi G, Nagy M. 2015. Coordinated behaviour in pigeon flocks. PLoS ONE 10, e0140558 ( 10.1371/journal.pone.0140558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Portugal SJ, Isaac R, Quinton KL, Reynolds SJ. 2010. Do captive waterfowl alter their behaviour patterns during their flightless period of moult? J. Ornithol. 151, 443–448. ( 10.1007/s10336-009-0474-3) [DOI] [Google Scholar]
- 95.Portugal SJ, Green JA, Butler PJ. 2007. Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): the importance of wing moult. J. Exp. Biol. 210, 1391–1397. ( 10.1242/jeb.004598) [DOI] [PubMed] [Google Scholar]
- 96.Portugal SJ, Thorpe SKS, Green JA, Myatt JP, Butler PJ. 2009. Testing the use/disuse hypothesis: pectoral and leg muscle changes in captive barnacle geese Branta leucopsis during wing moult. J. Exp. Biol. 212, 2403–2410. ( 10.1242/jeb.021774) [DOI] [PubMed] [Google Scholar]
- 97.Piersma T. 1988. Breast muscle atrophy and constraints on foraging during the flightless period of wing molting great crested grebes. Ardea 76, 96–106. ( 10.1007/BF00397858) [DOI] [Google Scholar]
- 98.Jehl JR. 1997. Cyclical changes in body composition in the annual cycle and migration of the eared grebe Podiceps nigricollis. J. Avian Biol. 28, 132 ( 10.2307/3677306) [DOI] [Google Scholar]
- 99.Gaunt AS, Hikida RS, Jehl JR. 1990. Rapid atrophy and hypertrophy of an avian flight muscle. Auk 107, 649–659. ( 10.2307/4087994) [DOI] [Google Scholar]
- 100.de Margerie E, Simonneau M, Caudal J-P, Houdelier C, Lumineau S.. 2015. 3D tracking of animals in the field using rotational stereo videography. J. Exp. Biol. 218, 2496–2504. ( 10.1242/jeb.118422) [DOI] [PubMed] [Google Scholar]
- 101.Jackson BE, Evangelista DJ, Ray DD, Hedrick TL. 2016. 3D for the people: multi-camera motion capture in the field with consumer-grade cameras and open source software. Biol. Open 5, 1334–1342. ( 10.1242/bio.018713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Theriault DH, Fuller NW, Jackson BE, Bluhm E, Evangelista D, Wu Z, Betke M, Hedrick TL. 2014. A protocol and calibration method for accurate multi-camera field videography. J. Exp. Biol. 217, 1843–1848. ( 10.1242/jeb.100529) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.