Abstract
Hypoxia is contributed in various pathophysiological conditions including obesity, cardiovascular diseases, and cancer. In cancer, hypoxia is a salient phenomenon and has been correlated with tumor progression, metastasis, and provoke resistance to therapies in cancer patients, which exert with stabilization of main effector, hypoxia inducible factor-1 alpha (HIF-1α). Therefore, therapeutic targeting of hypoxic responses in cancer is the potential approach to improve the better treatment efficacy. In the present study, we evaluated the effect of β-Escin (β-Es) on hypoxia-induced resistance to apoptosis and metastasis in human non–small-cell lung cancer cells. The MTT assay revealed that β-Es treatment decreased the A549 cells viability under cobalt chloride-induced hypoxia. Apoptotic proteins were analyzed by western blot that showed cancer cells treated with β-Es induced cell death in hypoxia condition as proteins compared with normoxia. Moreover, we observed that cobalt chloride induced hypoxia through the generation of intracellular reactive oxygen species and stabilized the transcriptional factor HIF-1α, which leads to cancer metastasis. This notion was supported by the migration, invasion, and adhesion assays. Furthermore, hypoxia increased the expression of transforming growth factor-β, and the activation of matrix metalloproteinases were suppressed by the treatment of β-Es as well as pretreatment with N-acetylcysteine (NAC). Therefore, we demonstrate that a concurrent activation of HIF-1α, transforming growth factor-β, and matrix metalloproteinases participate in hypoxia-induced metastasis and that β-Es prevent A549 cells metastasis by inhibition of reactive oxygen species.
Keywords: lung cancer, β-escin, hypoxia, TGF-β, ROS, MMPs
Introduction
Lung cancer is one of the most prevalent types of malignancies in both men and women and the second leading cause of cancer-related lethality around the world. In 2012, 1.58 million lung cancer mortalities were estimated globally [1]. Lung cancer patient’s deaths are intimately related to metastasis [2]. The existing treatment modalities such as surgery, radiotherapy, and chemotherapy have been used to treat lung cancer. However, only a substantial progress in the morbidity or mortality caused by lung cancer [3]. The resistance to radiotherapy or chemotherapy is a stagnant point in successful treatment in lung cancer victims. The recent data indicate that numerous human cancers evolve chemoresistance within the tumor microenvironment including lung cancer [4, 5]. Hypoxic microenvironment is one of the characteristic phenomena in most of the human solid tumor like lung cancer, and this isgenerated by an immoderate proliferation of cancer cells, irregular and inefficient of vascular networks. This hypoxic stress creates an adaptive mechanism on cancer cells survival, proliferation, and plays a vital role in migration and invasiveness in cancer cells via the remodeling of the extracellular matrix (ECM) [6–8].
Migration and invasion are two unwavering processes of tumor metastasis via the breakdown of the ECM components, which is mediated by a family of zinc- and calcium-dependent endopeptidases called matrix metalloproteinases (MMPs) [9, 10]. Accumulating evidence suggests that gelatinases group of MMPs such as MMP-2 (gelatinase A) and MMP-9 (gelatinase B) participate in numerous types of malignant tumor development and aggressiveness including non–small-cell lung cancer cells (NSCLC) [11]. Various types of stimuli have been connected with this process and promoted secretion, activation of these gelatinases in miscellaneous types of cancer [12]. Among them is transforming growth factor-β (TGF-β). The levels of TGF-β found to be enhanced in cancer patients and also act as a stimulant in cancer cells migration, invasiveness, differentiation, and ECM synthesis and it depends on the cell types. In cancer cells, the latent TGF-β participates in the invasiveness and metastasis of the prostate, mammary, and lung cancer at a metastasis (late) stage through upregulating MMPs [13, 14]. Especially, exposure of lung alveolar epithelial cells to hypoxia results in the excessive secretion of TGF-β [15]. Moreover, reactive oxygen species (ROS) instigate several cellular signal events that regulate diverse cellular functions including metastasis [16]. During hypoxia, the increased expression of hypoxia inducible factor-1alpha (HIF-1α) partaken in mitochondrial activity and ROS formation. Compelling evidences suggested that ROS plays a vital role in expression of HIF-1α and increases stabilization during hypoxia than normoxia thereby leading to the upregulation of TGF-β in hypoxic condition. Besides that, ROS can stimulate TGF-β secretion in many cell types and implicate in the synthesis of ECM proteinases, cell invasiveness, which all collectively elicit tumor progression and metastasis [17–19].
Recently, novel therapeutic agents are required to fight against the advanced stages of cancer. Plant-derived phytochemicals are the most indispensable sources of induced apoptosis and inhibiting metastasis in in vitro and in vivo cancer models [20]. β-Escin (β-Es) is a main active triterpene saponin, which is obtained from the seeds of Aesculus hippocastanum and has been demonstrated to pharmacological activities, including anti-inflammatory, antioxidant, and antiedematous effects [21]. Several studies have revealed that β-Es exhibited anticancer and antimetastatic activities against the several cancer models [22]. However, there is no direct evidence concerning the effects of β-Es on human lung cancer cells under hypoxia condition. Therefore, present study is focused to test the efficacy of β-Es treatment in lung adenocarcinoma cells exposed to the cobalt chloride (CoCl2)-mimicked hypoxic condition and examine the possible pathways.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) containing fetal bovine serum (FBS) and antibiotic–antimycotic solution were obtained from Gibco (MD, USA). Escin, CoCl2, trypsin, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT), Dimethyl sulfoxide (DMSO), N-acetylcysteine, 2′,7′-dichlorofluorescin diacetate (DCFH-DA), and gelatin were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Primary antibodies against Bax, Bad, Bid, Cytochrome-c (Cyt-c), cleaved Caspase-3 (Cas-3), Caspase-9 (Cas-9), and Bcl-2 antibodies were obtained from Cell Signaling Technologies (MA, USA). Primary antibody for TGFβ-1 was obtained from Santa-Cruz Biotechnology (CA, USA). Primary antibody for HIF-1α was obtained from Novus biological (USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit/mouse IgG secondary antibody was obtained from Genei.
Cell culture maintenance and hypoxia induction
Human NSCLC cell line A549 was routinely cultured in DMEM containing 10% FBS, and 1% antibiotic–antimycotic solution buffered at pH 7.4 (complete medium). Cells were reached to 80% confluence before being passaged or used for experiments. For hypoxia model, A549 cells were preincubated with CoCl2 to mimic the hypoxic condition by stabilizing HIF-1α. In the above cases, cells were grown at 37°C in a humidified atmosphere in the presence of consistent 5% CO2.
Cells viability assay
A549 cells viability were assessed by MTT method. The cells were seeded in 96-well plate at a density of 10 × 103cells/well and kept at 37°C for overnight. After the incubation period, the cells were treated with different concentration of the β-Es for the appropriate time and finally added with 50 μg of MTT solution/well and allowed to incubate at 37°C in the dark condition for another 2 h. Subsequently, the supernatant was removed and 100 μl of DMSO was added to dissolve the formazan. Absorbance was measured at the wavelength of 570 nm using an ELISA microplate reader.
Intracellular ROS assay
Generation of ROS was analyzed using DCFH-DA dye. At the end of experiment duration, the cells were stained with 5 μM of DCFH-DA and kept at 37°C for 15 min. Finally, the cells were washed twice with sterile phosphate-buffered saline (PBS) and then visualized under the fluorescence microscope and the images were captured (Nikon eclipse E200).
Scratch migration assay
A scratch migration assay was performed, as described previously [23]. A549 cells were seeded into the 12-well sterile tissue culture plate and allowed to reach 80% confluency. Then, A549 cells were starved in low-serum–containing DMEM (0.5% FBS) for 12 h. The monolayer was scratched with 0.2-ml micro tip and washed with sterile 1× PBS and treated with low-serum DMEM containing different concentration of β-Es for 24 h. At 0 and 24 h, images were captured on a photomicroscope (Motic, type 101). The ability of A549 cells migration was assessed by the wound closure rate using Tscratch software.
Transwell invasion assay
Cells were seeded at the density of 1 × 105 in the collagen-coated upper chamber of the transwell plate. Subsequently, the bottom of the chamber was filled with 10% of FBS-containing medium that was used as a chemoattractant. Under culturing conditions (37°C and 5% CO2), the cells were allowed to invade for 24 h. After the end of the experiment, the upper insert chambers were fixed and stained with crystal violet solution in 30% methanol, then washed slowly with PBS and the sterile cotton swabs were used to remove noninvasive cells from the upper chamber. The number of invasive cells was photographed (5 random fields). Subsequently, the stained cells were extracted with 30% acetic acid and optical density (OD) was measured at 590 nm. The results were performed in triplicates.
Cell adhesion assay
β-Es treated A549 cells were seeded at the density of 1 × 104 cells/well in collagen coated 96-well plate. After 60 min of incubation, the unattached cells were carefully removed and washed with sterile 1× PBS. The attached cells were fixed using 4% paraformaldehyde for 20 min, then stained with 0.4% crystal violet solution for 20 min, and then rinsed with PBS. The attached cells were counted under the light microscope in five randomly selected fields at ×10 magnification. Quantification of adhesiveness was calculated by the amount of crystal violet dye taken up by the attached cells and OD was measured at 590 nm by ELISA plate reader. Each experiment was executed in triplicates.
Protein extraction and immunoblotting analysis
Whole-cell proteins were extracted from experimental group of cells using Radioimmunoprecipitation assay (RIPA) lysis buffer (0.05 M Tris [pH 7.4], 0.15 M NaCl, 1% Nonidet P-40, 0.1% SDS, and 0.5% sodium deoxycholate) in the presence of protease inhibitors, and the protein concentration was estimated by CB-X protein assay kit (G-Biosciences, USA); 40 μg of proteins were loaded on standard SDS-PAGE gel and the resolved proteins were electrotransferred onto polyvinylidene difluoride membranes. The membranes were blocked using 5% BSA and probed with different primary antibodies for overnight at 4°C (at dilutions of 1:1000 to 1:3500); furthermore, the membranes were incubated for 1 h with the HRP-conjugated secondary antibodies at a dilution of 1:3000 to 1:5000. Finally, the immunoreactive protein bands were sensed by an enhanced chemiluminescence substrate (Pierce ECL, USA).
MMP-2 and 9 activities assay
Conditioned media were prepared from experimental cells and the protein was concentrated by 20% trichloroacetic acid. Equal amounts of nonreducing proteins were loaded on the 10% SDS-PAGE separating gel containing 0.1% gelatin. After electrophoresis, the gel was thoroughly washed with 2.5% of Triton X-100 for 1.5 h. Finally, the gel was developed in 10 mM CaCl2 containing developing buffer at 37°C for 36 h. Furthermore, the gel was stained with Coomassie Brilliant Blue R-250 (CBB) R-250 for 20 min and destained. MMP activity was identified as clear digestion bands over a blue background.
Statistical Evaluation
The data were expressed as mean ± standard deviation from at least triplicate-independent experiments. Statistical analysis of the data was performed using one-way analysis of variance followed by a post hoc Turkey test, in which P-values < 0.05 were considered statistically significant score.
β-Es inhibited the viability and induced caspase-dependent apoptosis in A549 under CoCl2-induced hypoxic condition
Tumor hypoxia denotes a pivotal role in the progression of tumor and toward resistance to therapy. In our experiment, we first confirmed that CoCl2 instigates hypoxia in A549 cells by the expression of the master regulator of HIF-1α. As shown in Figure 1A, 100 μM of CoCl2 can significantly induce the stabilization of HIF-1α protein in time-dependent manner in A549 cells. On the basis of this result, in this study we used 100 μM of CoCl2 to induce hypoxia in A549 cell. Next, the effect of β-Es on A549 cell viability was observed under normoxia and CoCl2-induced hypoxia condition. A549 were exposed to 24 h of incubation with different of β-Es under normoxia or hypoxia-mimicking condition (100 μM of CoCl2). The cell viability assay revealed that under normoxia condition A549 viability was decreased to 51.03% in 12.5 μM of β-Es-treated group for 24 h implying a half-maximal inhibitory concentration as of 12.5 μM. Interestingly, under the hypoxia condition, β-Es treatment induced stronger cytotoxicity in A549 cells that were viable for 24 h (Fig. 1C). Also, to substantiate the effect of β-Es on NSCLC cells under normoxia and hypoxia, we used 20 μM of cisplatin used as a positive control. Next we tested the apoptotic effect of β-Es under CoCl2-induced hypoxic condition. Treatment with different concentration of β-Es-induced apoptotic cell death with a significant increase in Cas-9 and 3 in CoCl2-induced hypoxic A549 cells (Fig. 1D). The obtained results on the activation of caspases show that β-Es induced apoptotic cell death in hypoxic A549 cells.
Figure 1.
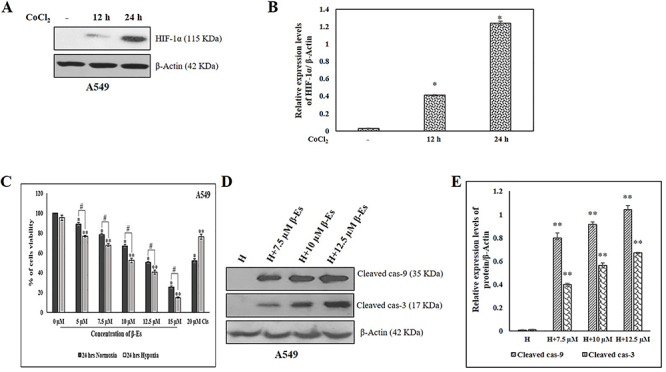
Effect of β-Es on the viability of A549 cells under normoxia and CoCl2 induced hypoxic condition. (A) A549 cells were incubated under 100 μM CoCl2 for 0, 12 and 24 h. Immunoblot shows HIF-1α expression. (B) Densitometric analysis of HIF-1α protein expression. (C) Effect of β-Es on A549 cells viability under normoxia and 100 μM CoCl2 induced hypoxic condition: A549 cells were prior to exposed the 100 μM CoCl2 for 2 h before treated to various concentration of β-Es treatment for 24 h. Cells viability was determined by MTT assay. (D) A549 cells were pre-incubated 100 μM of CoCl2 for 2 h before treated with β-Es (24 h) for the indicated concentration. At the end of the treatment, cells were lysed and equal amounts of proteins were loaded and western blotting for the detection of cleaved cas-9 and 3. (E) Expression of cleaved cas-9 and 3 were quantified using Image-J software and displayed in bar graph (mean ± SD, n=3). Results were normalized to β-actin. Data are expressed as mean α S.D. *p<0.05 as compared with normoxia; **p<0.05 as compared with hypoxia; #p<0.05 as compared with the β-Es treated normoxia and hypoxia group.
β-Es enhanced the apoptosis in A549 cells under CoCl2-induced hypoxic condition
To further corroborate the cells viability assay, the profundity of the apoptotic response in NSCLC cells under normoxia and hypoxic condition was tested. Figure 2A shows that the 100 μM of CoCl2-induced hypoxia (H) increased the higher expression of Bax, Bad, Bid, and Cyt-c and decreased the expression of anti-apoptotic protein Bcl-2 in CoCl2-induced hypoxia condition compared with normoxia. At the same time, cleaved Cas-3 and 9 were found to be unaffected by CoCl2-induced hypoxia condition compared with normoxia. Moreover, under CoCl2-induced hypoxia condition treatment with β-Es efficiently enhanced the higher expression of apoptotic proteins (Bax, Bad, Bid, Cas-3, Cas-9, and Cyt-c) in hypoxia condition treatment with 12.5 μM of β-Es in A549 than that of β-Es-treated–alone group, and the expression of Bcl-2 protein exhibited the opposite tendency in A549 cells.
Figure 2.
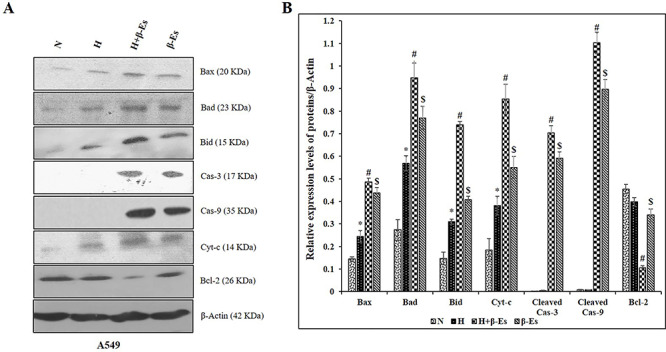
β-Es inhibits hypoxia induced apoptotic resistane in A549 cells. (A) A549 cells were treated with or without 12.5 μM β-Es, under normoxia/hypoxia for 24 h. Total protein were extracts and equal concentration of proteins were subjected to SDS PAGE and immunoblot was analysed. β-actin was used as the loading control. (B) The bar graph indicates the mean ± SD. *p<0.05 vs normoxia; #p<0.05 vs hypoxia; $p<0.05 vs H+β-Es.
β-Es repress CoCl2-induced HIF-1α stabilization through the suppression of ROS
Previous reports suggested that both ROS and master transcription factor HIF-1α are involved in the resistance to apoptosis and metastasis cascades and ROS are crucial molecules for stabilization of HIF-1α. The effect of β-Es in generation of ROS in A549 cells was measured using DCFH-DA dye. As shown in Figure 3A, ROS formation was increased in hypoxia-induced CoCl2 group as compared with normoxia. Consistently, various concentrations of β-Es treatment markedly suppressed the hypoxia-induced generation of ROS in A549 cells and the NAC (free-radical scavenger)-alone group is considered as the positive control. These results suggested that β-Es have the capability to reduce the hypoxia-induced generation of ROS. Based on these evidences, we hypothesized that β-Es might influence the destabilization of HIF-1α protein by western blot. The immunoblot results show that (Fig. 3B) the stabilization of HIF-1α was increased in CoCl2-induced hypoxic cells compared with the normoxia cells, whereas β-Es-treated cells significantly decreased the accumulation of HIF-1α.
Figure 3.
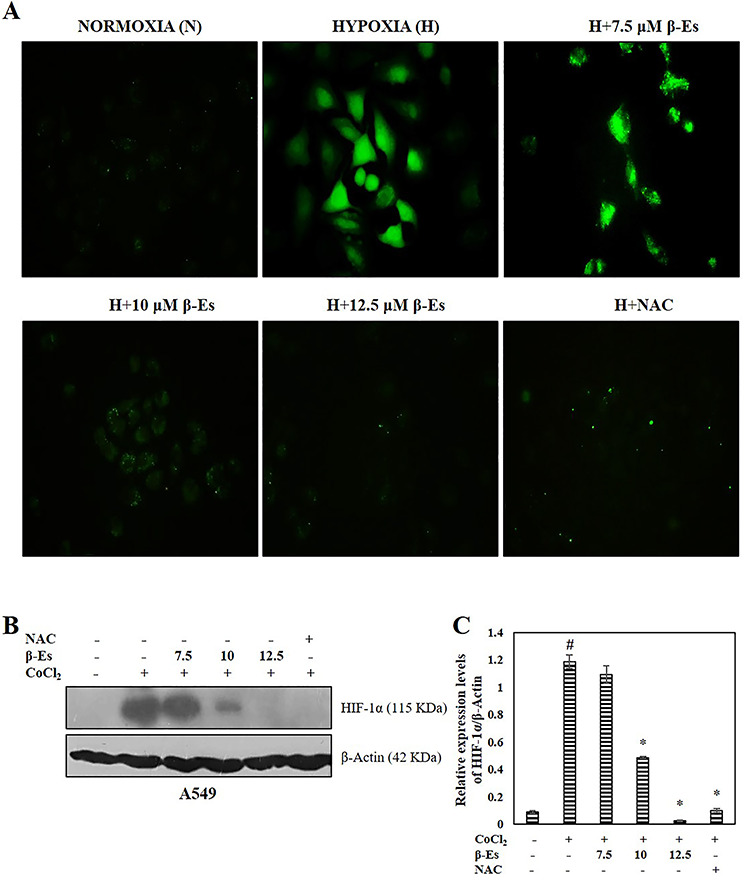
β-Es repress CoCl2 induced HIF-1a stabilization through the suppression of ROS (A) Fluorescence microscopic images of DCFH-DA stained β-Es treated A549 cells with indicated concentration (20 X magnification). 10?mM NAC used as a positive control. (B) Cell lysates were subjected to western blot analysis with HIF-1α antibodies. β-actin was used as the loading control. (C) A densitometric analysis was used to estimate the expression levels of HIF-1α. Significance: #p<0.05 vs normoxia; *p<0.05 vs hypoxia.
β-Es reduced hypoxia-induced migration, invasion, and adhesiveness in A549 cells
Generation of ROS has been primarily connected with cytotoxicity and apoptosis; nevertheless, studies have revealed the involvement of cancer metastasis. Based on the available reports, the effect of β-Es against hypoxia-induced tumor metastasis in experimental A549 cells was evaluated. A scratch assay was performed in order to confirm the cell migrating ability. Figure 4 indicated that the 100 μM of CoCl2-induced hypoxia cells was dramatically attaining the faster migration compared with the normoxia cells, whereas various concentration of β-Es or 5 mM of NAC-treated cells decelerate the wound closure as compared with the normoxia and hypoxia cells. Additionally, the invasion ability was examined (Fig. 5). Transwell invasion assay showed that β-Es-treated experimental cells inhibited the invasive property. Nevertheless, the CoCl2-induced hypoxic mimetic cells promoted the invasive ability compared with the normoxia. Consistent with these data, under the CoCl2-induced hypoxic condition, various concentrations of β-Es or NAC alone remarkably suppressed the hypoxia-induced invasiveness. Moreover, the adhesive property of A549 cells on basement protein-coated plate was investigated. The result show that hypoxia induced adhesion on collagen-coated plate compared with normoxia, whereas different concentration of β-Es or 5 mM of NAC-alone–treated groups significantly reduced the adhesion ability compared with normoxia and hypoxia (Fig. 6). These results illustrate that β-Es treatment remarkably suppressed the hypoxia-induced adhesion, migration, and invasion properties via suppression of ROS.
Figure 4.
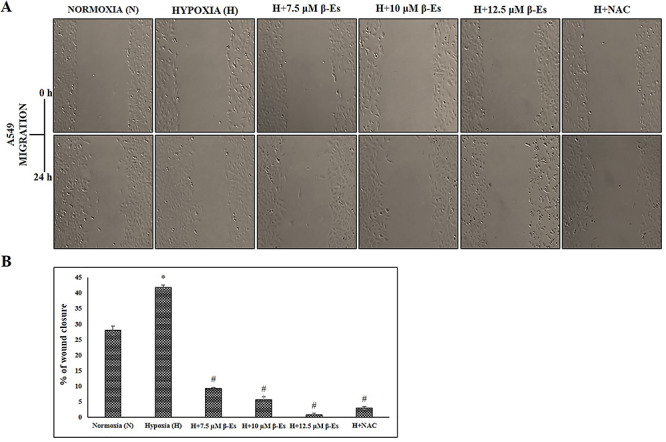
β-Es attenuates hypoxia induced migration ability in A549 cells (A) The migratory ability of A549 cells were assessed by scratch wound healing assay for 24 h. Representative wounds images were photographed after scratch (0 h) and after 24 h of healing through an inverted microscope (10 X magni?cation). The experiment was performed three times with similar results. (B) The percentage of wound closure area was quanified using TScratch software. Each bar indicates the mean ± SD of three independent experiments. *P<0.05 vs Normoxia and #P<0.05 vs Hypoxia.
Figure 5.
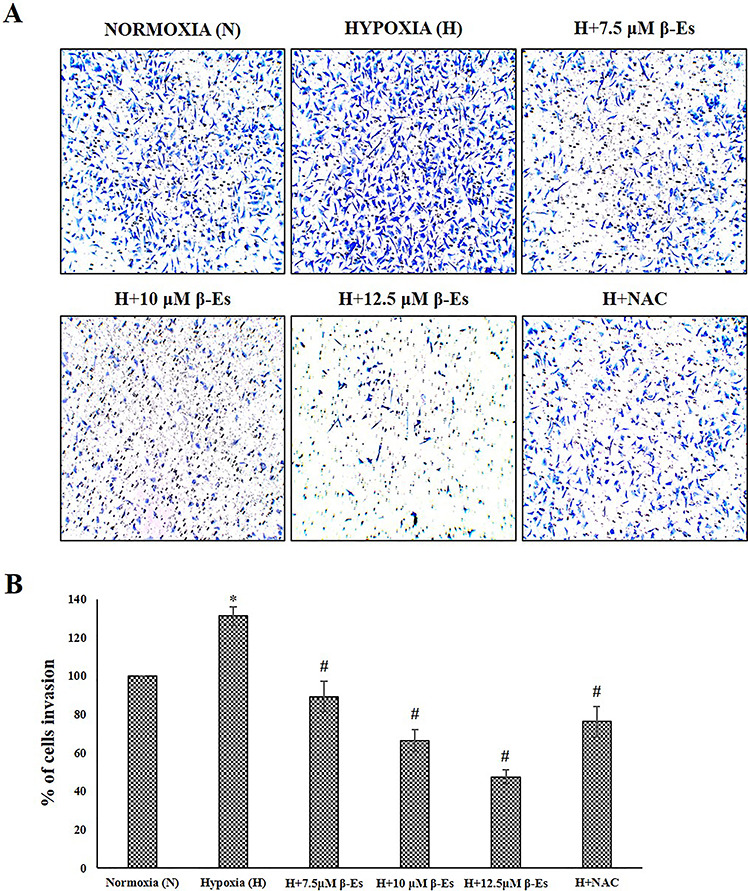
β-Es reduced invasiveness of A549 cells under hypoxia (A) The effect of β-Es treatment on invasion of A549 cells were measured using boyden chamber for 24 h. The images were obtained through an inverted microscope (10 X magni?cation). (B) The number of invading cells were quantified by solubilization of dye with 30% acetic acid and absorbance was read at 590nm. Data was obtained from triplicate experiments. *P<0.05 vs Normoxia and #P<0.05 vs Hypoxia. The experiment was performed three times with similar results.
Figure 6.
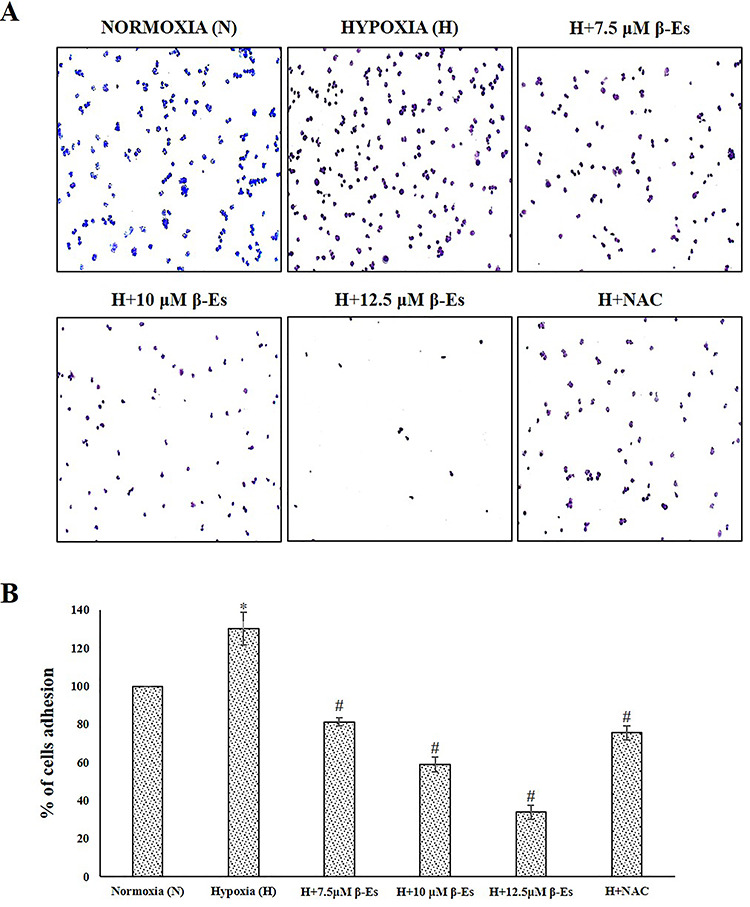
β-Es decreased hypoxia induced adhesiveness in A549 cells (A) β-Es treatment induced the inhibition of the A549 cell adhesion on collagen coated matrix for 60 min. (B) The number of adhering cells were quantified by solubilization of dye with 30% acetic acid and absorbance at 590 nm was measured. *P<0.05 vs Normoxia and #P<0.05 vs Hypoxia. Results are presented as the mean ± SD of three independent experiments.
β-Es suppresses TGF-β expression and MMPs activation under CoCl2-induced hypoxic condition
TGF-β and MMPs are prerequisite crucial mediators in ECM remodeling and cancer metastasis. Furthermore, since hypoxia is known to modulate the secretion of cytokines and growth factors in numerous cell types, it would be logical to determine the effect of β-Es on key component of the TGF-β. Following these observations as shown in Figure 7A and B, after 24 h, the protein level of TGF-β was augmented in a CoCl2-induced hypoxic cells compared with the normoxia cells, whereas β-Es-treated cells decreased the expression of TGF-β level as compared with the control and hypoxia cells. The effects of β-Es on the regulation of MMP-2 and 9 in A549 cells by zymography were shown. The result indicated that CoCl2-mimicked hypoxia model increased the activities of MMP-2 and 9 in A549 cells as compared with normoxia, whereas β-Es-treated experimental groups significantly decreased hypoxia-induced upregulation of MMP-2 and 9 activities (Fig. 7C).
Figure 7.
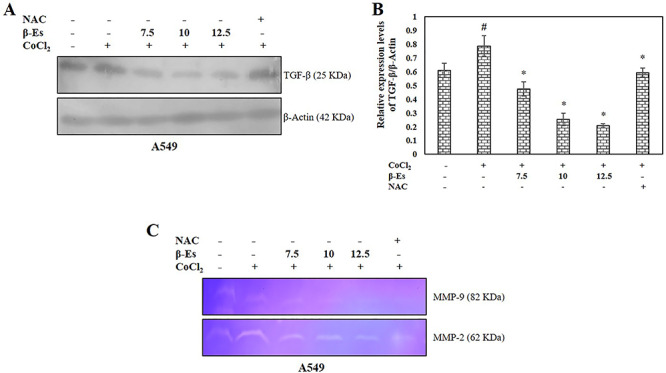
β-Es suppresses TGF-β expression and MMPs activation under CoCl2 induced hypoxic condition (A) Whole cell lysates of β-Es treated A549 cells were immunoblotted with antibodies for TGF-β under conditions of normoxia (N) or hypoxia (H) for 24 h. (B) The mean ± SD of values obtained from densitometric analysis of TGF-β (n=3). Significance: #p<0.05 vs normoxia; *p<0.05 vs hypoxia. (C) The MMP-2 and 9 activities: Cells were pretreated with or without 100 μM CoCl2 for 2 h before treated to β-Es for 24 h. The conditioned media were collected and analyzed by gelatin zymography.
Discussion
In solid tumors, the expeditious progression of tumor and metastasis events is tightly incorporated with the adjacent microenvironment. Growing body of evidences suggest that human solid tumors frequently faced the hypoxic microenvironment that permit tumor cells survival, metastasis, and affect radiotherapy and chemotherapy treatments [24, 25]. In this study, β-Es efficiently induced apoptotic cell death in A549 cells under CoCl2-mimicked hypoxic condition than normoxia. This is significant to clinical oncology, because hypoxia denotes a compelling therapeutic target due to its foremost impact on tumorigenesis and prevail resistance to novel modality treatments. In addition, the results of this study suggest that β-Es inhibits migration, invasion, and adhesion capability of hypoxic stress in NSCLC cell lines via disruption of intracellular ROS-dependent HIF-1α/TGF-β/MMPs 2 and 9.
In this study, CoCl2 was used to generate hypoxic condition and CoCl2 is widely used as an in vitro and in vivo chemical mimic of hypoxia agent by instigating the stabilization of HIF-1α through various mechanisms, such as inhibition of prolyl hydroxylases through generation of ROS, Co2+substitutes for Fe2+, and depletion of ascorbate, prevent HIF-1α bind to von hippel lindau (VHL) protein, and activation of PI3K-signaling pathway [26–29]. In the current study, the expression of HIF-1α gradually increased in time-dependent manner in A549 cells, confirming that hypoxia was provoked by the 100 μM CoCl2.
β-Es, a triterpene saponin, is involved in a variety of functions, which includes antioxidative, anti-inflammatory, and anticancer activities. Several studies indicate that β-Es effectively inhibited the proliferation of different types of cancer cells [30–33]. Nevertheless, currently there is no study about the effect of β-Es on NSCLC under the hassle hypoxic microenvironment. In this study, our findings showed that β-Es treatment effectively reduced the A549 cells viability under the CoCl2-mimicked hypoxia than normoxia, which connotes the potential of β-Es treatment, given that hypoxia instigate cancer resistance.
Drug resistance in cancer is a major hindrance for cure of cancer and can occur via inhibition of apoptotic process controlled by a cellular death mechanism [34, 35]. Hypoxic environment is toxic to both normal and cancer cells, depending on the cell type and exposure time [36]. Under mild hypoxia environment cancer cells undergo genetic and adaptive changes that allow them to escape cell death and even proliferate. Multiple studies demonstrated that during hypoxia and hypoxia-mimicked condition HIF-1α is stabilized and regulates several genes involved in apoptosis [37]. Furthermore, hypoxia upregulates the expression numerous anti-apoptotic proteins, such as the heat shock protein (HSP-70) and survivin, in a manner dependent of HIF-1α [38, 39]. In our experimental setup, 100 μM CoCl2 induced the expression of Bax, Bad, Bid, and induced the leakage of Cyt-c into cytosol. At the same time, cleaved Cas-3 and 9 were found to be unaffected by CoCl2-induced hypoxia condition compared with normoxia. Western blotting data found that increased expression of HSP-70 under CoCl2 mimicked hypoxia conditioning A549 cells compared with normoxia (data not shown). It is possible that the overexpression of HSP-70 prevent the recruitment of pro-Cas 9 to apoptotic protease-activating factor 1 (Apaf-1) apoptosome complex and, subsequently, prevent the apoptosis in CoCl2-exposed A549 cells. These data are in harmony with previous studies, which suggest that overexpression of HSP-70 associated with the inhibition of apoptosis under hypoxia and hypoxia-mimicking condition [40, 41]. In addition to, 12.5 μM of β-Es significantly enhanced the apoptosis in A549 cells than in β-Es-treated normoxia. These data indicate that β-Es induced pronounced activation of apoptosis in A549 cells in the presence of CoCl2-induced hypoxia condition. Indeed, several studies have suggested that β-Es induce apoptotic cell death, in several human cancers, including human osteosarcoma cells, leukemia Jurkat T cells, and lung cancer [42–44].
Numerous reports suggest that superfluous formation of intracellular ROS will lead to mitochondrial impairment and eventually cause cell death [45, 46]. On the contrary, generation of elevated level of ROS occurs at hypoxia and nonhypoxic circumstance, which might act as an intermediary to bring about cancer cells invasion and metastasis [47]. Furthermore, under hypoxic conditions, mitochondrial complex III may produce ROS, and this moderate level of mitochondrial ROS has been involved to stabilize the foremost transcription factors HIF-1α and plays a vital role in the activation of drug resistance and metastasis [48, 49]. In addition, Chachami et al. [27] indicated that ROS involved in HIF-1α stabilization in CoCl2-exposed airway smooth muscle cells. In line with these notions, we observed under CoCl2-induced hypoxic condition, treatment of A549 cells with β-Es or NAC, which results in decrease stabilization of HIF-1α, is associated with a reduce the accumulation of ROS generation (Fig. 3A and B). These data are consistent with previous studies, which stated that β-Es is, in part, connected to its antioxidant properties, which are mainly due to its ability to scavenge ROS [30].
Previous research evidences reported that hypoxia-mimicking agents persuade the generation of ROS, which involved in stimulating cancer cells migration and invasion [50, 51]. Therefore, the suppression of cancer cells metastasis could be partially related to the inhibition of ROS generation. This study demonstrates that under hypoxia, β-Es treatment decreased the migration, invasion, and adhesion capability of A549 cells as compared with the presence of NAC-alone group. Previous study has been demonstrated that β-Es had the proficiency on inhibition of migration and invasion in AGS gastric adenocarcinoma cells [52]. Also, studies suggest that β-Es inhibit the hypoxia-induced invasiveness in MCF-7 and MDA-MB-231 cells [53].
In addition to this, the active form of TGF-β and MMPs are a decisive regulator in cancer metastasis through the modulation of ECM [54]. Previous research evidences show that hypoxia and ROS have independently enhanced the TGF-β and MMPs expression [55–59]. Furthermore, Zhou et al. reported that HIF-1α and ROS are upstream of TGF-β production and that both HIF-1α and ROS are required for the production of TGF-β in hypoxia-exposed alveolar epithelial cells [15]. In line with these findings, the western blot analysis shows that CoCl2-mimicking hypoxia increased TGF-β expression, which was decreased with increasing concentration of β-Es. Moreover, the ROS scavenger NAC-alone–treated A549 cells prevented the hypoxia-induced upregulation of TGF-β. These data suggest that regulation of hypoxia-induced metastasis by β-Es may be partially prevented by TGF-β.
Tumor invasion is a sophisticated process and is often correlated with hydrolysis of ECM by an active form of proteolytic enzyme MMPs, particularly, MMPs 2 and 9 play a key role in ECM degradation and to facilitate cancer cells invasion into the blood or lymph vessels and circulate to other parts. The elevated levels of MMPs 2 and 9 were observed in late stages of cancer, which could be a prognostic factor in several human cancers [60, 61]. In line with these notions, β-Es was revealed to decrease the activities of MMPs 2 and 9 in A549 cells under hypoxic condition as evidenced by zymography analysis. These data indicated that β-Es may exert its antimigratory and anti-invasion effects by regulating MMP-2 and 9 activities.
In summary, this study shows that ROS is an important factor released by CoCl2-mimicked hypoxic A549 cells to regulate HIF-1α, TGF-β, and MMPs. Our findings suggest that strategies targeting ROS in hypoxia condition could be an NSCLC treatment. β-Es could target ROS and inhibit activation of HIF-1α, TGF-β, and MMPs and subsequent metastatic traits. Given the properties found in this study, β-Es can be a suitable candidate for treatment of NSCLC.
Funding
This work is supported by a student fellowship from University Grants Commission, New Delhi. India. Grant number is UGC-BSR (2014-2019)
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
This study is supported by Basic Scientific Research (BSR) scheme of University Grants Commission, New Delhi, India.
References
- 1. Didkowska J, Wojciechowska U, Manczuk M et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 2016;4:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichols L, Saunders R, Knollmann FD. Causes of death for patients with lung cancer. Arch Pathol Lab Med 2012;136:1552–7. [DOI] [PubMed] [Google Scholar]
- 3. Miller KD, Siegel RL, Lin CC et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- 4. Senthebane DA, Rowe A, Thomford NE et al. The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci 2017, 18, 7, 1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tredan O, Galmarini CM, Patel K et al. Drug resistance and the solid tumor microenvironment. J Nat Cancer Inst 2007;99:1441–54. [DOI] [PubMed] [Google Scholar]
- 6. Zhou J, Schmid T, Schnitzer S et al. Tumor hypoxia and cancer progression. Cancer Lett 2006;237:10–21. [DOI] [PubMed] [Google Scholar]
- 7. Salem A, Asselin MC, Reymen B et al. Targeting hypoxia to improve non–small cell lung cancer outcome. J Nat Cancer Inst 2017;28:14–30. [DOI] [PubMed] [Google Scholar]
- 8. Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 2014;14:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoon SO, Park SJ, Yun CH et al. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol 2003;36:128–37. [DOI] [PubMed] [Google Scholar]
- 10. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9–34. [DOI] [PubMed] [Google Scholar]
- 11. Merchant N, Nagaraju GP, Rajitha B et al. Matrix metalloproteinases: their functional role in lung cancer. Carcinogenesis 2017;38:766–80. [DOI] [PubMed] [Google Scholar]
- 12. Roomi MW, Monterrey JC, Kalinovsky T et al. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep 2009;21:1323–33. [DOI] [PubMed] [Google Scholar]
- 13. Kubiczkova L, Sedlarikova L, Hajek R et al. TGF-β–an excellent servant but a bad master. J Transl Med 2012;10:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Principe DR, Doll JA, Bauer J et al. TGF-β: Duality of function between tumor prevention and carcinogenesis. J Nat Cancer Inst 2014;106:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou G, Dada LA, Wu M et al. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol 2009;297:L1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta SC, Hevia D, Patchva S et al. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signalling 2012;16:1295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chandel NS, McClintock DS, Feliciano CE et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 2000;275:25130–8. [DOI] [PubMed] [Google Scholar]
- 18. Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate hypoxic signaling. CurrOpin Cell Biol 2009;21:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krstic J, Trivanovic D, Mojsilovic S et al. Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longevitv 2015;2015:654594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aung T, Qu Z, Kortschak R et al. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. IntJ Mol Sci 2017;18:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharm Res 2001;44:183–93. [DOI] [PubMed] [Google Scholar]
- 22. Cheong DH, Arfuso F, Sethi G et al. Molecular targets and anti-cancer potential of escin. Cancer Lett 2018;422:1–8. [DOI] [PubMed] [Google Scholar]
- 23. Lecomte N, Njardarson JT, Nagorny P et al. Emergence of potent inhibitors of metastasis in lung cancer via syntheses based on migrastatin. Proc Nat Acad Sci, 2011, 108 (37), 15074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walsh JC, Lebedev A, Aten E et al. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signalling 2014;21:1516–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muz B, Puente P, Azab F et al. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015;3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan Y, Hilliard G, Ferguson T et al. Cobalt inhibits the interaction between hypoxia-inducible factor-α and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-α. J Biol Chem 2003;278:15911–6. [DOI] [PubMed] [Google Scholar]
- 27. Chachami G, Simos G, Hatziefthimiou A et al. Cobalt induces hypoxia-inducible factor-1α expression in airway smooth muscle cells by a reactive oxygen species–and PI3K-dependent mechanism. Am J Resp Cell Mol Biol 2004;31:544–51. [DOI] [PubMed] [Google Scholar]
- 28. Salnikow K, Donald SP, Bruick RK et al. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem 2004;279:40337–44. [DOI] [PubMed] [Google Scholar]
- 29. Matsuura H, Ichiki T, Ikeda J et al. Inhibition of prolyl hydroxylase domain-containing protein downregulates vascular angiotensin II type 1 receptor. Hypertension 2011;58:386–93. [DOI] [PubMed] [Google Scholar]
- 30. Vašková J, Fejerčáková A, Mojžišová G et al. Antioxidant potential of Aesculus hippocastanum extract and escin against reactive oxygen and nitrogen species. Eur Rev Med Pharmacol Sci 2015;19:879–86. [PubMed] [Google Scholar]
- 31. Wang H, Zhang L, Jiang N et al. Anti-inflammatory effects of escin are correlated with the glucocorticoid receptor/NF-κB signaling pathway, but not the COX/PGF2α signaling pathway. Experiment Ther Med 2013;6:419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mojzisova G, Kello M, Pilatova M et al. Antiproliferative effect of β-escin-an in vitro study. Acta Biochim Pol 2016;63:79–87. [DOI] [PubMed] [Google Scholar]
- 33. Harford-Wright E, Bidere N, Gavard J. β-Escin selectively targets the glioblastoma-initiating cell population and reduces cell viability. Oncotarget 2016;7:66865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pommier Y, Sordet O, Antony S et al. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene 2004;23:2934–49. [DOI] [PubMed] [Google Scholar]
- 35. Fulda S. Tumor resistance to apoptosis. Int J Cancer 2009;124:511–5. [DOI] [PubMed] [Google Scholar]
- 36. Dai ZJ, Gao J, Ma XB et al. Up-regulation of hypoxia inducible factor-1α by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells. J Experim Clin Cancer Res 2012;31:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sermeus A, Genin M, Maincent A et al. Hypoxia-induced modulation of apoptosis and BCL-2 family proteins in different cancer cell types. PloS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhai C, Lv J, Wang K et al. HSP70 silencing aggravates apoptosis induced by hypoxia/reoxygenation in vitro. Experiment Ther Med 2019;18:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu D, Li DW, Xie J et al. Effect and mechanism of survivin on hypoxia-induced multidrug resistance of human laryngeal carcinoma cells. BioMed Res Int 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang WJ, Xia LM, Zhu F et al. Transcriptional upregulation of HSP70-2 by HIF-1 in cancer cells in response to hypoxia. Int J Cancer 2009;124:298–305. [DOI] [PubMed] [Google Scholar]
- 41. Saksonová S, Brodňanová M, Dibdiakova K et al. Cobalt chloride affects the death of SH-SY5Y cells induced by inhibition of ubiquitin proteasome system. Role of heat shock protein 70 and caspase 3. Gen Physiol Biophys 2018. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Z, Gao J, Cai X et al. Escin sodium induces apoptosis of human acute leukemia Jurkat T cells. Phytother Res 2011;25:1747–55. [DOI] [PubMed] [Google Scholar]
- 43. Zhu J, Yu W, Liu B et al. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Çiftçi GA, Işcan A, Kutlu M. Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cell lines. Cytotechnology 2015;6:7893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther 2008;7:1875–84. [DOI] [PubMed] [Google Scholar]
- 46. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 2016;1863:2977–92. [DOI] [PubMed] [Google Scholar]
- 47. Azimi I, Petersen RM, Thompson EW et al. Hypoxia-induced reactive oxygen species mediate N-cadherin and SERPINE1 expression, EGFR signalling and motility in MDA-MB-468 breast cancer cells. Sci Rep 2017;7:15140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 2010;16:5928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guzy RD, Hoyos B, Robin E et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 2005;1:401–8. [DOI] [PubMed] [Google Scholar]
- 50. Liu Y, Cui Y, Shi M et al. Deferoxamine promotes MDA-MB-231 cell migration and invasion through increased ROS-dependent HIF-1α accumulation. Cell Physiol Biochem 2014;33:1036–46. [DOI] [PubMed] [Google Scholar]
- 51. Zong L, Li J, Chen X et al. Lipoxin A4 attenuates cell invasion by inhibiting ROS/ERK/MMP pathway in pancreatic cancer. Oxid Med Cell Longev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee HS, Hong JE, Kim EJ, Kim SH. Escin suppresses migration and invasion involving the alteration of CXCL16/CXCR6 Axis in human gastric adenocarcinoma AGS cells. Nutr Cancer 2014;66:938–45. [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Xu X, Zhao P et al. Escin Ia suppresses the metastasis of triple-negative breast cancer by inhibiting epithelial-mesenchymal transition via down-regulating LOXL2 expression. Oncotarget 2016;7:23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: Functional interactions in tumor stroma-infiltrating myeloid cells. Sci World J 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng J, Wang X, Ran L et al. Hypoxia-inducible factor 1α regulates the transforming growth factor β1/SMAD family member 3 pathway to promote breast cancer progression. J Breast Cancer 2018;21:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee YL, Cheng WE, Chen SC et al. The effects of hypoxia on the expression of MMP-2, MMP-9 in human lung adenocarcinoma A549 cells. Eur Resp J 2014;44:P2699. [Google Scholar]
- 57. Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 2004;64:7464–72. [DOI] [PubMed] [Google Scholar]
- 58. Mori K, Uchida T, Yoshie T et al. A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS J 2019;286:459–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer 2019;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daniele A, Zito AF, Giannelli G et al. Expression of metalloproteinases MMP-2 and MMP-9 in sentinel lymph node and serum of patients with metastatic and non-metastatic breast cancer. Anticancer Res 2010;30:3521–7. [PubMed] [Google Scholar]
- 61. Voura EB, English JL, Hoi-Ying EY et al. Proteolysis during tumor cell extravasation in vitro: metalloproteinase involvement across tumor cell types. PLoS One 2013;8:e78413. [DOI] [PMC free article] [PubMed] [Google Scholar]


