Abstract
Early lagomorphs are central to our understanding of how the brain evolved in Glires (rodents, lagomorphs and their kin) from basal members of Euarchontoglires (Glires + Euarchonta, the latter grouping primates, treeshrews, and colugos). Here, we report the first virtual endocast of the fossil lagomorph Megalagus turgidus, from the Orella Member of the Brule Formation, early Oligocene, Nebraska, USA. The specimen represents one of the oldest nearly complete lagomorph skulls known. Primitive aspects of the endocranial morphology in Megalagus include large olfactory bulbs, exposure of the midbrain, a small neocortex and a relatively low encephalization quotient. Overall, this suggests a brain morphology closer to that of other basal members of Euarchontoglires (e.g. plesiadapiforms and ischyromyid rodents) than to that of living lagomorphs. However, the well-developed petrosal lobules in Megalagus, comparable to the condition in modern lagomorphs, suggest early specialization in that order for the stabilization of eye movements necessary for accurate visual tracking. Our study sheds new light on the reconstructed morphology of the ancestral brain in Euarchontoglires and fills a critical gap in the understanding of palaeoneuroanatomy of this major group of placental mammals.
Keywords: Euarchontoglires, lagomorphs, Palaeogene, endocast, evolution
1. Introduction
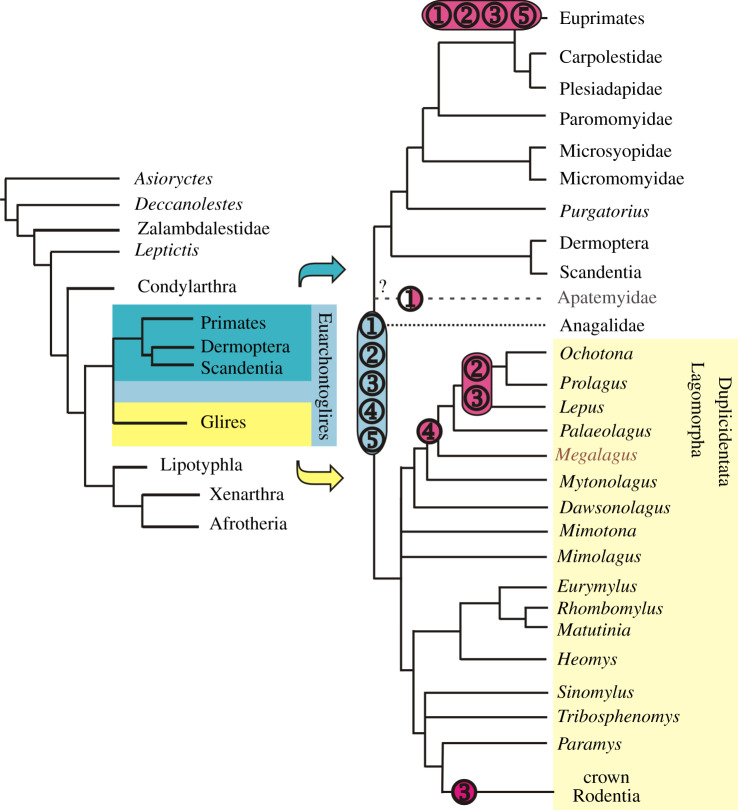
Euarchontoglires, one of the four modern placental clades recognized both in multigene (e.g. see [1]) and morphological studies (e.g. [2–4]), consists of Euarchonta (primates, scandentians, dermopterans and their fossil relatives) and Glires (lagomorphs, rodents and their kin; figure 1). The fossil record of Euarchonta begins with the North American Purgatorius; its first appearance is in the earliest Palaeocene, Puercan 1 North American Land Mammal Age (NALMA) [7]. The earliest record of Glires is Chinese Mimotona, a duplicidentate representative of the cohort (i.e. the group that includes all Glires related more closely to Lepus than Mus) [6], dated at 61.0 Ma, Shanghuanian Asian Land Mammal Age (ALMA), equivalent to Tiffanian 2 NALMA [8], which makes the fossil evidence for Duplicidentata only slightly less ancient than that for Euarchonta.
Figure 1.
Phylogenetic scheme of the Euarchontoglires relationships. Based on Meng et al. [5], Silcox et al. [4] and Fostowicz-Frelik & Meng [6], modified. Primitive and derived characters (in blue and pink, respectively): (1), size of the olfactory bulbs (blue, large; pink, small; white, extra large); (2), midbrain exposure (blue, large; pink, small); (3), neocorticalization (blue, small; pink, increased); (4), petrosal lobules (blue, small; pink, large); (5), encephalization quotient (EQ; blue, low; pink, high). (Online version in colour.)
However, there is very little fossil evidence for cranial structure in Lagomorpha prior to the late Eocene, with the sole exception of Dawsonolagus antiquus from the lower part of the Arshanto Formation (late Early Eocene) of Nei Mongol, China, represented by a partial skull with a completely absent basicranium [9]. Following the first radiation of the group in the early middle Eocene of central Asia (Irdinmanhan ALMA), lagomorphs quickly appeared in North America, where they have been present since the middle Eocene (ca. 42 Ma, late Uintan NALMA, [10]). By the end of the Eocene (Chadronian), North American lagomorphs were quite diverse (e.g. [10–13]). One of these lineages is represented by Megalagus, from the Eocene and Oligocene of North America [13]. Megalagus is a member of an early-branching lineage of stem lagomorphs; its closest relatives, Tachylagus and Mytonolagus, can be traced back to the middle Eocene (late Uintan NALMA) [10,14]. As such, Megalagus is the most basal lagomorph taxon for which the complete skull is known [6,13].
In this paper, we use high-resolution X-ray computed tomography (CT) data to provide, to our knowledge, the first description of a lagomorph digital endocast, based on the well-preserved skull of Megalagus turgidus. The evolutionary history of the lagomorph brain is almost entirely unknown, in contrast with our knowledge of other groups within Euarchontoglires (e.g. see review in [15]). Only the brain of the European rabbit (Oryctolagus cuniculus) has been studied in detail, using magnetic resonance imaging (MRI, e.g. [16] and [17]). However, this laboratory species represents a fairly recent (ca. 5.0 Ma for the genus) crown leporid radiation [18]. Previously published natural endocasts [19,20] of fossil lagomorphs also pertain to quite recent (Pliocene) taxa. As such, the Me. turgidus specimen studied here gives us the best available insight into the endocast morphology of early lagomorphs and yields data previously missing for this branch of Glires.
Basal duplicidentates branched off before the lineages of Rodentiaformes (i.e. Tribosphenomys and other Alagomyidae; see [21]) and paramyine rodents (figure 1; see also e.g. [22] and [5]: figure 74). Morphologically, duplicidentates (including lagomorphs) are in many ways the most basal and conservative modern members of Glires (e.g. [5,22,23]). For these reasons, the endocast of Megalagus may provide crucial information on the condition of the brain near the split between basalmost Glires and Euarchonta. This makes our study of the endocast of Megalagus pertinent not only to understanding brain evolution in Glires, but also in Euarchontoglires.
2. Description
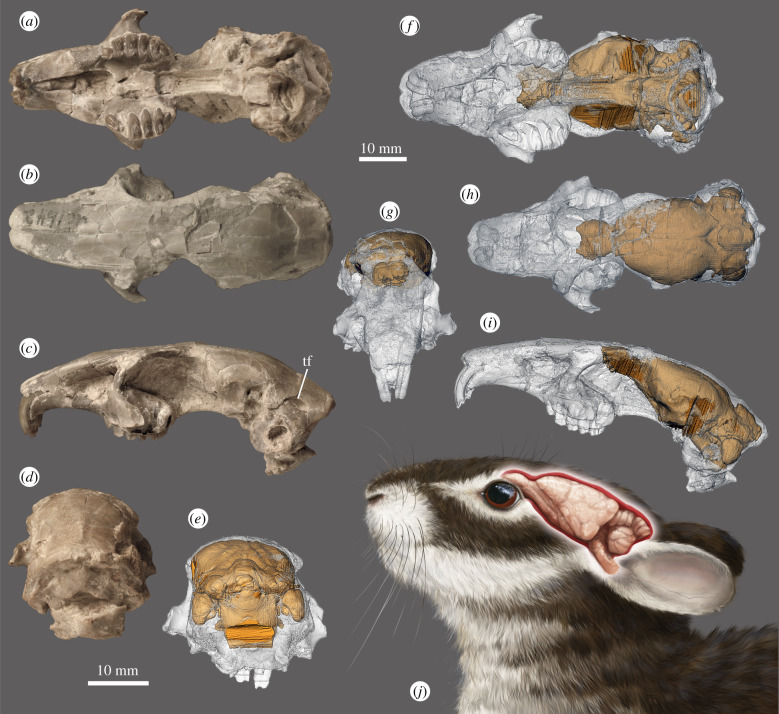
The endocast of Megalagus turgidus was extracted (see the electronic supplementary material) from high-resolution X-ray CT data of an almost complete (only the zygomatic arches are missing), undistorted skull (FMNH UC 1642; figure 2), dated to the earliest Orellan (early Oligocene; 33.7–32.00 Ma) [24] from the Brule Formation at Grime's Ranch, Sioux County, Nebraska [25].
Figure 2.
Cranium and endocast visualization of Megalagus turgidus (FMNH UC 1642) from the Brule Formation at Grime's Ranch, Sioux County, Nebraska. (a–d) External cranial morphology, (e–i) virtual endocast location in the cranium, and (j) life reconstruction of the Megalagus head with brain visualized (artist: Agnieszka Kapuścińska). (a,f) ventral; (b,h) dorsal; (c,i,j) lateral; (d,e) caudal; and (g) frontal views. Abbreviation: tf, temporal foramen. (online version in colour.)
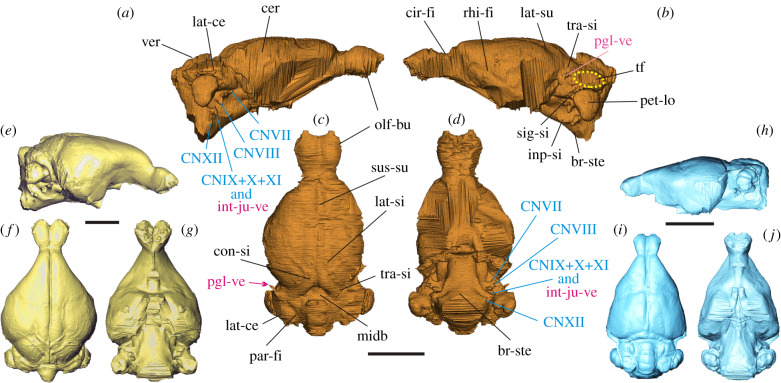
The endocast is elongate, with well-developed, ovoid and pedunculated olfactory bulbs (figure 3), which extend in the cranium to the area above the upper M1 and cover approximately two thirds of the length of the orbit (figure 2). The cerebral hemispheres are oval, gently rounded at the sides, slightly tapering anteriorly, with the maximum width near its mid-length. The frontal lobes in Megalagus do not overlap the olfactory bulbs and the circular fissure is relatively wide (figure 3).
Figure 3.
Digital endocasts of Megalagus turgidus (a–d), extant leporid Poelagus marjorita AMNH 51052 (e–g), and extant ochotonid, Ochotona princeps AMNH 40547 (h–j). Abbreviations: br-ste, brain stem; cer, cerebrum; cir-fi, circular fissure; CNVII, cranial nerve VII (facial nerve); CNVIII, cranial nerve VIII (vestibulocochlear nerve); CNIX, cranial nerve IX (glossopharyngeal nerve); CNX, cranial nerve X (vagus nerve); CNXI, cranial nerve XI (accessory nerve); CNXII, cranial nerve XII (hypoglossal nerve); con-si, confluence of sinuses; inp-si, inferior petrosal sinus; int-ju-ve, internal jugular vein; lat-ce, lateral lobe of cerebellum; lat-si, lateral sinus; lat-su, lateral sulcus; midb, midbrain; olf-bu, olfactory bulbs; par-fi, paramedian fissure; pet-lo, petrosal lobule; pgl-ve, postglenoid vein; rhi-fi, rhinal fissure; sig-si, sigmoid sinus; sus-su, superior sagittal sulcus; tf, temporal foramen; tra-si, transverse sinus; ver, vermis. Colour code: blue, nerves; pink, blood vessels; black, brain structures; (a,b,e and h), lateral; (c,f and i) dorsal; (d,g and j), ventral views. Surface rendering of Megalagus endocast available in [26]. (Online version in colour.)
The brain of Megalagus was almost entirely lissencephalic, with the exception of weakly invaginated lateral sulci that extend the length of the cerebrum just lateral to the sagittal sinus. The anterior and posterior extremities of the lateral sulci diverge laterally, while in the mid-length the sulci approach the superior sagittal sinus (figure 3). The rhinal fissure, marking the division between the palaeocortex and neocortex [27,28], is visible in Megalagus as a weak sulcus. It runs longitudinally on both sides of the endocast (the right side, being damaged, does not show its whole course; figure 3). There is no apparent Sylvian sulcus in Megalagus. The superior sagittal sinus is visible in the caudal half of the endocast of Megalagus, whereas in the rostral half there is a superior sagittal sulcus, but the sinus is not apparent, which suggests that it would have been partially buried in the meninges. In extant lagomorphs, the superior sagittal sinus is continuous with the transverse sinus leading to the sigmoid sinus, which is continuous with the internal jugular vein. In Megalagus, the transverse sinus is strong and well developed, but the area where it would be expected to merge with the sigmoid sinus is obscured by a large, elongate temporal foramen (as described in [25], p. 509), located between the squamosal, parietal and the mastoid exposure of the petrosal (figure 2). A temporal foramen this large is unknown for any extant lagomorph. On the endocast, the temporal foramina are apparent as large flat areas on top of the lateral lobes of the cerebellum (figure 3).
The cerebrum does not cover the cerebellum in Megalagus turgidus (figure 3); thus, a portion of the midbrain of Megalagus is exposed. The anteroposterior extension of the midbrain in Megalagus is less expansive than observed in the basal Glires Rhombomylus (see [5]: figure 50), which has a very broadly exposed midbrain. There are no clearly defined colliculi. The dorsal surface of the cerebellum exhibits a well-defined vermis that is separated from the lateral lobes by the paramedian fissures (figure 3). The lateral lobes of the cerebellum are large, although their precise form is somewhat obscured by the impression of the temporal foramen on the endocast. The cerebellar part of the endocast in Megalagus is positioned slightly ventral to the cerebral hemispheres (figure 3). This configuration is caused by the arching of the skull, which is expressed strongly both in leporids [29] and in Megalagus (figure 2), and is much weaker in Ochotona [6]. The petrosal lobules (comprised the paraflocculi) are large, with a rounded and smooth, teardrop-shaped (tapering ventrally) lateral surface. They are aligned with the lateral lobes and the vermis in dorsal view. Excluding the petrosal lobules, the cerebellum of Megalagus is slightly narrower than the cerebrum (table 1).
Table 1.
Measurements and parameters of the endocast of Megalagus turgidus (FMNH UC 1642). (Calculations of encephalization quotient (EQ) and masses of olfactory bulbs and petrosal lobules based on an estimated body mass of Me. turgidus of 2325 g; reconstructed data for NS and NS/TS in italics.)
| measurement (abbreviation); values in mm | |
|---|---|
| total length (TL) | 37.76 |
| olfactory bulb length (OL) | 8.32 |
| olfactory bulb width (OW) | 9.60 |
| olfactory bulb height (OH) | 5.61 |
| neocortex maximum height (NMH) | 10.21 |
| cerebrum total length (CRML) | 19.20 |
| cerebrum maximum width (CRMW) | 18.24 |
| cerebrum maximum height (CRMH) | 12.80 |
| cerebellum length (vermis) (CLML) | 9.28 |
| cerebellum width (without petrosal lobules) (CLW) | 16.32 |
| ratio; values in % | |
| OL/TL | 22.03 |
| CRML/TL | 50.85 |
| CLML/TL | 24.58 |
| CLW/CRMW | 89.47 |
| OW/CRMW | 52.63 |
| OW/CLW | 58.82 |
| NMH/CRMH | 79.77 |
| surfaces (abbreviation); values in mm2 | |
| total endocast area (TS) | 4108.10 |
| neocortical surface area (NS) | 779.86 |
| neocortical surface area (one side) (NS1) | 389.93 |
| volumes (abbreviation); values in mm3 | |
| total endocast (TV) | 7052.78 |
| olfactory bulbs (OV) | 280.10 |
| petrosal lobules (PLV) | 162.59 |
| ratio; values in % | |
| NS/TS | 18.98 |
| OV/TV | 3.97 |
| PLV/TV | 2.31 |
| mass; values in mg | |
| olfactory bulb mass | 266.76 |
| petrosal lobule mass | 154.85 |
| Jerison's EQ | 0.31 |
| Eisenberg's EQ | 0.39 |
The ventral aspect of the cranium is damaged, which does not allow for the reconstruction of some of the more rostral structures on the endocast (e.g. the optic tracts). The hypophyseal fossa is not clearly demarcated, with the relevant region actually being concave rather than convex on the endocast (figure 3).
The brainstem is well preserved (slightly curved ventrally), thus the more posterior vascular foramina and casts of some cranial nerves can be reconstructed. The casts of the passageways for cranial nerves VII (facial) and VIII (vestibulocochlear) are visible on both sides, located rostral to the petrosal lobules (figure 3). Similarly, a cast of the jugular foramen, through which the internal jugular vein and the IX (glossopharyngeal), X (vagus) and XI (accessory) cranial nerves would have passed, is positioned rostroventral to the petrosal lobule (figure 3). Finally, there is a cast of the hypoglossal foramen for cranial nerve XII (hypoglossal) on the brainstem, expressed better on the left side of the endocast (figure 3).
3. Comparisons with extant and fossil Lagomorpha
Based on comparisons to endocasts of extant members of Lagomorpha (electronic supplementary material, figure S3), the endocast morphology of Megalagus can be interpreted to exhibit an array of primitive and derived characters, the latter mostly in a nascent form, representing features developed further in crown lagomorphs. The primitive characters displayed by Megalagus include large olfactory bulbs that are separated from the cerebrum by a wide circular fissure, partly exposed midbrain, and generally much less expanded cerebral hemispheres, in comparison with recent lagomorphs and archaeolagines (see the electronic supplementary material, figure S3, [20]).
Compared to extant lagomorphs, the endocast of Megalagus (especially in dorsal and ventral views) shows a greater similarity to leporids (and to exclusively fossil archaeolagines) than to ochotonids. As in leporids, the cerebral hemispheres are round rather than triangular in outline, which contrasts with the shape of the hemispheres in extant ochotonids (figure 3). Additionally, the Megalagus endocast shows a slight downward bending (mostly at the brain stem), reflecting the arched profile of the cranium, also characteristic of leporids (figure 2; electronic supplementary material, figure S4). In lateral view, however, the Megalagus endocast exhibits a much flatter (less swollen) cerebrum, which resembles more closely that of Ochotona (figure 3; electronic supplementary material, figure S3I–K). The endocast of Megalagus is consistent with modern lagomorph taxa in its location in the cranium, with the maximal width being at the level of the posterior root of the zygomatic arch (see [20]). The location of the anterior edge of the olfactory bulbs matches that observed in extant leporids (above P4–M2 alveolus; electronic supplementary material, figure S4A), but it is more posterior than in modern Ochotona (above P3; electronic supplementary material, figure S4B).
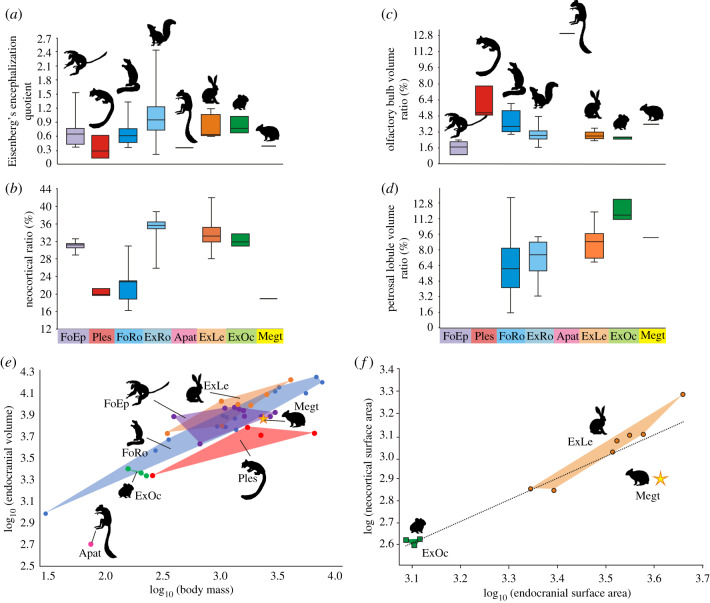
The olfactory bulbs of Megalagus are larger in terms of their volume relative to the endocranial volume and their width relative to the cerebrum maximal width than those of all extant lagomorphs for which data are currently available (figure 4c; electronic supplementary material, figure S5; table 1; electronic supplementary material, table S2). On the other hand, the volume of the olfactory bulbs relates to the estimated body mass groups Megalagus with extant leporids (electronic supplementary material, figure S5). In terms of the length of the olfactory bulbs relative to the endocast length (OL/TL, table 1), Megalagus overlaps with some of the Lepus species (i.e. Lepus americanus, electronic supplementary material table S2) and it is close to the archaeolagine Hypolagus brachygnathus and fossil ochotonid Prolagus meyeri, for which the relative length of the olfactory bulbs is ca. 20% [19]. Among the extant lagomorphs, only the leporid Poelagus marjorita markedly exceeds Megalagus in this respect, whereas the rest of the studied taxa exhibit lower values for the OL/TL ratio (table 1; electronic supplementary material, table S2). Thus, such an elongation of the olfactory bulbs seems to be generally typical of major lagomorph lineages, including Megalagus, Prolaginae and extant leporids.
Figure 4.
Comparisons of endocranial metrics in Euarchontoglires. (a) Encephalization quotient (EQ) based on Eisenberg's [30] equation; (b) neocortical surface area ratio (NS/ESx100); (c) olfactory bulb volume ratio (OV/TVx100); (d) petrosal lobule volume ratio (PV/TVx100); (e) bivariate plot of log10 (endocranial volume) versus log10 (body mass); and (f) bivariate plot of log10 (neocortical surface area) versus log10 (endocranial surface area). Abbreviations: Apat, Apatemyidae; ExLe, extant leporids; ExO, extant ochotonids; ExRo, extant rodents; FoEp, fossil euprimates; Megt, Megalagus turgidus; Ples, Plesiadapiformes. (Online version in colour.)
The cerebral hemispheres of Megalagus are less laterally expanded than in modern lagomorphs and archaeolagines, thus its endocast lacks the overall teardrop shape displayed by the crown taxa (figure 3; electronic supplementary material, figure S3). In Megalagus, the circular fissure is broader than in ochotonids, and much broader than in leporids, in which the cerebrum almost touches the olfactory bulbs owing to the swollen cerebral hemispheres (figure 3; electronic supplementary material, figure S3).
The endocasts in all lagomorph taxa studied here are lissencephalic (smooth). The lateral sulci are only weakly present on the Megalagus endocast (figure 3; electronic supplementary material, figure S2). The rhinal fissure in the rostral half of the cerebrum in Megalagus is similarly positioned to that of modern lagomorphs, but in the temporal region its course is more dorsal than in living leporids (electronic supplementary material, figure S3), indicating much lesser neocorticalization, as also reflected in the relatively lower neocortical ratio (figure 4; electronic supplementary material, table S2).
In Megalagus, the midbrain is more broadly exposed than in extant lagomorphs, although the difference is slight. Overall, extant leporids have less exposed midbrain than Ochotona, probably owing to the characteristic arched form of the leporid cranium, and to greater development of the transverse sinus, which partly covers the midbrain (electronic supplementary material, figure S3). The colliculi are variably visible in extant lagomorph endocasts (electronic supplementary material, figure S3). In Megalagus, they are indiscernible (figure 3), similar to those in most leporids, whereas the caudal colliculi are more apparent in Ochotona (electronic supplementary material, figure S3).
The cerebellum in modern lagomorphs (both leporids and ochotonids) is markedly narrower than the cerebrum (electronic supplementary material, figure S3, table S2). By contrast, in Megalagus the cerebrum and cerebellum are of similar width (table 1). The petrosal lobules are very well developed in all lagomorphs, including Megalagus (figures 2 and 3, electronic supplementary material, figure S3). The volume of the petrosal lobules relative to that of the whole endocast in Megalagus is within the range of values known for extant leporids (figure 4; electronic supplementary material, figure S5), although it is still lower than in extant ochotonids (figure 4d). On the other hand, the volume of the petrosal lobules in relation to the estimated body mass is lower in Megalagus than in any modern lagomorph (electronic supplementary material, figure S6A).
4. Comparisons with other Euarchontoglires
The morphology of the Megalagus endocast is similar in many ways to that observed in early members of Euarchontoglires (electronic supplementary material, figure S7), such as Plesiadapiformes [31–34], Ischyromyidae (e.g. see [35–37]) and Rhombomylus, a eurymylid [5]. These similarities include well-defined, uncovered and elongated olfactory bulbs, partly exposed midbrain and prominent petrosal lobules.
The endocast volume relative to the estimated body mass for Megalagus places it on the margin of the distribution of fossil euprimates and close to ischyromyid rodents and plesiadapiforms, but separate from the extant lagomorphs (figure 4). The relative length of the olfactory bulbs (OL/TL) in Megalagus is higher than in most of the ischyromyid rodents (apart from a borderline case of Paramys copei) [37] and early representatives of modern rodent groups, such as sciurids or aplodontids) [38,39]. This ratio overlaps partly with the values observed for the Palaeocene–Eocene Plesiadapiformes (electronic supplementary material, table S4) [31–33] and early Eocene eurymylid Rhombomylus (ca 22%). The olfactory bulb relative volume (OV/TV ratio) gives a slightly different picture, with the value for Megalagus (table 1) being within the range of ischyromyid rodents (figure 4c) [39]; this contrast reflects some difference in the shape of the bulbs, with them being more elongate relative to their volume compared to early fossil rodents. The OV/TV ratio value is higher than in early representatives of the modern crown rodents [37–39], but slightly lower than in Plesiadapiformes (electronic supplementary material, table S3) [31,32].
The relative length of the cerebral hemispheres (CRML/TL) is comparable in Megalagus (51%; table 1), Ischyromyidae and Plesiadapiformes (electronic supplementary material, table S4), the latter showing the greatest observed range, with Plesiadapis showing the lowest values (42–45.5%) and Microsyops displaying proportionally the longest cerebrum (54.0%). This primitive brain architecture shared by Megalagus with Ischyromyidae and Plesiadapiformes is in contrast with more derived modern lineages of Glires [39] and euprimates [40], which all exhibit greater relative cerebral development (the CRML/TL ratio on average is over 60%).
Another feature shared by Megalagus, ischyromyid rodents [35,37], Plesiadapiformes [31,32] and early members of the modern rodent clades [38,39] is an exposed midbrain. The extent of the midbrain exposure in Megalagus is much less than in Plesiadapis tricuspidens [33] and Rhombomylus [5], as well as in some of the Ischyromyidae (Paramys, Notoparamys and Ischyromys) [35,37], in which the colliculi are visible to some degree. It resembles more closely that of the virtual endocast of Microsyops annectens, in which the colliculi are not visible, but a patch of midbrain is exposed, and the rest of the midbrain is grooved by the venous sinuses rather than the cerebrum [32] (also see [41]). However, it is worth noting that exposure of the colliculi is known to vary intraspecifically (e.g. in Mi. annectens and Ischyromys typus) [32,36].
The cerebellum of Megalagus is only marginally narrower than the cerebrum, a common trait among early fossil Euarchontoglires, excluding fossil euprimates (e.g. see [33,34,39,42]). The difference between the cerebrum and cerebellum width is greater in more derived fossil taxa (e.g. Cedromus wilsoni) [38], in extant representatives of modern Glires (compare figure 3; electronic supplementary material, figure S3), and in living and fossil euprimates [40]. The vermis and cerebellar hemispheres are generally similarly developed in Megalagus as in Ischyromyidae and Plesiadapiformes, but the relative size of the petrosal lobules is distinctly larger in Megalagus (figure 4d; electronic supplementary material, table S3) than in extant and fossil rodents.
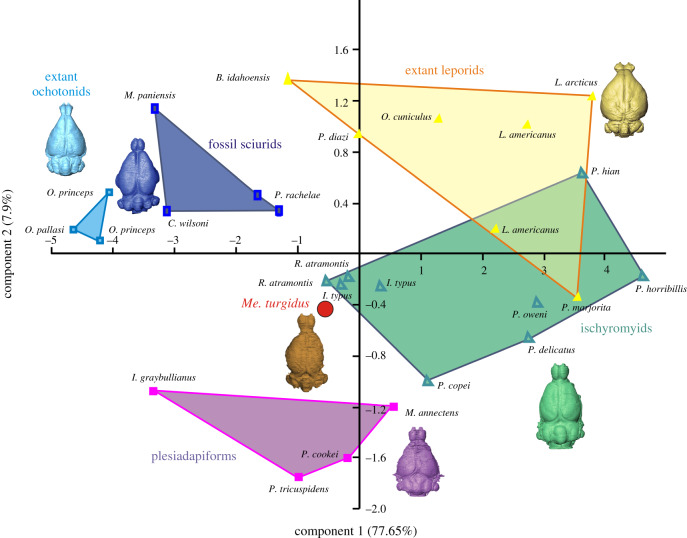
5. Principal component analysis
A principal component analysis was performed based on nine measurements of the endocasts for 24 species of extant lagomorphs, fossil rodents, plesiadapiforms and Megalagus (electronic supplementary material, table S4). Principal component 1 and 2 (PC1 and PC2) represent 77.65% and 7.9% of the variance, respectively (electronic supplementary material, figure S8), whereas principal components 3–5 represent a further 12% of the variance (electronic supplementary material, figure S8). All the variables are positively correlated with PC1 (electronic supplementary material, figure S8), which implies that it represents a proxy for the size of the endocast. PC2 is most strongly correlated with the olfactory bulb length (−0.57) and width (0.58). Generally, an endocast with a high PC2 score has relatively short but wide olfactory bulbs (electronic supplementary material, table S4).
In the plot of PC1 versus PC2, Megalagus falls between smaller ischyromyids (Ischyromys) and plesiadapiforms (figure 5). Extant leporids and larger ischyromyid rodents partly overlap in the morphospace, while extant ochotonids are grouped more closely with the representatives of fossil sciurids (figure 5). Megalagus is relatively separated from extant lagomorphs (both Ochotonidae and Leporidae, although closer to the latter) and sciurids, which points to its morphological difference from the modern groups of Glires. Overall, Megalagus plots near the centre of the morphospace of the studied Euarchontoglires, and this equidistant position suggests its morphological consistency with the endocast architecture characteristic of basal groups of Euarchontoglires.
Figure 5.
Results of principal component analysis of nine endocast characters in 24 species of fossil and extant lagomorphs, ischyromyids, fossil sciurids and plesiadapiforms. Note the central position of Megalagus in the morphospace, closer to Ischyromyidae and Plesiadapiformes rather than to extant Lagomorpha. For raw data, see the electronic supplementary material. (Online version in colour.)
6. Discussion
The endocranial morphology of Megalagus can be interpreted from several perspectives; first, it represents an early stage of brain evolution in lagomorphs, and second, more broadly, a source of information relevant to understanding the form of the brain in Euarchontoglires in general.
Compared to extant lagomorphs and rodents, including early fossil representatives of modern crown rodents (e.g. Oligocene Cedromus) [38], the encephalization quotients (EQ) of Megalagus and the rest of the sampled basal members of Euarchontoglires are generally lower (figure 4a; electronic supplementary material, table S3). The neocortical ratio shows similar values for Megalagus (19%), ischyromyid rodents (17–23%) and plesiadapiforms (20–24%), all of which display smaller relative area of the neocortex (figure 4; table 1; electronic supplementary material, tables S2, S3) than in the crown members of Glires, and in both fossil and extant members of Euprimates, in which the ratio value is generally over 30% ([38–40], figure 13). These observations suggest that expansion of the brain through neocorticalization happened multiple times in the evolution of Euarchontoglires, and that the common ancestor of this group probably had a relatively small neocortex [33]. With respect to the olfactory bulbs, Megalagus is similar to plesiadapiforms and early fossil rodents in their size (length) relative to the rest of the brain (electronic supplementary material, table S4), with relatively larger bulbs than observed in living lagomorphs, or fossil or living euprimates (figure 4c). This similarity suggests that, in this way, Megalagus is also similar to what might be inferred for the primitive ancestor of Euarchontoglires. The one issue with that interpretation is the relatively much larger olfactory bulbs of apatemyids. This part of the endocast in Labidolemur and Carcinella (see [42] and [43], respectively) match in proportions and overall shape the olfactory bulbs of the late Cretaceous eutherians (e.g. Asioryctes) [44]. Whether this similarity is a matter of ancestor–descendent relationship or convergent evolution remains uncertain. It is also worth noting that the contrast with living lagomorphs is not apparent when olfactory bulb size is considered in relation to body mass rather than endocranial volume (electronic supplementary material, figure S5), which suggests that the apparent difference in relative olfactory bulb size might be attributable to expansions in other parts of the brain rather than reduction in the apparatus for olfaction.
In Megalagus, the anterior extremities of its olfactory bulbs reach the area over M1; thus, they are more posteriorly located in the cranium compared to modern lagomorphs (up to P3–P4; electronic supplementary material, figure S4) and rodents (up to the diastema) [39]. With respect to the position of the olfactory bulbs, Megalagus is similar to the middle-to-late Eocene ischyromyids (Rapamys and Ischyromys) [35,36], and to the early Eocene paromomyid plesiadapiform Ignacius [31], and so might be primitive in this way relative to modern lagomorphs. In ischyromyids, stratigraphically younger genera have more anteriorly located olfactory bulbs, but the bulbs extend no farther than M1, while in the fossil representatives of the modern rodents the olfactory bulbs are positioned rather at P4 (e.g. Cedromus) [38]. Silcox et al. [32] suggested that the relative position of the olfactory bulbs in plesiadapiforms may be related to the length of the muzzle, especially the diastema. However, in early Glires (both ischyromyids, and lagomorphs), the diastema length does not change significantly; thus, the explanation for the shifting relative position of the front of the brain has to be different.
In all Palaeogene taxa studied herein the midbrain is exposed, although to varying degrees. The relatively well-exposed midbrain, which is partially visible between the posteriorly diverging and relatively long cerebral hemispheres, was characteristic of stem placentals, such as Asioryctes, Barunlestes, Kennalestes and Zalambdalestes [44,45]. As such, this trait of Megalagus is likely to be an archaic (plesiomorphic) feature, comprising part of the morphotype of early Euarchontoglires.
In summary, the stem groups of Euarchontoglires share features that include relatively large olfactory bulbs, an at least partially exposed midbrain, the cerebellum with relatively large petrosal lobes, low EQ and a small neocortex. The similarities in taxa that range from the late early Palaeocene Plesiadapis to the early Oligocene Megalagus suggest that this general architecture remained relatively stable, especially in Lagomorpha. In that context, the endocast structure of Me. turgidus fills the gap in our knowledge of the primitive brain morphology for Lagomorpha, arguably the most basal branch of Glires, and as such it gives us an insight into the ancestral brain architecture of Euarchontoglires.
Supplementary Material
Acknowledgements
We are grateful to W. Simpson (FMNH) for access to the Megalagus specimen, E. Westwig (formerly AMNH) for making Romerolagus diazi available for study, J.O. Thostenson (formerly AMNH) for CT-scanning of the AMNH and FMNH lagomorph specimens at the AMNH, and D. Boyer for facilitating the scanning at the Shared Materials Instrumentation Facility (SMIF), Duke University. We thank the artists A. Kapuścińska who made a reconstruction of the Megalagus head and F. Ippolito for the photos of the skull. Thanks are due to three anonymous reviewers, whose comments substantively improved this paper.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.0vt4b8gwg [26].
Authors' contributions
Ł.F.F. conceived the study; Ł.F.F. and M.T.S. designed and outlined research; all authors studied specimens, gathered and analysed data; S.L.T., Ł.F.F. and M.T.S. wrote the paper, all authors discussed and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The present work was supported by a Marie Skłodowska-Curie Actions: Individual Fellowship (H2020-MSCA-IF-2018-2020; grant no. 792611) to O.C.B., a Natural Sciences and Engineering Research Council of Canada (NSERC) CGS grant to M.M.L., an NSERC Discovery Grant to M.T.S., and a National Science Centre (Cracow, Poland) grant no. 2015/18/E/NZ8/00637 to Ł.F.F.
References
- 1.Murphy WJ, et al. 2001. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 294, 2348–2351. ( 10.1126/science.1067179) [DOI] [PubMed] [Google Scholar]
- 2.Bloch JI, Silcox MT, Boyer DM, Sargis EJ. 2007. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proc. Natl Acad. Sci. USA 104, 1159–1164. ( 10.1073/pnas.0610579104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher RA, Bennett N, Lehmann T. 2009. The new framework for understanding placental mammal evolution. Bioessays 31, 853–864. ( 10.1002/bies.200900053) [DOI] [PubMed] [Google Scholar]
- 4.Silcox MT, Bloch JI, Boyer DM, Houde P. 2010. Cranial anatomy of Paleocene and Eocene Labidolemur kayi (Mammalia: Apatotheria), and the relationships of the Apatemyidae to other mammals. Zool. J. Linn. Soc. 160, 773–825. ( 10.1111/j.1096-3642.2009.00614.x) [DOI] [Google Scholar]
- 5.Meng J, Hu Y, Li C-K. 2003. The osteology of Rhombomylus (Mammalia, Glires): implications for phylogeny and evolution of Glires. Bull. Am. Mus. Nat. Hist. 275, 1–247. () [DOI] [Google Scholar]
- 6.Fostowicz-Frelik Ł, Meng J. 2013. Comparative morphology of premolar foramen in lagomorphs (Mammalia: Glires) and its functional and phylogenetic implications. PLoS ONE 8, e79794 ( 10.1371/journal.pone.0079794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox RC, Scott CS. 2011. A new, early Puercan (earliest Paleocene) species of Purgatorius (Plesiadapiformes, Primates) from Saskatchewan, Canada. J. Paleontol. 85, 537–548. ( 10.1666/10-059.1) [DOI] [Google Scholar]
- 8.Wang Y-Q, Li C-K, Li Q, Li D-S. 2016. A synopsis of Paleocene stratigraphy and vertebrate paleontology in the Qianshan Basin, Anhui, China. Vertebrat. PalAsiatic. 54, 89–120. [Google Scholar]
- 9.Li C-K, Meng J, Wang Y-Q. 2007. Dawsonolagus antiquus, a primitive lagomorph from the Eocene Arshanto Formation, Nei Mongol, China. Bull. Carnegie Mus. 39, 97–110. ( 10.2992/0145-9058(2007)39[97:DAAPLF]2.0.CO;2) [DOI] [Google Scholar]
- 10.Dawson MR. 2008. Lagomorpha. In Evolution of tertiary mammals of North America (eds Janis CM, Gunnell GF, Uhen MD), pp. 293–310. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Dawson MR. 1958. Later Tertiary Leporidae of North America. Vertebrata 6, 1–75. [Google Scholar]
- 12.Fostowicz-Frelik Ł, Tabrum AR. 2009. Leporids (Mammalia, Lagomorpha) from the Diamond O Ranch local fauna, latest middle Eocene of southwestern Montana. Ann. Carnegie Mus. 78, 253–271. ( 10.2992/007.078.0303) [DOI] [Google Scholar]
- 13.Fostowicz-Frelik Ł. 2013. Reassessment of Chadrolagus and Litolagus (Mammalia: Lagomorpha) and a new genus of North American Eocene lagomorph from Wyoming. Am. Mus. Novit. 3773, 1–76. ( 10.1206/3773.2) [DOI] [Google Scholar]
- 14.Storer JE. 1992. Tachylagus, a new lagomorph from the Lac Pelletier lower fauna (Eocene: Duchesnean) of Saskatchewan. J. Vert. Paleontol. 12, 230–235. ( 10.1080/02724634.1992.10011452) [DOI] [Google Scholar]
- 15.Silcox MT, López-Torres S. 2017. Major questions in the study of primate origins. Ann. Rev. Earth Planet. Sci. 45, 113–137. ( 10.1146/annurev-earth-063016-015637) [DOI] [Google Scholar]
- 16.Muñoz-Moreno E, Arbat-Plana A, Batalle D, Soria G, Illa M, Prats-Galino A, Eixarch E, Gratacos E. 2013. A magnetic resonance image based atlas of the rabbit brain for automatic parcellation. PLoS ONE 8, e67418 ( 10.1371/journal.pone.0067418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müllhaupt D, Augsburger H, Schwarz A, Fischer G, Kircher P, Hatt J-M, Ohlerth S. 2015. Magnetic resonance imaging anatomy of the rabbit brain at 3T. Acta Vet. Scand. 57, 47 ( 10.1186/s13028-015-0139-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Martínez N. 2008. The lagomorph fossil record and the origin of the European rabbit. In Lagomorph biology: evolution, ecology, and conservation (eds Alves PC, Ferrand N, Hackländer K), pp. 27–46. Berlin, Germany: Springer. [Google Scholar]
- 19.Sych L. 1967. Fossil endocranial cast of Hypolagus brachygnathus Kormos (Leporidae, Mammalia). Acta Zool. Crac. 12, 27–30. [Google Scholar]
- 20.Czyżewska T. 1985. Natural endocranial casts of Hypolagus brachygnathus Kormos, 1934 (Leporidae, Lagomorpha) from Węże I near Działoszyn. Acta Zool. Crac. 29, 1–12. [Google Scholar]
- 21.Asher RJ, Smith MR, Rankin A, Emry RJ. 2019. Congruence, fossils and the evolutionary tree of rodents and lagomorphs. R. Soc. Open Sci. 6, 190387 ( 10.1098/rsos.190387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng J, Wyss AR. 2001. The morphology of Tribosphenomys (Rodentiaformes, Mammalia): phylogenetic implications for basal Glires. J. Mamm. Evol. 8, 1–71. ( 10.1023/A:1011328616715) [DOI] [Google Scholar]
- 23.Fostowicz-Frelik Ł. 2017. Convergent and parallel evolution in early Glires (Mammalia). In Evolutionary biology: self/nonself evolution, species and complex traits evolution, methods and concepts (ed. Pontarotti P.), pp. 199–216. Berlin, Germany: Springer. [Google Scholar]
- 24.Prothero DR, Emry RJ. 2004. The Chadronian, Orellan, and Whitneyan North American land mammal ages. In Late Cretaceous and Cenozoic mammals of North America (ed. Woodburne MO.), pp. 156–168. New York, NY: Columbia University Press. [Google Scholar]
- 25.Olson EC. 1942. The skull of Megalagus turgidus (Cope). Am. J. Sci. 240, 505–511. ( 10.2475/ajs.240.7.505) [DOI] [Google Scholar]
- 26.Fostowicz-Frelik Ł, López-Torres S, Bertrand OC, Lang MM, Silcox MT. 2020. Data from: Cranial endocast of the stem lagomorph Megalagus and brain structure of basal Euarchontoglires, Dryad Digital Repository ( 10.5061/dryad.0vt4b8gwg) [DOI] [PMC free article] [PubMed]
- 27.Jerison HJ. 2012. Digitalized fossil brains: Neocorticalization. Biol. Ther. Dent. 6, 383–392. [Google Scholar]
- 28.Long A, Bloch JI, Silcox MT. 2015. Quantification of neocortical ratios in stem primates. Am. J. Phys. Anthropol. 157, 363–373. ( 10.1002/ajpa.22724) [DOI] [PubMed] [Google Scholar]
- 29.Wible JR. 2007. On the cranial anatomy of the Lagomorpha. Bull. Carnegie Mus. 39, 213–234. ( 10.2992/0145-9058(2007)39[213:OTCOOT]2.0.CO;2) [DOI] [Google Scholar]
- 30.Eisenberg JF. 1981. The mammalian radiations: an analysis of trends in evolution, adaptation, and behavior, 610 p Chicago, IL: University of Chicago Press. [Google Scholar]
- 31.Silcox MT, Dalmyn CK, Bloch JI. 2009. Virtual endocast of Ignacius graybullianus (Paromomyidae, Primates) and brain evolution in early Primates. Proc. Natl Acad. Sci. USA 106, 10 987–10 992. ( 10.1073/pnas.0812140106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silcox MT, Benham AE, Bloch JI. 2010. Endocasts of Microsyops (Microsyopidae, Primates) and the evolution of the brain in primitive primates. J. Hum. Evol. 58, 505–521. ( 10.1016/j.jhevol.2010.03.008) [DOI] [PubMed] [Google Scholar]
- 33.Orliac MJ, Ladevèze S, Gingerich PD, Lebrun R, Smith T. 2014. Endocranial morphology of Palaeocene Plesiadapis tricuspidens and evolution of the early primate brain. Proc. R. Soc. B 281, 20132792 ( 10.1098/rspb.2013.2792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gingerich PD, Gunnell GF. 2005. Brain of Plesiadapis cookei (Mammalia, Proprimates): surface morphology and encephalization compared to those of Primates and Dermoptera. Contr. Mus. Paleontol. Univ. Michigan 31, 185–195. [Google Scholar]
- 35.Bertrand OC, Amador-Mughal F, Lang MM, Silcox MT. 2019. New virtual endocasts of Eocene Ischyromyidae and their relevance in evaluating neurological changes occurring through time in Rodentia. J. Mamm. Evol. 26, 345–371. ( 10.1007/s10914-017-9425-6) [DOI] [Google Scholar]
- 36.Bertrand OC, Silcox MT. 2016. First virtual endocast of a fossil rodent: Ischyromys typus (Ischyromyidea, Oligocene) and brain evolution in rodents. J. Vert. Paleontol. 36, e1096275 ( 10.1080/02724634.2016.1096275) [DOI] [Google Scholar]
- 37.Bertrand OC, Amador-Mughal F, Silcox MT. 2016. Virtual endocasts of Eocene Paramys (Paramyinae): oldest endocranial record for Rodentia and early brain evolution in Euarchontoglires. Proc. R. Soc. B 283, 20152316 ( 10.1098/rspb.2015.2316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand OC, Amador-Mughal F, Silcox MT. 2017. Virtual endocast of the early Oligocene Cedromus wilsoni (Cedromurinae) and brain evolution in squirrels. J. Anat. 230, 128–151. ( 10.1111/joa.12537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertrand OC, Amador-Mughal F, Lang MM, Silcox MT. 2018. Virtual endocasts of fossil Sciuroidea: brain size reduction in the evolution of fossoriality. Palaeontology. 61, 919–948. ( 10.1111/pala.12378) [DOI] [Google Scholar]
- 40.Harrington AR, Silcox MT, Yapuncich GS, Boyer DM, Bloch JI. 2016. First virtual endocast of adapiform primates. J. Hum. Evol. 99, 52–78. ( 10.1016/j.jhevol.2016.06.005) [DOI] [PubMed] [Google Scholar]
- 41.Szalay FS. 1969. Mixodectidae, Microsyopidae and the insectivore-primate transition. Bull. Am. Mus. Nat. Hist. 140, 193–330. [Google Scholar]
- 42.Silcox MT, Dalmyn CK, Hrenchuk A, Bloch JI, Boyer DM, Houde P. 2011. Endocranial morphology of Labidolemur kayi (Apatemyidae, Apatotheria) and its relevance to the study of brain evolution in Euarchontoglires. J. Vert. Paleontol. 31, 1–12. ( 10.1080/02724634.2011.609574) [DOI] [Google Scholar]
- 43.Koenigswald von W, Ruf I, Gingerich P. 2009. Cranial morphology of a new apatemyid, Carcinella sigei n. gen. n. sp. (Mammalia, Apatotheria) from the late Eocene of southern France. Palaeontographica Abt A: Paläozoologie - Stratigraphie 288, 53–91. ( 10.1127/pala/288/2009/53) [DOI] [Google Scholar]
- 44.Kielan-Jaworowska Z. 1984. Evolution of the therian mammals in the late Creataceous of Asia. Part VI. Endocranial casts of eutherian mammals. Palaeontol. Pol. 46, 157–171. [Google Scholar]
- 45.Kielan-Jaworowska Z, Trofimov BA. 1986. Endocranial cast of the Cretaceous eutherian mammal Barunlestes. Acta Palaeontol. Pol. 31, 137–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fostowicz-Frelik Ł, López-Torres S, Bertrand OC, Lang MM, Silcox MT. 2020. Data from: Cranial endocast of the stem lagomorph Megalagus and brain structure of basal Euarchontoglires, Dryad Digital Repository ( 10.5061/dryad.0vt4b8gwg) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.0vt4b8gwg [26].