Abstract
Background/Aims
Disaccharidase assay is used for assessing carbohydrate intolerance in children, but its usefulness in adults is not known. The aim of this study is to assess the prevalence of disaccharidase deficiency in patients with unexplained gastrointestinal symptoms.
Methods
A retrospective review of adults with chronic (> 1 year) abdominal symptoms and negative imaging and endoscopy/colonoscopy and who completed bowel symptom questionnaire and duodenal biopsy for lactase, maltase, sucrase, and palatinase was performed. A subset also underwent 25 g lactose breath test (LBT).
Results
One hundred twenty patients (females = 83) were evaluated, of whom 48 also underwent LBT. Fifty-six (46.7%) patients had enzyme deficiency; 44 (36.7%) had single (either lactase or maltase), 1 had 3 enzyme deficiencies, 11 (9.2 %) had all 4 disaccharidase enzyme (pan-disaccharidase) deficiency, and 64 (53.0%) had normal enzyme levels. Baseline prevalence and severity of 11 gastrointestinal symptoms were similar between normal and single enzyme deficiency groups. The sensitivity and specificity of LBT was 78.3% and 72.0%, respectively and overall agreement with lactase deficiency was 75.0%.
Conclusions
Isolated disaccharidase deficiency occurs in adults, usually lactase and rarely maltase, and pan-disaccharidase deficiency is rare. Baseline symptoms or its severity did not predict enzyme deficiency.
Keywords: Breath tests, Disaccharidase, Lactose intolerance, Sucrase-isomaltase deficiency
Introduction
After oral ingestion, dietary carbohydrates are digested and absorbed from the gastrointestinal (GI) tract, and provide 40-60% of the average caloric intake in humans.1 The main carbohydrates ingested in the human diet are starch, sucrose, and lactose and these sugars are broken down into monosaccharides by various enzymes, including disaccharidases (carbohydrate-specific hydrolases) that are produced by the brush-border membrane and enterocytes lining the small intestine.1
Disaccharidase deficiency can cause malabsorption of carbohydrates. The unabsorbed sugars can serve as an osmotic load in the small bowel, drawing fluid into the lumen and leading to intestinal distension and rapid propulsion into the colon. This can produce diarrhea, gas, bloating, flatulence, and abdominal pain which are symptoms typically reported by patients with carbohydrate intolerance. Over the last decade, measurement of disaccharidase levels, notably lactase, sucrase, maltase, and palatinase from small bowel biopsies obtained during an upper endoscopy, has been increasingly used to assess disccharidase deficiency, particularly in children.2
The incidence of sucrase-isomaltase deficiency has been estimated at 0.2% in North America and up to 10.0% in Greenland Eskimos.3 In contrast, the incidence of lactase deficiency varies between 15.0-80.0%, depending on the ethnicity of the population.4 While carbohydrate intolerance is a well-established but rare cause of chronic abdominal pain in young children, its frequency and associated symptoms in adults, especially non-lactase deficiency has not been well described. Furthermore, the problem of carbohydrate malabsorption and/or intolerance is important because of the rising consumption of sugars in the United States.5-7 Given the apparent lack of compensatory enzyme production, the clinical implications of disaccharidase deficiency in patients with unexplained abdominal pain may be significant.6 It is possible that disaccharidase deficiency may have been under-recognized in the United States, particularly in adults, but there has been no systematic assessment of its prevalence or symptom profiles.
Our aims were 3-fold: (1) to examine the clinical utility of disaccharidase testing in patients presenting with unexplained GI symptoms; (2) to correlate GI symptoms with enzyme deficiency; and (3) to assess the diagnostic correlation of lactase enzyme deficiency with the lactose breath test (LBT).
Materials and Methods
We retrospectively examined the medical records of patients who underwent esophagogastroduodenoscopy with small bowel biopsies for disaccharidase testing that were performed by 2 gastroenterologists, and data were extracted from chart review and collated into an Excel spreadsheet. Our study population included 120 adult patients, at least 18 years of age, who presented to the Digestive Health Center at Augusta University Medical Center over 3 years, with at least 1 year of unexplained upper and lower GI symptoms, including abdominal pain, bloating, nausea and vomiting. All of these patients had normal upper and lower endoscopy with biopsy, normal abdominal computed axial topography scan, normal stool tests and normal hematology and biochemistry profile. All patients completed a 11-symptom questionnaire to record baseline GI symptoms.7 Patients with a history of upper GI surgery, including small bowel resections, known celiac disease or olmesartan use were excluded from the study.
Additionally, a subset of patients (n = 48) who underwent LBT, in addition to disaccharidase assay were also included. All of these patients had a glucose breath test to evaluate small intestinal bacterial overgrowth, and only if glucose breath test was negative, they had lactose breath test. In this subgroup, we performed a correlative assessment of breath testing with lactase deficiency, and assessed the sensitivity, specificity, positive and negative predictive value, and agreement of LBT with lactase deficiency. The study was approved by IRB number 993793-2.
Disaccharidase Assay
Two biopsy specimens were obtained from the second portion of the duodenum for disaccharidase testing. The tissue fragments were placed in a dry, empty specimen container. Tissue samples were sent to Kaleida Health Children’s Hospital Laboratory, Buffalo, NY, USA. The biopsies were analyzed for levels of sucrase, maltase, lactase, and palatinase levels using the Dahlqvist method as follows: an intestinal homogenate is incubated with the appropriate disaccharide.8,9 The disaccharidase activity is then interrupted by the addition of Tris, and the glucose liberated is measured with a glucose oxidase reagent.8,9 Disaccharidase activity is then expressed as micromoles of disaccharide hydrolyzed per minute per gram of protein.8,9 Normative data from this laboratory were based on fasting levels of disaccharidase levels (lactase > 15.0 μM/min/g, sucrase > 25.0 μM/min/g, maltase > 100.0 µM/min/g and palatinase > 5.0 µM/min/g) and these ranges were used to determine whether enzyme deficiency was present in our patients.10 Enzyme values that were below the normal cutoff were considered diagnostic of one or more disaccharidase deficiency. Previous studies of disaccharidase levels have also relied on this assay and normative data.10-12
Lactose Breath Testing
After a 12-hour overnight fast, patients ingested a mixture of 25 g lactose dissolved in 250 mL water. End expiratory alveolar breath samples were collected at baseline and at 30-minute intervals after ingestion of lactose over 5 hours and analyzed for hydrogen and methane levels (QuinTron Instrument Company Inc, Milwaukee, WI, USA). Baseline symptom profiles as well as symptoms experienced during the test were recorded on validated questionnaires.7 An increase of 20 ppm of hydrogen or 15 ppm of methane or a combined increase of at least 20 ppm of hydrogen and methane above baseline values were considered positive breath test for lactose intolerance.13
Measurements and Statistical Methods
The prevalence of one or more abnormal disaccharidase levels were assessed. We also compared the prevalence of proton pump inhibitor use, diabetes, previous GI surgery and patient symptom profiles with the presence or absence of disaccharidase deficiency. Baseline abdominal symptom prevalence and their severity were also assessed and compared between the normal enzyme, single deficiency, and pan disaccharidase deficiency groups using ANOVA test with Bonferroni correction. Kruskal-Wallis test was used to test the differences in age and body mass index as they were not normally distributed. Sensitivity, specificity, positive and negative predictive values for the LBT, and agreement between LBT and lactase mucosal assay results were calculated and compared. All statistical analyses were performed in SAS version 9.4 (SAS institute, Cary, NC, USA).
Results
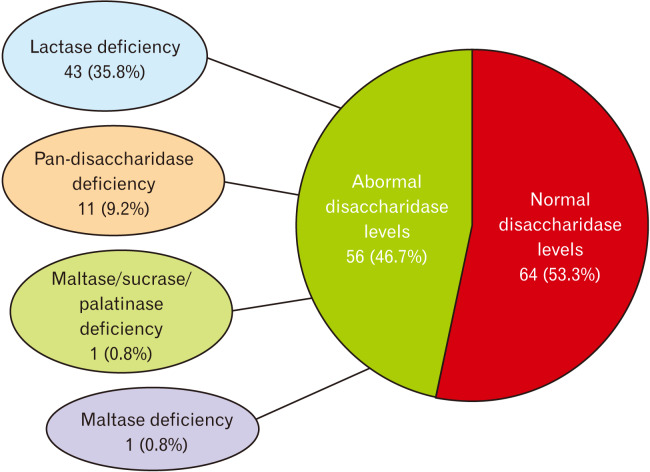
A total of 120 patients were included in this analysis. Their mean age was 48.1 years (20-67), and 83 (69.2%) patients were females. There were no demographic differences between normal and disaccharidase deficient cohorts, and this included the presence of diabetes, proton pump inhibitor use and previous surgery (Table 1). We found that 56/120 (46.7%) patients had one or more disaccharidase enzyme deficiency (Fig. 1). Of these, 44 were deficient in only one enzyme: isolated lactase deficiency was seen in 43 (35.8%) patients and maltase in 1 (0.8%) patient. Eleven subjects (9.2%) were deficient in all 4 disaccharidases that were assayed and were categorized as pan-disaccharidase deficiency (Fig. 1). One patient exhibited combined maltase, sucrase, and palatinase deficiency (0.8%).
Table 1.
Baseline Characteristics of Normal Enzyme vs Single Disaccharidase Enzyme Deficient vs Pan-disaccharidase Enzyme Deficient Patients
| Characteristics | Total (N = 120 [100.0%]) | Normal (n = 64 [53.3%]) | Pan-disaccharidase (n = 11 [9.2%]) | Single deficiency (n = 45 [37.5%])a | Raw P-value | Bonferroni adjustment |
|---|---|---|---|---|---|---|
| Gender | 0.478 | |||||
| Female | 83 (69.2) | 44 (68.8) | 6 (54.6) | 33 (73.3) | ||
| Male | 37 (30.8) | 20 (31.3) | 5 (45.5) | 12 (26.7) | ||
| Pain | 0.027 | 0.324 | ||||
| Yes | 93 (77.5) | 51 (79.7) | 5 (45.5) | 37 (82.2) | ||
| No | 27 (22.5) | 13 (20.3) | 6 (54.6) | 8 (17.8) | ||
| Cramping | 0.012 | 0.144 | ||||
| Yes | 71 (59.2) | 42 (65.6) | 2 (18.2) | 27 (60.0) | ||
| No | 49 (40.8) | 22 (34.4) | 9 (81.8) | 18 (40.0) | ||
| Bloating | 0.838 | > 0.99 | ||||
| Yes | 99 (82.5) | 54 (84.4) | 9 (81.8) | 36 (80.0) | ||
| No | 21 (17.5) | 10 (15.6) | 2 (18.2) | 9 (20.0) | ||
| Fullness | 0.030 | 0.360 | ||||
| Yes | 85 (70.8) | 47 (73.4) | 4 (36.4) | 34 (75.6) | ||
| No | 35 (29.2) | 17 (26.6) | 7 (63.6) | 11 (24.4) | ||
| Nausea | 0.070 | 0.840 | ||||
| Yes | 69 (57.5) | 41 (64.1) | 3 (27.3) | 25 (55.6) | ||
| No | 51 (42.5) | 23 (35.9) | 8 (72.7) | 20 (44.4) | ||
| Belching | 0.050 | 0.600 | ||||
| Yes | 61 (50.8) | 37 (57.8) | 2 (18.2) | 22 (48.9) | ||
| No | 59 (49.2) | 27 (42.2) | 9 (81.8) | 23 (51.1) | ||
| Indigestion | 0.003 | 0.036 | ||||
| Yes | 69 (57.5) | 41 (64.1) | 1 (9.1) | 27 (60.0) | ||
| No | 51 (42.5) | 23 (35.9) | 10 (90.9) | 18 (40.0) | ||
| Diarrhea | 0.071 | 0.852 | ||||
| Yes | 71 (59.2) | 41 (64.1) | 3 (27.3) | 27 (60.0) | ||
| No | 49 (40.8) | 23 (35.9) | 8 (72.7) | 18 (40.0) | ||
| Gas | 0.053 | 0.636 | ||||
| Yes | 90 (75.0) | 51 (79.7) | 5 (45.5) | 34 (75.6) | ||
| No | 30 (25.0) | 13 (20.3) | 6 (54.6) | 11 (24.4) | ||
| Constipation | 0.931 | > 0.99 | ||||
| Yes | 61 (50.8) | 33 (51.6) | 6 (54.6) | 22 (48.9) | ||
| No | 59 (49.2) | 31 (48.4) | 5 (45.5) | 23 (51.1) | ||
| Vomiting | 0.807 | > 0.99 | ||||
| Yes | 43 (35.8) | 24 (37.5) | 3 (27.3) | 16 (35.6) | ||
| No | 77 (64.2) | 40 (62.5) | 8 (72.7) | 29 (64.4) | ||
| Weight loss | 0.480 | > 0.99 | ||||
| Yes | 10 (8.3) | 5 (7.8) | 3 (27.3) | 2 (4.4) | ||
| No | 110 (91.7) | 59 (92.2) | 8 (72.7) | 43 (95.6) | ||
| PPI | 0.090 | 0.807 | ||||
| Yes | 53 (44.2) | 33 (51.5) | 2 (18.2) | 18 (40.0) | ||
| No | 67 (55.8) | 31 (48.4) | 9 (81.8) | 27 (60.0) | ||
| Diabetes | 0.649 | 0.807 | ||||
| Yes | 20 (16.7) | 10 (15.6) | 1 (9.1) | 9 (20.0) | ||
| No | 100 (83.3) | 54 (84.4) | 10 (90.9) | 36 (80.0) | ||
| Prior GI surgery | 0.070 | 0.807 | ||||
| Yes | 31 (25.8) | 22 (34.4) | 2 (18.2) | 7 (15.6) | ||
| No | 89 (74.2) | 42 (65.6) | 9 (81.8) | 38 (84.4) | ||
| Age (yr) | 49.5 (34.0-63.0) | 46.5 (30.5-59.5) | 42.0 (41.0-65.0) | 52.0 (36.0-65.0) | 0.223 | |
| BMI (kg/m2) | 26.4 (21.0-31.1) | 26.4 (20.8-30.8) | 24.7 (18.7-37.1) | 26.6 (21.8-31.2) | 0.977 | |
We included 1 patient with maltase, sucrase, and palatinase deficiency in this group.
PPI, proton pump inhibitor; GI, gastrointestinal; BMI, body mass index.
Data are presented as number (%) or median (interquartile range).
Figure 1.
The prevalence of normal and abnormal disaccharidase enzyme levels.
Prevalance of Gastrointestinal Symptoms With Disaccharidase Deficiency
The prevalence of baseline symptoms in all 120 patients were as follows: bloating (82.5%), pain (77.5%), gas (75.0%), fullness (70.8%), cramping (59.1%), diarrhea (59.1%), nausea (57.5%), indigestion (57.5%), belching (50.8%), vomiting (35.8%), and weight loss (8.3%). Patients with normal enzymes (64/120, 53.3%) shared a similar baseline symptom profile to the total patient population (Table 1); bloating (84.4%), pain (79.7%), gas (79.7%), fullness (73.4%), cramping (65.6%), diarrhea (64.0%), nausea (64.1%), indigestion (64.1%), belching (57.8%), vomiting (37.5%), and weight loss (7.8%). Fewer patients with normal enzymes reported vomiting (37.5%) and weight loss (7.8%) (Table 1). Likewise, single disaccharidase deficient patients (45/120) also had similar symptom(s) prevalence; abdominal pain (82.2%), bloating (80.0%), fullness (75.6%), gas (75.6%), indigestion (60.0%), cramping (60.0%), and nausea (55.6%) (Table 1). Single disaccharidase deficient patients reported less vomiting (35.6%) and weight loss (4.4%). The prevalences of various baseline symptoms were not statistically different between subjects with normal enzymes and those with single enzyme deficiency (Table 1). Also, we examined the severity of each symptom and found that the mean symptom severity scores were not significantly different between the normal enzyme, single, and pan-disaccharidase deficient groups (Table 2).
Table 2.
Baseline Symptom Severity of Patients With Normal Enzymes vs Single Disaccharidase Deficiency vs Pan-disaccharidase Deficiency Patients
| Symptoms | Total (N = 120 [100.0%]) | Normal (n = 64 [53.3%]) | Pan-disaccharidase (n = 11 [9.2%]) | Single deficiency (n = 45 [37.5%])a | Raw P-value | Bonferroni adjustment |
|---|---|---|---|---|---|---|
| Pain | 6.1 (3.1) | 6.5 (2.7) | 4.7 (4.0) | 5.8 (3.4) | 0.207 | > 0.99 |
| Cramping | 4.7 (3.4) | 5.2 (3.3) | 4.3 (3.8) | 4.0 (3.4) | 0.251 | > 0.99 |
| Bloating | 6.3 (3.2) | 6.5 (2.9) | 6.3 (3.7) | 6.1 (3.5) | 0.851 | > 0.99 |
| Fullness | 6.1 (3.3) | 6.3 (3.2) | 5.9 (3.3) | 5.9 (3.6) | 0.865 | > 0.99 |
| Nausea | 4.6 (3.2) | 5.3 (3.2) | 2.4 (2.5) | 4.1 (3.2) | 0.023 | 0.253 |
| Belching | 4.1 (3.2) | 4.5 (3.3) | 3.9 (2.8) | 3.6 (3.2) | 0.432 | > 0.99 |
| Indigestion | 4.8 (3.5) | 5.2 (3.3) | 2.1 (3.2) | 4.8 (3.7) | 0.069 | 0.759 |
| Diarrhea | 3.9 (3.4) | 4.3 (3.4) | 3.1 (3.7) | 3.5 (3.5) | 0.469 | > 0.99 |
| Gas | 5.6 (3.0) | 5.9 (2.7) | 5.0 (3.3) | 5.2 (3.3) | 0.522 | > 0.99 |
| Constipation | 4.8 (3.6) | 5.3 (3.5) | 4.3 (4.4) | 4.1 (3.7) | 0.329 | > 0.99 |
| Vomiting | 2.0 (2.9) | 2.8 (3.2) | 0.3 (1.0) | 1.2 (2.2) | 0.010 | 0.110 |
We included 1 patient with maltase, sucrase, and palatinase deficiency in this group.
Data are presented as mean (SD).
Pan-disaccharidase Deficiency Group
The most common symptoms reported by the pan-disaccharidase deficiency cohort were bloating (81.8%) and constipation (54.5%) at baseline (Table 1). However, they reported relatively less cramping (18.2%), pain (54.5%), fullness (36.4%), nausea (7.3%), belching (18.2%), diarrhea (27.3%), and gas (45.5%) when compared to both normal and single disaccharidase deficient patients, but these data were not significant. They also reported more weight loss (27.3%) when compared to single disaccharidase deficient patient (4.4%) or normal group (7.8%) but this was not significant.
Diagnostic Performance of Lactose Breath Test
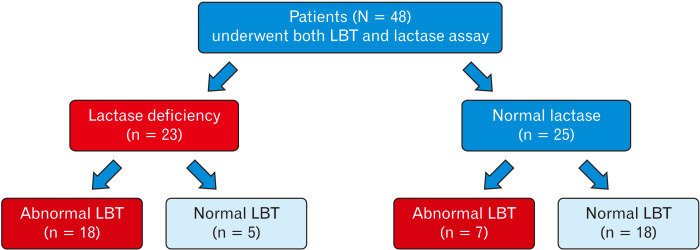
Of the 48 patients who had both tests, 23 (47.9%) had decreased lactase enzyme levels suggesting lactase deficiency, and in this group, 18/23 (78.3%) had abnormal LBT (Fig. 2). In the normal lactase enzyme group, 7/25 (28.0%) had abnormal LBT (Fig. 2). The overall diagnostic agreement between lactase deficiency and lactose breath test was 75.0%, sensitivity was 78.3%, and specificity was 72.0%. The positive predictive value was 72.0% and negative predictive value was 78.3%. These data suggest good diagnostic agreement, specificity, and sensitivity for the LBT.
Figure 2.
Flow chart of patients who underwent both the lactose breath test (LBT) and the lactase enzyme deficiency assay testing.
Discussion
Although disaccharidase deficiency has been reported in children,2,3,6 its prevalence in adults has not been systematically assessed. We found that lactase was the most common enzyme deficiency with a prevalence of 35.8% that was comparable to the pediatric study. Interestingly, the next highest prevalence of disccharidase deficiency (9.2%) involved all 4 enzymes and these subjects were categorized as pan-disaccharidase deficient. Previous pediatric studies also described a similar prevalence of pan-disaccharidase deficiency,2,3,6 although 1 pediatric study reported a prevalence of 44.7% for lactase, 7.6% for sucrase, 3.5% for sucrase-isomaltase, and 3.2% for pan-disaccharidase deficiency.14 Interestingly, they found a significant positive correlation between disaccharidase testing frequency and the number of disaccharidase deficiencies found,14 suggesting that increased awareness and testing for these deficiencies may be useful in patients with unexplained gastrointestinal symptoms who may otherwise be labeled as having irritable bowel syndrome; the same may be true in adults. Also, even in children, aside from lactase deficiency, other single disaccharidase deficiencies are rare.15
In our cohort, pan-disaccharidase deficient patients reported a higher prevalence of weight loss than single disaccharidase deficient patients, but a larger cohort is required to evaluate its significance. Interestingly, they had less cramping, fullness, belching, and indigestion compared to patients with normal enzymes although not significant. In contrast, chronic abdominal pain was an important feature in pediatric studies of pan-disaccharidase deficiency,6 whereas this was seen in 45.5% of our pan-disaccharidase population and similar to those with normal enzyme levels. The prevalence of abdominal symptoms could not differentiate between those with or without enzyme deficiency.
Carbohydrate malabsorption has been implicated in the pathogenesis of chronic abdominal pain and also in the pathogenesis of irritable bowel syndrome, unexplained gas, and bloating in adults.6,7,16-19 The enterocytes lining the intestinal mucosa form the brush border which secrete the disaccharidase enzymes.20 In one pediatric study, 49.0% patients were found to have one or more disaccharidase deficiencies,6 of these, 37.0% were lactase deficient, 21.0% had sucrase deficiency and 21.0% had glucoamylase or maltase, 8.0% had palatinase and 4.9% had pan-disaccharidase deficiency.6 Although this prevalence of pan-disaccharidase deficiency is lower than 9.2% seen in our study, a population study of 27 875 mucosal biopsy analysis also reported a prevalence of 8.0%.21 Thus, whether adults acquire this at a later stage in life or this is persistent from childhood or is secondary to small intestinal bacterial overgrowth is unclear. In contrast to the pediatric studies, the prevalence of sucrase deficiency was significantly low in our adult population, although lactase deficiency was similar. It is therefore unclear whether children recover their ability to synthesize brush border disaccharidases as they grow into adulthood or whether our sample represents a new, de novo population that has developed this deficiency as adults is unclear. While sucrase breath testing is another non-invasive test option, it may be difficult to distinguish between sucrose and fructose intolerance, and the breath test may be falsely positive with coexisting small intestinal bacterial overgrowth. Additionally, when sucrase is deficient, both our study and study by El-Chammas et al6 show that it occurs alongside pan-disaccharidase deficiency, and rarely in isolation. Thus, our findings in adults show similar and comparable prevalence of disaccharidase deficiencies as seen in children.
Lactase is the most common disaccharidase deficiency worldwide.4 Although congenital lactase deficiency is recognized early in life,14,19,22 the mechanism that leads to adult onset lactase deficiency is unclear.19 It may be an acquired phenomenon unmasked by an enteric infection or gene polymorphism.19,22-24 The high prevalence of lactase deficiency could be due to the ethnic distribution of our population, approximately 25.0% were AFrican Americans. In a recent systematic review, the prevalence of lactose intolerance was 36.0%, which is similar to our study.22 We observed that LBT had an excellent diagnostic yield with good specificity, sensitivity, and predictive values compared to lactase enzyme assay and that the diagnostic performance of LBT was similar to those reported previously.23 However, mucosal lactase deficiency may be patchy and could be missed by biopsy sampling, and some patients with normal lactase enzyme levels may have a false positive lactose breath test due to bacterial overgrowth. In clinical practice, one ought to also consider cost implication. Because LBT is significantly less expensive (approximately $88.00-189.00) when compared to pan-disaccharidase mucosal assay testing ($208.00 plus cost of endoscopy and biopsy), non-invasive, and the genetic testing is expensive and not widely available, we recommend LBT as the preferred and most cost-effective test for evaluation of lactose intolerance. It is important to point out that we used the 25 g lactose dose for LBT, as recommended by the North American Consensus,13 and that higher rates of sensitivity/specificity (> 90%) have been reported when using a higher dose (50 g).24
The enzyme levels used to adjudicate disaccharidase deficiencies were based on normative data in fasting patients, and therefore applicable to our patients who were fasting for endoscopy. In previous case reports of adult disaccharidase deficiencies, it was found that a lactose free diet did not lead to depressed brush border lactase activity, with the corollary being true as well, as was similar in the case of sucrase. However, maltase does seem to increase with increased daily carbohydrate intake.15 These observations suggest that enzyme deficiencies may be partly restored by repeated carbohydrate challenge.
Our study has few limitations. This was a retrospective analysis of prospectively collected data. We used questionnaires to collect baseline symptoms that allows for recall bias; however, this questionnaire has been validated.7 Additionally, we are a major referral center for functional GI disorders, and may have encountered patients with more severe disease, and our findings may represent an overestimation of disaccharidase deficiency in adults. However, our prevalence data for disaccharidase deficiency is similar to those in a pediatric community study.6 The biopsies for disaccharidase assays were collected by 2 gastroenterologists and analyzed in one laboratory, decreasing the collection and reporting errors. Also, the Dalqvist method of disaccharidase assay is a widely used commercial option for measurement of endoscopic samples. We did not evaluate a control group of asymptomatic subjects to examine the prevalence of disaccharidase deficiency in the community, but in clinical practice only symptomatic patients are likely to undergo this assessment. Finally, our study was not designed to address treatment outcomes with dietary interventions, following a diagnosis of disaccharidase deficiency.
In conclusion, our study demonstrates that disaccharidase deficiencies are frequently encountered in adults with unexplained symptoms; 9.2% may have pan-disaccharidase deficiency, and 35.8% have lactase deficiency. Isolated sucrase deficiency is rare and usually part of the pan-disaccharidase deficiency spectrum. Therefore, we recommend the assessment of disaccharidase enzyme levels in patients with unexplained GI symptoms.
Acknowledgments
Acknowledgements: This study was presented at DDW 2017, Chicago and Dr Viswanathan was awarded the AGA Fellow Award; Gastroenterology 2017;152:S7-S8. We thank Mrs Helen Smith for her excellent secretarial support.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Satish S C Rao: study concept and design, study recruitment, data analysis and interpretation, manuscript preparation, critical revision, and important intellectual content and final approval; Lavanya Viswanathan: study design, data acquisition, data analysis and interpretation, study recruitment, and manuscript preparation; Kevin Kennedy: data analysis and statistical analysis; Amol Sharma: study recruitment, data interpretation, and manuscript preparation; Yun Yan: data acquisition and analysis, and statistical analysis; and Enoe Jimenez: data acquisition. All authors have approved the final version being submitted.
References
- 1.Russo P, Ruchell ED, Piccoli DA. 2nd ed. Springer; New York, NY: 2014. Pathophysiology of pediatric gastrointestinal and liver disease; pp. 19–154. [DOI] [Google Scholar]
- 2.Blomme B, Gerlo E, Hauser B, Vandenplas Y. Disaccharidase activities in Belgian children: reference intervals and comparison with non-Belgian caucasian children. Acta Paediatr. 2003;92:806–810. doi: 10.1111/j.1651-2227.2003.tb02537.x. [DOI] [PubMed] [Google Scholar]
- 3.Marcadier JL, Boland M, Scott CR, et al. Congenital sucrase-isomaltase deficiency: identification of a common inuit founder mutation. CMAJ. 2015;187:102–107. doi: 10.1503/cmaj.140657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vesa TH, Marteau P, Korpela R. Lactose intolerance. J Am Coll Nutr. 2000;19:165S–175S. doi: 10.1080/07315724.2000.10718086. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Agriculture and US Department of Health and Human Services. Dietary guidelines for Americans. 7th ed. US Government Printing Office; Washington, DC: 2011. [Google Scholar]
- 6.El-Chammas K, Williams SE, Miranda A. Disaccharidase deficiencies in children with chronic abrominal pain. JPEN J Parenter Enteral Nutr. 2017;41:463–469. doi: 10.1177/0148607115594675. [DOI] [PubMed] [Google Scholar]
- 7.Choi YK, Kraft N, Zimmerman B, Jackson M, Rao SS. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol. 2008;42:233–238. doi: 10.1097/MCG.0b013e31802cbc2f. [DOI] [PubMed] [Google Scholar]
- 8.Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968;22:99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- 9.Dahlqvist A. Assay of intestinal disaccharidases. Enzymol Biol Clin. 1970;1:52–66. doi: 10.1159/000458348. [DOI] [PubMed] [Google Scholar]
- 10.Malis F, Lojda Z, Fric P, Jodl J. Disaccharidases in celiac disease and mucoviscidosis. Some correlations between histological, histochemical and biochemical studies. Digestion. 1972;5:40–48. doi: 10.1159/000197173. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez P, Canada FJ, Jimenez-Barbero J, Martin-Lomas M. Substrate specificity of small-intestinal lactase: study of the steric effects and hydrogen bonds involved in enzyme-substrate interaction. Carbohydr Res. 1995;271:31–42. doi: 10.1016/0008-6215(95)00034-Q. [DOI] [PubMed] [Google Scholar]
- 12.Townley RR, Khaw KT, Schwachman H. Quantitative assay of disaccharidase activities of small intestinal mucosal biopsy specimens in infancy and childhood. Pediatrics. 1965;36:911–921. [PubMed] [Google Scholar]
- 13.Rezaie A, Buresi M, Lembo A. Hydrogen and methane-based breath testing in gastrointestinal disorders: the north American consensus. Am J Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols BL, Jr, Adams B, Roach CM, Ma CX, Baker SS. Frequency of sucrase deficiency in mucosal biopsies. J Pediatr Gastroenterol Nutr. 2012;55(suppl 2):S28–S30. doi: 10.1097/01.mpg.0000421405.42386.64. [DOI] [PubMed] [Google Scholar]
- 15.Karnsakul W, Luginbuehl U, Hahn D, et al. Disaccharidase activities in dyspeptic children: biochemical and molecular investigations of maltase-glucoamylase activity. J Pediatr Gastroenterol Nutr. 2002;35:551–556. doi: 10.1097/00005176-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Sibley E. Carbohydrate intolerance. Curr Opin Gastroenterol. 2004;20:162–167. doi: 10.1097/00001574-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Amieva-Balmori M, Coss-Adame E, Rao NS, Dávalos-Pantoja BM, Rao SSC. Diagnostic utility of carbohydrate breath tests for SIBO, fructose and lactose intolerance. Dig Dis Sci. 2020;65:1405–1413. doi: 10.1007/s10620-019-05889-9. [DOI] [PubMed] [Google Scholar]
- 18.Cooper BT, Scott J, Hopkins J, Peters TJ. Adult onset sucrase-isomaltase deficiency with secondary disaccharidase deficiency resulting from severe dietary carbohydrate restriction. Dig Dis Sci. 1983;28:473–477. doi: 10.1007/BF02430538. [DOI] [PubMed] [Google Scholar]
- 19.Gerbault P, Liebert A, Itan Y, et al. Evolution of lactase persistence: an example of human niche construction. Philos Trans R Soc B Biol Sci. 2011;366:863–877. doi: 10.1098/rstb.2010.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border. J Cell Biol. 2014;207:441–451. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haberman Y, Di Segni A, Loberman-Nachum N, et al. Congenital sucrase-isomaltase deficiency: a novel compound heterozygous mutation causing aberrant protein localization. J Pediatr Gastroenterol Nutr. 2017;64:770–776. doi: 10.1097/MPG.0000000000001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storhaug CL, Fosse SK, Fadnes LT. County, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:738–746. doi: 10.1016/S2468-1253(17)30154-1. [DOI] [PubMed] [Google Scholar]
- 23.Hovde Ø, Farup PG. A comparison of diagnostic tests for lactose malabsorption--which one is the best? BMC Gastroenterol. 2009;9:82. doi: 10.1186/1471-230X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marton A, Xue X, Szilagyi A. Meta-analysis: the diagnostic accuracy of lactose breath hydrogen or lactose tolerance tests for predicting the North European lactase polymorphism C/T-13910. Aliment Pharmacol Ther. 2012;35:429–440. doi: 10.1111/j.1365-2036.2011.04962.x. [DOI] [PubMed] [Google Scholar]