Abstract
Arboviruses are among the most important emerging pathogens due to their increasing public health impact. In Kenya, continued population growth and associated urbanization are conducive to vector spread in both urban and rural environments, yet mechanisms of viral amplification in vector populations is often overlooked when assessing risks for outbreaks. Thus, the characterization of local arbovirus circulation in mosquito populations is imperative to better inform risk assessments and vector control practices. Aedes species mosquitoes were captured at varying stages of their life cycle during different seasons between January 2014 and May 2016 at four distinct sites in Kenya, and tested for chikungunya (CHIKV), dengue (DENV) and Zika (ZIKV) viruses by RT-PCR. CHIKV was detected in 45 (5.9%) and DENV in 3 (0.4%) mosquito pools. No ZIKV was detected. Significant regional variation in prevalence was observed, with greater frequency of CHIKV on the coast. DENV was detected exclusively on the coast. Both viruses were detected in immature mosquitoes of both sexes, providing evidence of transovarial transmission of these arboviruses in local mosquitoes. This phenomenon may be driving underlying viral maintenance that may largely contribute to periodic re-emergence among humans in Kenya.
Author summary
Transovarial transmission, or vertical transmission, is the spread of a pathogen from parent to offspring. It has been observed that some mosquito-borne viruses can be transmitted from female mosquitoes to their offspring during follicle development or during oviposition. The occurrence of transovarial transmission is evident in the presence of virally infected male mosquitoes, which typically do not take bloodmeals, and the presence of virus in immature mosquitoes of any sex. Transovarial transmission aids in the amplification of mosquito-borne viruses in the environment by increasing the number of infected mosquitoes in a given region, thus expanding the possibility of viral transmission to humans. The combination of transovarial transmission and the preservation of viable eggs during dry seasons may trigger sudden amplification of the virus after rainy periods, resulting in an outbreak. This study provides some of the first evidence of transovarial transmission of chikungunya and dengue viruses in Aedes aegypti mosquitoes in Africa during interepidemic periods, which has important implications for local virus persistence and epidemic patterns.
Introduction
Arthropod–borne viruses (arboviruses) comprise some of the most important emerging pathogens due to their geographic spread and increasing impact on vulnerable human populations. Over 100 arboviruses are known to cause pathology in humans, creating a significant global health burden, yet the transmission, epidemiology, and incidence of arbovirus-related human disease remain poorly defined, particularly in sub-Saharan Africa. Kenya has had multiple arbovirus outbreaks in the past 2 decades including yellow fever [1, 2], chikungunya [3], Rift Valley fever [4–6] and dengue fever [7], which have resulted in significant effects to local economies and community health [8–13]. Thus, the characterization of arboviral circulation in the rapidly changing Kenyan environments that support vector proliferation is essential to better inform human risk assessment and vector control practices.
Transovarial transmission (TOT) is a mechanism by which infective female mosquitoes pass the virus to their offspring via their eggs. TOT is believed to be a mechanism by which arboviruses maintain a local presence during environmental conditions that are adverse for mosquito proliferation, e.g. during dry seasons and winter. Quiescence of vertically infected mosquito eggs in response to environmental conditions may contribute to viral persistence or re-emergence within a region [14]. A recent review of modeling studies suggests that, for DENV, TOT is likely not an important mechanism of viral propagation in Southeast Asia and the Americas, but rather asymptomatic infections in humans and the movement of people account for DENV persistence [15]. Others theorize that selective pressures arise from different vector-virus system combinations that influence the success of horizontal and vertical transmission in a population, as well as overall virulence [16, 17]. Although comprehensive evidence exists demonstrating that TOT of CHIKV and DENV occurs in many distinct endemic regions, complex mechanisms and a lack of field data lead to an incomplete understanding of the contributions of TOT to viral persistence during interepidemic times. In particular, the propagation and maintenance of DENV and CHIKV in Africa and the role of TOT in this viral maintenance is not well understood. This study describes the prevalence and spatiotemporal distribution of CHIKV, DENV, and ZIKV in field-caught mosquitoes in Kenya. We further investigate the rate of TOT of these viruses to determine its possible contribution to viral maintenance in this endemic setting.
Methods
Ethics statement
The study protocol was approved by the Stanford University Institutional Review Board (Protocol ID #31488) and the Kenya Medical Research Institute (KEMRI) National Scientific and Ethical Review Committee (SSC # 2611). Meetings were held at all four sites with local government administrators (village elders, chiefs, and assistant chiefs) and with the local residents in each sub-location to introduce the research study and staff to the public. Written informed consent was obtained from the adults who volunteered to participate in Human Landing Catches (HLC) before they were trained and began sampling for mosquitoes. Oral consent was obtained from household heads to sample mosquitoes within their houses and their compounds.
Mosquito collection
Study sites: This study was conducted in four sites in Kenya: two western sites, Kisumu (urban) and Chulaimbo (rural) in Kisumu County, and two coastal sites, Ukunda (urban) and Msambweni (rural) in Kwale County (Fig 1). Kisumu is the third largest city in Kenya located at the shore of Lake Victoria. Chulaimbo village is located 19 kilometers from Kisumu. Ukunda is an emerging urban center located 30 kilometers south of Mombasa in Kwale County along the Indian Ocean coastline. Msambweni village is also in Kwale County, near the shore of the Indian Ocean. It is located 30 kilometers south of Ukunda nearer the Tanzanian border. The climate in both regions is tropical with a bimodal monthly rainfall pattern, with a long rainy period from March to June and a short rainy period from August to October. Sampling for Aedes mosquitoes in each of the four sites was done in a selected area of approximately 1.5 x 1.0 km.
Fig 1. Map of study sites in Kenya.
Created in QGIS 2.18.11 using MapBox.
The mosquito collection methods have been previously described in detail [18, 19] and are briefly summarized here:
Human Landing Catches (HLC): Two homesteads were selected in each of the sites for sampling of blood-seeking mosquitoes using HLC both indoors and outdoors. Paired teams worked together, with one exposing the legs, and the other collecting mosquitoes landing on their partner’s legs. Team members changed roles hourly. Captured mosquitoes were placed in collection vessels provided with 10% sugar solution on cotton wool. Vessels were transported on ice to insectaries at KEMRI at Kisian in Kisumu County (for the western sites) and the Vector Borne Disease Control Unit in Msambweni County Referral Hospital, Kwale County (for coastal sites).
Prokopack Automated Aspirators: Twenty houses were randomly selected in each of the sites and sampled indoors and outdoors monthly for resting mosquitoes using Prokopack aspirators [20]. Sampling was performed simultaneously, both indoors and outdoors, for 20 minutes by a pair of entomology team members. The collection vessel, which was fitted with a wire mesh on the underside, was used on the aspirator and was capped and removed at the end of each sampling period. Mosquito specimens were stored on ice and transported to each insectary as described above.
Biogents-Sentinel traps (BGS): Two houses were selected in each of the four sites for adult mosquito sampling using Biogents (BGS)-traps (Biogents AG Weissenburgstr 22 93055 Regensburg, Germany). Initially, the BGS traps were set indoors in the sitting room without lure. In May 2015, the houses were changed, the trap location was changed from indoors to open, secure verandas, and the traps were baited with CO2. The BGS trap was set to sample mosquitoes for five consecutive days every month. Trapped specimens were collected daily and stored and transported as above.
Sampling of immature mosquitoes: Pupal and Larval sampling: Forty houses in each of the four study sites were selected by random sampling: 20 houses for larval sampling and 20 for ovitrap sampling. Houses were assessed for immature mosquito infestation on a monthly basis over the study period. All natural and artificial water-holding containers in and around each household were inspected for mosquito larvae and pupae. All pupae and larvae (3rd and 4th instars) from positive containers were collected with pipettes and ladles [19, 21]. Water from large containers was first sieved and mosquito samples were placed in a white plastic tray with some water from which the immatures were pipetted. Mosquito samples were placed in 10mL Falcon tubes (Fisher Scientific, Waltham, MA) and/or Whirl-pak plastic bags (Nasco, Fort Atkinson, WI), labeled, and taken to the insectaries. There, immature mosquitoes were reared in 200 mL plastic cups under laboratory conditions at an average temperature of 28.15 ± 1.8ºC and relative humidity of 80.9 ± 6.3%, and larvae were fed on TetraMinbaby fish food until adult mosquitoes emerged.
Ovitrap Sampling: Ovitraps were placed in 20 households that were randomly selected as fixed sampling points in each of our four study sites. Each ovitrap consisted of a black plastic cup filled with 350 mL of tap, borehole, or rainwater. The inside of the cup was lined by a brown paper towel that was partially submerged. Eggs were laid on the damp paper towel just above the water line. Ovitraps were set once a week for the duration of the study. To obtain the eggs, the paper towel in each trap was removed, placed into a plastic storage bag and transported to the insectary facilities. Each paper towel was examined under the dissecting microscope for identification of Aedes species eggs. To confirm the species, the eggs were submerged in tap water for hatching and the larvae reared to adults under the conditions described above.
Mosquito identification: In the insectaries, once mature, all mosquitoes were killed by placing them at -20°C for 15 minutes. They were then sorted by genus (Aedes spp., Anopheles spp., or Culex spp.) and sex, using a standard identification key [22]. Further analyses described only detail data pertaining to Aedes spp. collected, with only Ae. aegypti specimens pooled and tested by methods described below.
Sample storage and transport for viral testing: A single leg was removed from each mosquito specimen and transferred to a 2mL Cryotube (Sarstedt, Rommelsdorf, Germany) with 0.5mL of RNA later (Sigma-Aldrich, St. Louis, MO) to preserve any viral nucleic acids. Samples were stored at -80°C until they were shipped to Stanford, CA, USA on dry ice for processing.
Collection of rainfall data
To monitor rainfall at each of our sampling sites, one rain gauge (HOBO Onset data loggers, Onset Computer Corporation 470 Bourne, MA USA), was installed at each of the four sites to collect daily rainfall. The sites were: Chulaimbo Sub-District Hospital (Chulaimbo) (0°02'16.5"S, 34°38'20.1"E), Jaramogi Oginga Odinga Teaching and Referral Hospital (Kisumu) (0°05'17.5"S, 34°46'13.6"E), Msambweni County Referral Hospital (Msambweni) (4°28′12″S, 39°28′48″E) and Diani Health Centre (Ukunda) (4°16'44.5"S, 39°35'25.0"E). Data from rain gauges were downloaded monthly.
Sample processing
Mosquito legs taken from mosquitoes that had been sorted by species, sex, trap type, and date of capture, were grouped into 714 pools of ~25 individual legs (range 1–36). Specimens of each type (sex etc.) were independently isolated, and all instruments were cleaned and decontaminated with 10% bleach solution between isolations to mitigate any potential for cross-contamination. Each pool of mosquito legs was then transferred into a 2.0 mL microcentrifuge tube with six 2 mm ceramic lysis beads (OMNI International GA USA) with 700 μL of MagMAX Lysis/Binding Solution Concentrate (Applied Biosystems, CA, USA). Samples were then homogenized in a Bead Ruptor 24 (OMNI International, GA, USA), according to the following program: Strength = 5.65, Time = 0:30, Cycles = 2, Delay = 0:05. Sample lysates were then centrifuged at 15,000 rpm for 6 minutes at room temperature to remove debris. Nucleic acid isolation was then performed using MAGMAX 96-Viral Isolation Kit (Life Technologies, CA, USA) according to the manufacturer’s instructions. The manufacturer’s decontamination protocol was performed after every run to prevent nucleic acid transfer between samples.
Molecular testing
Pooled legs of Aedes aegypti mosquitoes were tested for the presence of ZIKV, CHIKV, and DENV RNA with a triplex real-time reverse transcription PCR (rRT-PCR), as previously described [23]. Briefly, ZIKV-CHIKV-DENV rRT-PCR reactions were performed using the SuperScript III Platinum One-Step qRT-PCR kit (Life Technologies) with a total reaction volume of 25 μL and 5 μL of eluate. Reactions were performed on a Rotor-Gene Q instrument (Qiagen). Thresholds were set according to the published validation, and all exponential curves that crossed the threshold prior to cycle 45 were considered positive [24]. Negative controls containing no RNA template were included on every run of the assay, and amplicon curves were not observed in any of these controls. To validate our assay results, we performed monoplex PCR assays, using the same primer sets included in the multiplex reaction, on a subset of 18 CHIKV positive samples, and all 3 of the DENV positive samples to confirm infection.
Statistical analysis
Multi-way chi-square (χ2) analyses were performed to determine statistical differences among the levels of positivity for the recovered samples from different trap types. Maximum likelihood estimates of viral infection rates were calculated using the Poolscreen methodology developed by Katholi and Unnasch [25].
Results
Prevalence of infection
Between January 2014 and May 2016 mosquitoes were collected using multiple trapping methods at 4 study sites in Kenya; Chulaimbo (rural) and Kisumu (urban) in the west of the country, and Msambweni (rural) and Ukunda (urban) on the coast (Fig 1).
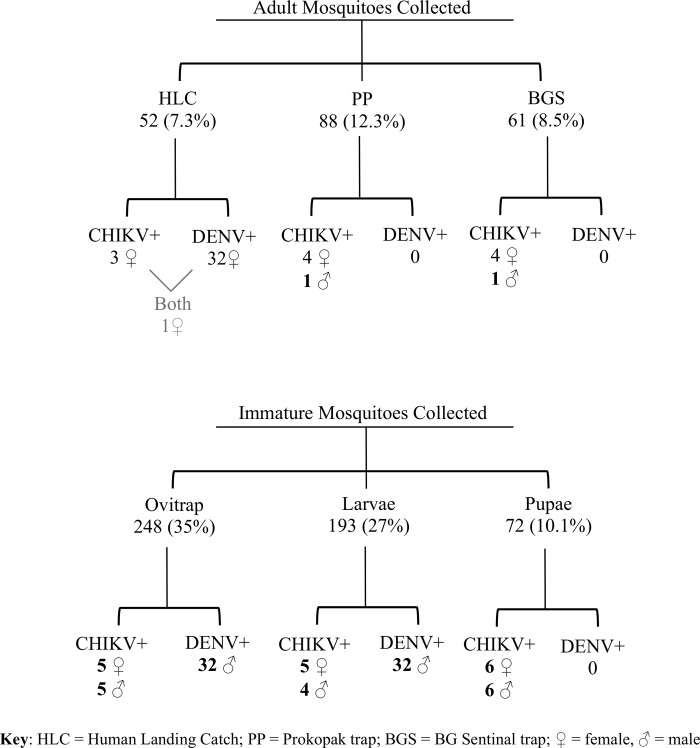
A total of 714 pools of Aedes aegypti species legs were tested. Of these 180 (25%) were caught in Chulaimbo, 123 (17%) in Kisumu, 178 (25%) in Msambweni, and 233 (33%) in Ukunda (Fig 2). The distribution of mosquito leg pools amongst each of the trap types was as follows: Ovitraps—248/714 pools (35.0%), Larval—193 (27.0%), Pupal—72 (10.1%), BGS—61 (8.5%), Prokopack—88 (12.3%) HLC—52 (7.3%) (Fig 2).
Fig 2. Flow chart describing pools of Ae. aegypti mosquito legs by age, sex, and virus detected.
All mosquito leg pools described were collected from four distinct study sites: Kisumu and Chulaimbo (urban and rural sites, respectively, in western Kenya), and Ukunda and Msambweni (urban and rural sites, respectively, in coastal Kenya) between January 2014 and May 2016.
Mosquito infection rates
Of the 714 pools tested, 43 (5.9%) were positive for CHIKV (Table 1, Fig 2) and 3 pools (0.4%) were positive for DENV, including 1 pool that was positive for both viruses (Table 2, Fig 2). ZIKV was not detected in in any mosquito pool tested in this study.
Table 1. Chikungunya.
Poolscreen estimates for the minimum infection rate for CHIKV [16]. Kisumu and Ukunda represent the urban sites for the West and Coast, respectively. Rural collection sites were located in Chulaimbo and Msambweni for the West and Coast, respectively.
| Sites | No. Pools | No. positive pools | Estimated infection rate (95% CI) |
|---|---|---|---|
| All | 714 | 43 | 0.49% (0.34–0.68%) |
| West | 321 | 17 | 0.33% (0.18–0.54%) |
| West rural | 180 | 9 | 0.28% (0.12–0.53%) |
| West urban | 123 | 8 | 0.42% (0.17–0.84%) |
| Coast | 411 | 25 | 0.70% (0.43–1.10%) |
| Coast rural | 178 | 13 | 0.85% (0.43–1.50%) |
| Coast urban | 233 | 12 | 0.58% (0.28–1.00%) |
| All rural | 369 | 22 | 0.46% (0.27–0.71%) |
| All urban | 347 | 20 | 0.51% (0.29–0.81%) |
Table 2. Dengue.
Poolscreen estimates for the minimum infection rate for DENV [16]. Kisumu and Ukunda represent the urban sites for the West and Coast, respectively. Rural collection sites were located in Chulaimbo and Msambweni for the West and Coast, respectively.
| Sites | No. pools | No. positive pools | Estimated infection rate (95% CI) |
|---|---|---|---|
| All | 714 | 3 | 0.03% (0.01–0.10%) |
| West | 321 | 0 | 0.00% (0.00–0.03%) |
| West rural | 180 | 0 | 0.00% (0.00–0.06%) |
| West urban | 123 | 0 | 0.00% (0.00–0.10%) |
| Coast | 395 | 3 | 0.08% (0.02–0.24%) |
| Coast rural | 178 | 2 | 0.13% (0.02–0.44%) |
| Coast urban | 233 | 1 | 0.05% (0.00–0.25%) |
| All rural | 369 | 2 | 0.04% (0.00–0.14%) |
| All urban | 347 | 1 | 0.03% (0.00–0.13%) |
Spatiotemporal distribution
Of the 45 arbovirus-positive pools 17/45 (37.7%) were from mosquitoes from western Kenya and 28/45 (62.2%) were from the coast. All three of the DENV positive mosquito samples were from the coastal sites. The maximum-likelihood CHIKV infection rate for the coast was 0.7% (95% CI 0.43–1.10%), compared to 0.33% (95% CI 0.18% - 0.54%) in the west (Table 1). The infection rates for both CHIKV and DENV on the coast exceeded the upper confidence intervals for infection rate in the west, confirming regional variation in the circulation of CHIKV and DENV. Although the rural study sites of Chulaimbo and Msambweni yielded more arbovirus-positive pools than their urbanized counterparts, the calculated infection rates did not significantly differ between rural and urban sites (Tables 1 and 2).
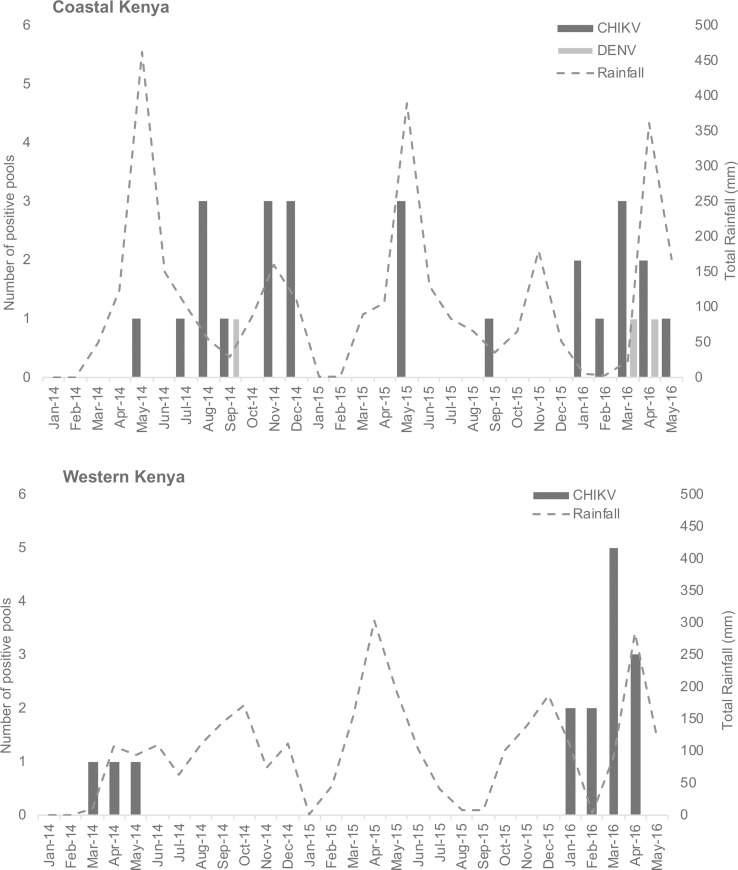
CHIKV was identified in pools of both immature and mature mosquitoes and across all years and seasons of the study (Fig 2). CHIKV and DENV were not detected in western Kenya in the latter half of 2014 nor in 2015, although the detection of CHIKV resumed in the early part of 2016. On the coast, both viruses were more frequently detected throughout the study, with peaks of infected mosquitoes roughly corresponding to peak periods of rainfall (Fig 3). Of note, 22/42 (52.3%) of the CHIKV positive pools and 2/3 (66.6%) of the DENV positive samples were collected between January and May 2016.
Fig 3. Temporal distribution of arbovirus positive mosquito leg pools and total rainfall across each study region.
Mosquitoes and rainfall data collected between January 2014 and May 2016. Further study site details within western and coastal Kenya can be found in Fig 1.
Life cycle stages
When grouped either as field-caught adult samples (those caught by BGS, HLC and Prokopack) or as immature form samples (caught by larval and pupal sampling or by ovitraps) the contribution of each group to the total number of positives is proportional to its size. Legs from adult mosquitoes accounted for 28% of the total sample pools tested (Table 3), and contributed 27.9% of all positive pools (Fig 2). Of the sample pools collected and tested, 72% were captured as immature mosquitoes and reared to adulthood prior to leg removal and pooling, contributing 72.1% of the positive pools. Due to the collection yield rates, adult pools were generally smaller (mean 7.2 individuals) than the overall study average pool size (15.2) and the immature form pool size (18.4). When expressed as a maximum likelihood infection rate [25], i.e., the estimated number of mosquitoes infected per 1,000 tested, the rate of CHIKV positivity was higher in adult mosquitoes (mean 61.8/1,000, range 30–100) than in immature mosquitoes (mean 9.0/1,000, range 2–24).
Table 3. The numbers of total Ae. aegypti pools caught by each collection method. The number of CHIKV positive pools of legs is shown in parentheses (). The number of DENV positives is shown in brackets [] The sex distribution of positive samples is given in the sub-rows.
* One of the pools of mosquito legs from female mosquitoes was caught by HLC was positive for both viruses.
| Collection type | No. of pools tested (Pos) | % of total pools | % of positive pools |
|---|---|---|---|
| BGS Sentinel | 61 (4) | 8.5 | 9.3 |
| Male | 1 | ||
| Female | 3 | ||
| Human Landing Catch | 52 (3) [32] | 7.3 | 9.3 |
| Male | 0 | ||
| Female | 3 [32] * | ||
| Prokopack Aspirator | 88 (4) | 12.3 | 9.3 |
| Male | 1 | ||
| Female | 3 | ||
| Ovitrap | 248 (10) [32] | 35.0 | 23.3 |
| Male | 5 [32] | ||
| Female | 5 | ||
| Larval Collection | 193 (9) [32] | 27.0 | 20.9 |
| Male | 4 [32] | ||
| Female | 5 | ||
| Pupal Collection | 72 (12) | 10.1 | 27.9 |
| Male | 6 | ||
| Female | 5 | ||
| Total | 714 (43) [3] | 100 | 100 |
Ovitrap samples accounted for 35% of all pools tested, but with a proportionally smaller number of positives (23.3%). Conversely, pools of legs collected from pupal mosquitoes reared to adulthood contributed only 10.1% to the specimen total, but had 27.9% of positives. Indeed, the positivity rate in the larval collection was significantly increased in pairwise comparisons to both ovitraps (χ2 = 7.90, p = 0.005) and larval collection (χ2 = 10.35, p = 0.001). Rates for ovitraps and collected larvae did not significantly differ (χ2 = 0.12, p = 0.74). Likewise, there were no significant differences among the adult groups (χ2 = 0.63, p = 0.73) and no differences in positivity rates among adults and larvae (χ2 = 1.73, p = 0.79).
In our adult mosquito samples, 9/11 (81.8%) of positive samples were bloodmeal-seeking female mosquitoes, with one positive male pool each from the BGS sentinel traps and the Prokopack aspirator trapping. No male mosquitoes caught by HLC were positive for arboviruses. However, in the immature form samples, arbovirus positivity was relatively evenly distributed between sexes (Fig 2).
Sample pools from each sex and lifecycle stage were found to be positive for both DENV and CHIKV. Of note, two of the three DENV positive pools were from male adult mosquitoes that had been reared in the laboratory from eggs or larvae (one pool each) collected in Msambweni, suggesting that DENV was vertically transmitted to these mosquitoes. The third DENV positive pool were adult female mosquitoes caught by HLC in Ukunda, suggesting that DENV was continuing to circulate at a low level at that time.
Discussion
Results of our study suggest that both DENV and CHIKV, but particularly CHIKV, remains in circulation at low levels in Kenyan vectors in both rural and urban settings. The viruses are maintained through all seasons, although peaks of infected mosquito abundance coincide with periods of increased rainfall. An association between rainfall and CHIKV incidence has been documented in other settings [26], but our detection of CHIKV in immature mosquitoes across all years and seasons of the study now indicates that this virus can be maintained by vertical transmission mechanisms during environmental conditions that are adverse for mosquito proliferation. While evidence of DENV TOT in Kenya has recently been described during epidemic times [7], the present study is the first to describe vertical transmission of CHIKV in wild Ae. aegypti mosquitoes and DENV during interepidemic times in Africa.
Extensive evidence for the maintenance of DENV and CHIKV by TOT mechanisms in endemic regions illustrates the stability of the DENV and CHIKV in wild mosquito populations (S1 Table). Evidence from experimental infections of Aedes spp. mosquitoes with CHIKV has demonstrated the potential for transovarial transmission of the virus [27–29]. Experimental evidence of TOT of CHIKV was debated in the 1980s [30], but has been repeatedly observed in laboratory settings with Aedes spp. (S1 Table) [27–29, 31–34]. The isolation of CHIKV viral RNA (but not viable virus) in artificially infected Aedes aegypti has been previously reported by Wong et al. [29]. In contrast Vazeille et al. reported on the absence of vertical transmission of the East/Central/South African (ECSA) strain of the virus in Aedes albopictus in their studies of experimental infections [35]. CHIKV TOT has been confirmed in wild mosquito populations recently, with a report by Jain et al. identifying CHIKV in field-caught immature Aedes aegypti mosquitoes in India [36], and a report by Thavara et al. finding CHIKV in Aedes aegypti and Aedes albopictus adult males in Thailand [37], both providing further evidence of vertical transmission in nature.
Our findings suggested the MIR for CHIKV was higher in adult mosquitoes as compared to immature mosquitoes. This might be expected if, in addition to vertical transmission of the virus (as suggested by our findings), adults may have also taken blood meals from CHIKV-positive humans. In addition, venereal infection of females by virus-positive male mosquitoes has been described as another potential route for infection of adult females [33]. Interestingly, the rate of infection of mosquitoes caught in larval collection was significantly higher than in the ovitraps. Bara et al. [38] suggest a mechanism of horizontal DENV transfer in Aedes mosquitoes, by which larvae may became infected by the ingestion of material from dead, vertically-infected immature mosquitoes or dead, infected adults [39, 40], yet such suggestions have yet to be documented with published data. In addition to its potential contribution to viral persistence, this mechanism may explain the proportionally increased rate of infection in our pupal samples.
We detected DENV in immature mosquito specimens and adult male pools, suggesting transovarial transmission of the virus in our samples. Thorough investigations have described DENV TOT in wild mosquitoes in a many affected geographic regions [7, 41–63] (S1 Table), suggesting an important role in the interepidemic maintenance of DENV. Although TOT mechanisms are thought not to be an important method of viral transmission of DENV in SE Asia and the Americas [15], the significant genetic divergence of Ae. aegypti mosquitoes from these regions compared to those in Africa has been documented [64]. Thus, it is possible that transovarial transmission mechanisms are of greater importance to DENV persistence in Africa compared to the rest of the tropics. Moreover, differences in the vertical transmission rate of DENV between Aedes aegypti and Aedes albopictus have been suggested previously [15], hence it is likely that that mosquito species also plays a defining role in the rate of vertical transmission of CHIKV. Future research comparing vertical transmission rates of different arboviruses in competent vector species could help to inform local vector and risk management policies. In future mosquito collections, we will endeavor to differentiate between different Aedes species in order to expand the understanding of species-specific TOT contributions to the global arboviral burden.
The increased presence of CHIKV in coastal Kenya, as observed in this study, is in agreement with previous studies that have shown high rates of alphavirus transmission in this region [65]. In addition, we only detected DENV-infected mosquitoes in the coastal study sites. This finding is in accord with previous epidemiologic findings on the regional variation in incidence of flavivirus infections in Kenya [12, 66–68]. During our 2014 to 2016 sampling period described in this study, we were also simultaneously collecting blood samples from child participants within acutely ill and healthy cohorts as a part of a larger, longitudinal study aiming to determine the incidence and prevalence of DENV and CHIKV in children in coastal and western Kenya, which has been previously described [10, 69]. Briefly, a primary diagnosis of acute DENV or CHIKV infection accounted for 24% (n = 93/385 participants) of the participants tested by RT-PCR [69]. These data specifically highlight the incidence of clinical CHIKV and DENV disease in Kenyan children, with a greater frequency of confirmed incident CHIKV cases from western Kenya than from the coast [69]. This is the opposite of the viral prevalence in mosquitoes that we have reported here. It is possible that CHIKV is persistently maintained in the mosquito population via transovarial transmission, particularly in regions where a larger proportion of the human population have some immunity to the virus. Moreover, in such regions of relatively higher immunity, local residents are hence less likely to present with symptomatic infections [70, 71]. We have previously noted that the presence of CHIKV in coastal Kenya may be misinterpreted by the presence of a closely related alphavirus, O’nyong’nyong virus [65], which is transmitted by anopheline mosquitoes [65], yet indistinguishable from CHIKV when utilizing common serological diagnostic tools due to antibody cross-reactivity [72]. Yet, repeated discovery of arboviruses in competent vectors and in human populations reiterates the public health importance of these findings, and the need for better integration into local vector control measures and community education.
Our study had limitations. An inherent sampling bias existed in our study due to the relative difficulty in capturing adult mosquitoes compared to eggs and larvae. We have, however adjusted for this by expressing our data proportionally and, where relevant, as minimum infective rate. As with all studies, it is possible that some variation was introduced by the different trap operators and entomologists at the different study sites. We minimized such effects by ensuring that all personnel were briefed and trained together, by rotating staff between the 4 sites, and by holding regular conference calls between all staff to ensure consistency of our protocols and methodologies. This study focused on the detection of viral RNA from pooled mosquito legs, and data presented in this manuscript is not representative of potential live virus that may have been contained within our samples. We were unable to recover viable CHIKV and DENV in the laboratory as the test samples were stored in a lysis buffer, which rendered them unsuitable for inoculation into cell culture. However, we did confirm the presence of CHIKV in a subset of our samples using individual monoplex reactions, using the same primers as used in the multiplex reaction. The template used in these confirmatory monoplex assays was generated in a separate reaction to that used for the multiplex assay. Additionally, initial mosquito specimen processing occurred in laboratories that did not contain any live virus, and molecular testing was conducted in a laboratory in which structural measures including a one-way work flow and separate room for nucleic acid isolation and detection are instituted to mitigate the risk of contamination. Thus, we are confident that our positive findings are not the result of cross-contamination. Further confirmation of vertical transmission of CHIKV is important to the elucidation of the mechanisms by which the virus is sustained outside of its human host. To achieve this, future research should involve the appropriate collection and storage of mosquito samples so that live virus, when present, can be isolated and characterized.
Our findings highlight the health implications of regional variation in arboviral circulation, and underscore the importance of understanding mechanisms of arboviral transmission to local risk assessment and healthcare management. Importantly, we have provided evidence that CHIKV and DENV are vertically transmitted in Ae. aegypti mosquitoes, likely facilitating the maintenance of baseline levels of virus in the study locations. Low-level circulation may explain the lack of seasonal patterns in CHIKV and DENV epidemics in Kenya, yet regularly occurring TOT is likely to contribute to local viral maintenance and a continuous, low-level incidence of human infections that establish a higher baseline immunity to these viruses within the local population.
Supporting information
NS = Not Specified.
(XLSX)
Acknowledgments
We would like to thank our field teams and entomologists in Kenya for the collection and identification of our mosquito samples.
Data Availability
All relevant data are available at https://purl.stanford.edu/tr890xw5305.
Funding Statement
This research was supported by National Institutes of Health (NIH) grant R01AI102918 (ADL). The funders had no role in the collection, analysis, or reporting of the data.
References
- 1.Okello GBA AN, Ouma J, Cherogony SC, Tukei PM, Ochieng W, Den Boer JW, Sanders EJ. Outbreak of yellow fever in Kenya. The Lancet. 1993;341(8843):489. [DOI] [PubMed] [Google Scholar]
- 2.Sanders EJ, Marfin AA, Tukei PM, Kuria G, Ademba G, Agata NN, et al. First recorded outbreak of yellow fever in Kenya, 1992–1993. I. Epidemiologic investigations. Am J Trop Med Hyg. 1998;59(4):644–9. 10.4269/ajtmh.1998.59.644 [DOI] [PubMed] [Google Scholar]
- 3.Sergon K, Njuguna C, Kalani R, Ofula V, Onyango C, Konongoi LS, et al. Seroprevalence of Chikungunya virus (CHIKV) infection on Lamu Island, Kenya, October 2004. Am J Trop Med Hyg. 2008;78(2):333–7. [PubMed] [Google Scholar]
- 4.Mwaengo D, Lorenzo G, Iglesias J, Warigia M, Sang R, Bishop RP, et al. Detection and identification of Rift Valley fever virus in mosquito vectors by quantitative real-time PCR. Virus Res. 2012;169(1):137–43. 10.1016/j.virusres.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 5.Woods CW, Karpati AM, Grein T, McCarthy N, Gaturuku P, Muchiri E, et al. An outbreak of Rift Valley fever in Northeastern Kenya, 1997–98. Emerg Infect Dis. 2002;8(2):138–44. 10.3201/eid0802.010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention. Rift Valley fever outbreak—Kenya, November 2006—January 2007. Morb Mortal Wkly Rep. 2007;56(4):73–6. [PubMed] [Google Scholar]
- 7.Lutomiah J, Barrera R, Makio A, Mutisya J, Koka H, Owaka S, et al. Dengue Outbreak in Mombasa City, Kenya, 2013–2014: Entomologic Investigations. PLoS Negl Trop Dis. 2016;10(10):e0004981 10.1371/journal.pntd.0004981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossi-Soyster EN, Cook EAJ, de Glanville WA, Thomas LF, Krystosik AR, Lee J, et al. Serological and spatial analysis of alphavirus and flavivirus prevalence and risk factors in a rural community in western Kenya. PLoS Negl Trop Dis. 2017;11(10):e0005998 10.1371/journal.pntd.0005998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudo ES, Ali S, Antonio VS, Chelene IR, Chongo I, Demanou M, et al. Seroepidemiological Studies of Arboviruses in Africa. Adv Exp Med Biol. 2018;1062:361–71. 10.1007/978-981-10-8727-1_25 [DOI] [PubMed] [Google Scholar]
- 10.Hortion J, Mutuku FM, Eyherabide AL, Vu DM, Boothroyd DB, Grossi-Soyster EN, et al. Acute Flavivirus and Alphavirus Infections among Children in Two Different Areas of Kenya, 2015. Am J Trop Med Hyg. 2019;100(1):170–3. 10.4269/ajtmh.18-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaBeaud AD, Sutherland LJ, Muiruri S, Muchiri EM, Gray LR, Zimmerman PA, et al. Arbovirus prevalence in mosquitoes, Kenya. Emerg Infect Dis. 2011;17(2):233–41. 10.3201/eid1702.091666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu DM, Banda T, Teng CY, Heimbaugh C, Muchiri EM, Mungai PL, et al. Dengue and West Nile Virus Transmission in Children and Adults in Coastal Kenya. Am J Trop Med Hyg. 2017;96(1):141–3. 10.4269/ajtmh.16-0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vu DM, Mutai N, Heath CJ, Vulule JM, Mutuku FM, Ndenga BA, et al. Unrecognized Dengue Virus Infections in Children, Western Kenya, 2014–2015. Emerg Infect Dis. 2017;23(11):1915–7. 10.3201/eid2311.170807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diniz DFA, de Albuquerque CMR, Oliva LO, de Melo-Santos MAV, Ayres CFJ. Diapause and quiescence: dormancy mechanisms that contribute to the geographical expansion of mosquitoes and their evolutionary success. Parasit Vectors. 2017;10(1):310 10.1186/s13071-017-2235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunnill M, Boots M. How important is vertical transmission of dengue viruses by mosquitoes (Diptera: Culicidae)? J Med Entomol. 2016;53(1):1–19. 10.1093/jme/tjv168 [DOI] [PubMed] [Google Scholar]
- 16.Ebert D, Herre EA. The evolution of parasitic diseases. Parasitol Today. 1996;12(3):96–101. 10.1016/0169-4758(96)80668-5 [DOI] [PubMed] [Google Scholar]
- 17.Lambrechts L, Scott TW. Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc Biol Sci. 2009;276(1660):1369–78. 10.1098/rspb.2008.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndenga BA, Mutuku FM, Ngugi HN, Mbakaya JO, Aswani P, Musunzaji PS, et al. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS One. 2017;12(12):e0189971 10.1371/journal.pone.0189971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngugi HN, Mutuku FM, Ndenga BA, Musunzaji PS, Mbakaya JO, Aswani P, et al. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasit Vectors. 2017;10(1):331 10.1186/s13071-017-2271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46(6):1256–9. 10.1603/033.046.0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chadee DD, Doon R, Severson DW. Surveillance of dengue fever cases using a novel Aedes aegypti population sampling method in Trinidad, West Indies: the cardinal points approach. Acta Trop. 2007;104(1):1–7. 10.1016/j.actatropica.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 22.Gillett J, Smith J. Common African mosquitoes and their medical importance. London: William Heinemann Medical Books Limited; 1972. [Google Scholar]
- 23.Waggoner JJ, Gresh L, Mohamed-Hadley A, Ballesteros G, Davila MJ, Tellez Y, et al. Single-Reaction Multiplex Reverse Transcription PCR for Detection of Zika, Chikungunya, and Dengue Viruses. Emerg Infect Dis. 2016;22(7):1295–7. 10.3201/eid2207.160326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waggoner JJ, Ballesteros G, Gresh L, Mohamed-Hadley A, Tellez Y, Sahoo MK, et al. Clinical evaluation of a single-reaction real-time RT-PCR for pan-dengue and chikungunya virus detection. J Clin Virol. 2016;78:57–61. 10.1016/j.jcv.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katholi CR, Unnasch TR. Important experimental parameters for determining infection rates in arthropod vectors using pool screening approaches. Am J Trop Med Hyg. 2006;74(5):779–85. [PubMed] [Google Scholar]
- 26.Wiwanitkit S aWV. Chikungunya virus infection and relationship to rainfall, the relationship study from southern Thailand. Journal of Arthropod-Borne Diseases. 2013;7(2):185–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal A, Dash PK, Singh AK, Sharma S, Gopalan N, Rao PV, et al. Evidence of experimental vertical transmission of emerging novel ECSA genotype of Chikungunya Virus in Aedes aegypti. PLoS Negl Trop Dis. 2014;8(7):e2990 10.1371/journal.pntd.0002990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chompoosri J, Thavara U, Tawatsin A, Boonserm R, Phumee A, Sangkitporn S, et al. Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes. Parasit Vectors. 2016;9:227 10.1186/s13071-016-1505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong HV, Vythilingam I, Sulaiman WY, Lulla A, Merits A, Chan YF, et al. Detection of Persistent Chikungunya Virus RNA but not Infectious Virus in Experimental Vertical Transmission in Aedes aegypti from Malaysia. Am J Trop Med Hyg. 2016;94(1):182–6. 10.4269/ajtmh.15-0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mourya DT. Absence of transovarial transmission of Chikungunya virus in Aedes aegypti & Ae. albopictus mosquitoes. Indian J Med Res. 1987;85:593–5. [PubMed] [Google Scholar]
- 31.Hailin Z YZ, Zhuqing M. Transovarial transmission of chikungunya virus in Aedes albopictus and Aedes aegypti mosquitoes. Chin J Virol. 1993;9:222–7. [Google Scholar]
- 32.Honorio NA, Wiggins K, Eastmond B, Camara DCP, Alto BW. Experimental Vertical Transmission of Chikungunya Virus by Brazilian and Florida Aedes Albopictus Populations. Viruses. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mavale M, Parashar D, Sudeep A, Gokhale M, Ghodke Y, Geevarghese G, et al. Venereal transmission of chikungunya virus by Aedes aegypti mosquitoes (Diptera: Culicidae). Am J Trop Med Hyg. 2010;83(6):1242–4. 10.4269/ajtmh.2010.09-0577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zytoon EM, el-Belbasi HI, Matsumura T. Transovarial transmission of chikungunya virus by Aedes albopictus mosquitoes ingesting microfilariae of Dirofilaria immitis under laboratory conditions. Microbiol Immunol. 1993;37(5):419–21. 10.1111/j.1348-0421.1993.tb03232.x [DOI] [PubMed] [Google Scholar]
- 35.Vazeille M, Mousson L, Failloux AB. Failure to demonstrate experimental vertical transmission of the epidemic strain of Chikungunya virus in Aedes albopictus from La Reunion Island, Indian Ocean. Mem Inst Oswaldo Cruz. 2009;104(4):632–5. 10.1590/s0074-02762009000400017 [DOI] [PubMed] [Google Scholar]
- 36.Jain J, Kushwah RBS, Singh SS, Sharma A, Adak T, Singh OP, et al. Evidence for natural vertical transmission of chikungunya viruses in field populations of Aedes aegypti in Delhi and Haryana states in India-a preliminary report. Acta Trop. 2016;162:46–55. 10.1016/j.actatropica.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 37.Thavara U, Tawatsin A, Pengsakul T, Bhakdeenuan P, Chanama S, Anantapreecha S, et al. Outbreak of chikungunya fever in Thailand and virus detection in field population of vector mosquitoes, Aedes aegypti (L.) and Aedes albopictus Skuse (Diptera: Culicidae). Southeast Asian J Trop Med Public Health. 2009;40(5):951–62. [PubMed] [Google Scholar]
- 38.Bara JJ, Clark TM, Remold SK. Susceptibility of larval Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to dengue virus. J Med Entomol. 2013;50(1):179–84. 10.1603/me12140 [DOI] [PubMed] [Google Scholar]
- 39.Miller BR, DeFoliart GR, Hansen WR, Yuill TM. Infection rates of Aedes triseriatus following ingestion of La Crosse virus by the larvae. Am J Trop Med Hyg. 1978;27(3):605–8. 10.4269/ajtmh.1978.27.605 [DOI] [PubMed] [Google Scholar]
- 40.Turell MJ, Linthicum KJ, Beaman JR. Transmission of Rift Valley fever virus by adult mosquitoes after ingestion of virus as larvae. Am J Trop Med Hyg. 1990;43(6):677–80. 10.4269/ajtmh.1990.43.677 [DOI] [PubMed] [Google Scholar]
- 41.Angel A, Angel B, Joshi V. Rare occurrence of natural transovarial transmission of dengue virus and elimination of infected foci as a possible intervention method. Acta Trop. 2016;155:20–4. 10.1016/j.actatropica.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 42.Angel B, Joshi V. Distribution and seasonality of vertically transmitted dengue viruses in Aedes mosquitoes in arid and semi-arid areas of Rajasthan, India. J Vector Borne Dis. 2008;45(1):56–9. [PubMed] [Google Scholar]
- 43.Arunachalam N TS, Thenmozhi V, Rajendran R, Paramasivan R, Manavalan R, Ayanar K, Tyagi BK. Natural vertical transmission of dengue viruses by Aedes aegypti in Chennai, Tamil Nadu, India. Indian J Med Res. 2008;127(4):395–7. [PubMed] [Google Scholar]
- 44.Cecilio SG, Junior WF, Totola AH, de Brito Magalhaes CL, Ferreira JM, de Magalhaes JC. Dengue virus detection in Aedes aegypti larvae from southeastern Brazil. J Vector Ecol. 2015;40(1):71–4. 10.1111/jvec.12134 [DOI] [PubMed] [Google Scholar]
- 45.Cruz LC, Serra OP, Leal-Santos FA, Ribeiro AL, Slhessarenko RD, Santos MA. Natural transovarial transmission of dengue virus 4 in Aedes aegypti from Cuiaba, State of Mato Grosso, Brazil. Rev Soc Bras Med Trop. 2015;48(1):18–25. 10.1590/0037-8682-0264-2014 [DOI] [PubMed] [Google Scholar]
- 46.da Costa CF, Dos Passos RA, Lima JBP, Roque RA, de Souza Sampaio V, Campolina TB, et al. Transovarial transmission of DENV in Aedes aegypti in the Amazon basin: a local model of xenomonitoring. Parasit Vectors. 2017;10(1):249 10.1186/s13071-017-2194-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Figueiredo ML, de CGA, Amarilla AA, de SLA, de SOA, de Araujo RF, et al. Mosquitoes infected with dengue viruses in Brazil. Virol J. 2010;7:152 10.1186/1743-422X-7-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutta P, Khan SA, Chetry S, Dev V, Sarmah CK, Mahanta J. First evidence of dengue virus infection in wild caught mosquitoes during an outbreak in Assam, Northeast India. J Vector Borne Dis. 2015;52(4):293–8. [PubMed] [Google Scholar]
- 49.Guedes DR, Cordeiro MT, Melo-Santos MA, Magalhaes T, Marques E, Regis L, et al. Patient-based dengue virus surveillance in Aedes aegypti from Recife, Brazil. J Vector Borne Dis. 2010;47(2):67–75. [PubMed] [Google Scholar]
- 50.Günther J, Martinez-Munoz JP, Perez-Ishiwara DG, Salas-Benito J. Evidence of vertical transmission of dengue virus in two endemic localities in the state of Oaxaca, Mexico. Intervirology. 2007;50(5):347–52. 10.1159/000107272 [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez-Bugallo G, Rodriguez-Roche R, Diaz G, Vazquez AA, Alvarez M, Rodriguez M, et al. First record of natural vertical transmission of dengue virus in Aedes aegypti from Cuba. Acta Trop. 2017;174:146–8. 10.1016/j.actatropica.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 52.Hull B, Tikasingh E, de Souza M, Martinez R. Natural transovarial transmission of dengue 4 virus in Aedes aegypti in Trinidad. Am J Trop Med Hyg. 1984;33(6):1248–50. 10.4269/ajtmh.1984.33.1248 [DOI] [PubMed] [Google Scholar]
- 53.Khin MM, Than KA. Transovarial transmission of dengue 2 virus by Aedes aegypti in nature. Am J Trop Med Hyg. 1983;32(3):590–4. 10.4269/ajtmh.1983.32.590 [DOI] [PubMed] [Google Scholar]
- 54.Martinez NE, Dzul-Manzanilla F, Gutierrez-Castro C, Ibarra-Lopez J, Bibiano-Marin W, Lopez-Damian L, et al. Natural vertical transmission of dengue-1 virus in Aedes aegypti populations in Acapulco, Mexico. J Am Mosq Control Assoc. 2014;30(2):143–6. 10.2987/14-6402.1 [DOI] [PubMed] [Google Scholar]
- 55.Martins VE, Alencar CH, Kamimura MT, de Carvalho Araujo FM, De Simone SG, Dutra RF, et al. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceara, Brazil. PLoS One. 2012;7(7):e41386 10.1371/journal.pone.0041386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohani A, Aidil Azahary AR, Malinda M, Zurainee MN, Rozilawati H, Wan Najdah WM, et al. Eco-virological survey of Aedes mosquito larvae in selected dengue outbreak areas in Malaysia. J Vector Borne Dis. 2014;51(4):327–32. [PubMed] [Google Scholar]
- 57.Serufo JC, de Oca HM, Tavares VA, Souza AM, Rosa RV, Jamal MC, et al. Isolation of dengue virus type 1 from larvae of Aedes albopictus in Campos Altos city, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1993;88(3):503–4. 10.1590/s0074-02761993000300025 [DOI] [PubMed] [Google Scholar]
- 58.Thavara U, Siriyasatien P, Tawatsin A, Asavadachanukorn P, Anantapreecha S, Wongwanich R, et al. Double infection of heteroserotypes of dengue viruses in field populations of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and serological features of dengue viruses found in patients in southern Thailand. Southeast Asian J Trop Med Public Health. 2006;37(3):468–76. [PubMed] [Google Scholar]
- 59.Thenmozhi V, Tewari SC, Manavalan R, Balasubramanian A, Gajanana A. Natural vertical transmission of dengue viruses in Aedes aegypt in southern India. Trans R Soc Trop Med Hyg. 2000;94(5):507 10.1016/s0035-9203(00)90067-1 [DOI] [PubMed] [Google Scholar]
- 60.Thongrungkiat S, Maneekan P, Wasinpiyamongkol L, Prummongkol S. Prospective field study of transovarial dengue-virus transmission by two different forms of Aedes aegypti in an urban area of Bangkok, Thailand. J Vector Ecol. 2011;36(1):147–52. 10.1111/j.1948-7134.2011.00151.x [DOI] [PubMed] [Google Scholar]
- 61.Thongrungkiat S, Wasinpiyamongkol L, Maneekan P, Prummongkol S, Samung Y. Natural transovarial dengue virus infection rate in both sexes of dark and pale forms of Aedes aegypti from an urban area of Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2012;43(5):1146–52. [PubMed] [Google Scholar]
- 62.Velandia-Romero ML, Olano VA, Coronel-Ruiz C, Cabezas L, Calderon-Pelaez MA, Castellanos JE, et al. Dengue virus detection in Aedes aegypti larvae and pupae collected in rural areas of Anapoima, Cundinamarca, Colombia. Biomedica. 2017;37(0):193–200. 10.7705/biomedica.v37i0.3584 [DOI] [PubMed] [Google Scholar]
- 63.Zeidler JD, Acosta PO, Barreto PP, Cordeiro Jda S. Dengue virus in Aedes aegypti larvae and infestation dynamics in Roraima, Brazil. Rev Saude Publica. 2008;42(6):986–91. 10.1590/s0034-89102008005000055 [DOI] [PubMed] [Google Scholar]
- 64.Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, Bossin H, et al. Worldwide patterns of genetic differentiation imply multiple 'domestications' of Aedes aegypti, a major vector of human diseases. Proc Biol Sci. 2011;278(1717):2446–54. 10.1098/rspb.2010.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LaBeaud AD, Banda T, Brichard J, Muchiri EM, Mungai PL, Mutuku FM, et al. High rates of o'nyong nyong and Chikungunya virus transmission in coastal Kenya. PLoS Negl Trop Dis. 2015;9(2):e0003436 10.1371/journal.pntd.0003436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Konongoi L, Ofula V, Nyunja A, Owaka S, Koka H, Makio A, et al. Detection of dengue virus serotypes 1, 2 and 3 in selected regions of Kenya: 2011–2014. Virol J. 2016;13(1):182 10.1186/s12985-016-0641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mease LE, Coldren RL, Musila LA, Prosser T, Ogolla F, Ofula VO, et al. Seroprevalence and distribution of arboviral infections among rural Kenyan adults: a cross-sectional study. Virol J. 2011;8:371 10.1186/1743-422X-8-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutherland LJ, Cash AA, Huang YJ, Sang RC, Malhotra I, Moormann AM, et al. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg. 2011;85(1):158–61. 10.4269/ajtmh.2011.10-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waggoner J, Brichard J, Mutuku F, Ndenga B, Heath CJ, Mohamed-Hadley A, et al. Malaria and chikungunya detected using molecular diagnostics among febrile Kenyan children. Open Form Infect Dis. 2017;4(3):oxf110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Queyriaux B, Simon F, Grandadam M, Michel R, Tolou H, Boutin JP. Clinical burden of chikungunya virus infection. Lancet Infect Dis. 2008;8(1):2–3. 10.1016/S1473-3099(07)70294-3 [DOI] [PubMed] [Google Scholar]
- 71.Sissoko D, Moendandze A, Malvy D, Giry C, Ezzedine K, Solet JL, et al. Seroprevalence and risk factors of chikungunya virus infection in Mayotte, Indian Ocean, 2005–2006: a population-based survey. PLoS One. 2008;3(8):e3066 10.1371/journal.pone.0003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pezzi L, Reusken CB, Weaver SC, Drexler JF, Busch M, LaBeaud AD, et al. GloPID-R report on Chikungunya, O'nyong-nyong and Mayaro virus, part I: Biological diagnostics. Antiviral Res. 2019;166:66–81. 10.1016/j.antiviral.2019.03.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NS = Not Specified.
(XLSX)
Data Availability Statement
All relevant data are available at https://purl.stanford.edu/tr890xw5305.