Abstract
Benzyl butyl phthalate (BBP) is a persistent environmental pollutant. BBP exposure and the possible effects on human neural tube defects (NTDs) remain elusive. In this study, we found that the detection ratio of positive BBP and its metabolites in maternal urine was obviously higher in NTDs’ population than that in normal controls by GC-MS (P < 0.01, P < 0.05, respectively). Animal experiments showed that BBP treatment induced developmental toxicity in chick embryo by enhancing the levels of oxidative stress and cell apoptosis (P < 0.01). More interestingly, the supplement of high-dose choline (CHO, 10 5 μg/mL) could partially restore the teratogenic effects of BBP by inhibiting the occurrence of oxidative stress. Our data collectively suggest that BBP exposure may disturb neural tube development by strengthening oxidative stress. CHO can partially restore the toxicity effects of BBP. This study may provide new insight for NTD prevention.
Keywords: BBP, human NTDs, oxidative stress, body fluid, chick embryo
Introduction
Neural tube defects (NTDs) are one of the most common crippling birth defects with an overall incidence in the United States of America of 1–2 per 1000 births [1]. China, which has the largest population in the world also has the highest incidence of birth defects in which NTDs are in the top five [2]. There are three common types of NTDs: encephalocele, anencephaly, and spina bifida cystica (open spina bifida), which are considered to be resulting from the failure of normal neural tube closure between the third and fourth week of embryonic development [3, 4]. A range of genetic and environmental factors are thought to be responsible for NTDs; environmental factors include nutritional deficiencies [5, 6], chemical exposures [7], air pollution [8], etc.
Benzyl butyl phthalate (BBP) is one kind of phthalic acid esters (PAEs), which has a close relationship with reproductive defects [9]. Studies showed that BBP retarded the outgrowth of fibroblasts from newborn rat cerebellum [10, 11]. The weight of uterus and ovary declined significantly when the immature female rats were administered BBP (200 mg/kg) orally from postnatal day 21 daily for 20 days [12]. These results suggest that BBP may affect the development of embryos in animals. However, up to now, limited information was available about the possible link between BBP and the occurrence of NTDs.
Some studies reported that NTDs were associated with oxidative stress in mouse and rats [13–15]. Oxidative stress from psychosocial stress might affect neurodevelopment in autism [16] and inhibit the expression of Pax-3, which is essential for neural tube closure [15]. PAE exposure altered the peroxidase level and the malondialdehyde (MDA) production on abalone embryonic development [17], suggesting that oxidative stress may be involved in PAE-induced abnormal embryonic development. However, whether NTDs induced by BBP were associated with reactive oxygen species is still largely unknown.
In this study, the distribution of BBP and its metabolites monobenzyl phthalate (MBZP) was detected in urine from mothers whose babies had NTDs and age-matched controls. Furthermore, reactive oxygen species (ROS) level and oxidative stress indicators were detected in BBP-treated chick embryos. Recovery experiments by nutritional supplements were also performed to observe the changes of indicators described above following BBP exposure. The study may provide new insights into prevention of NTDs at early stage of pregnancy.
Method and Materials
Sample collections
A population-based embryo development defects survey was performed in Family Planning Technique Service Station in Qian’xi, Hebei Province. The station provides family planning technique service, fertility guidance and contraceptive counseling, premarital counseling, and newlywed health care, premarital health care, and medical examination. Mothers whose babies had NTDs and age-matched controls were surveyed by questionnaire which contained the general condition. The inclusion criteria included that fetus was found to be spina bifida or anencephaly by B-mode ultrasound, and participants or their family members signed an informed consent. The exclusion criteria included that ultrasound diagnosis of fetal congenital spina bifida accompanied by other malformations in pregnant women; serum HIV and/or syphilis antibody were positive; patients with a history of underlying diseases, severe cardiovascular disease, diabetes, severe infections, etc.; refusal to sign informed consent; and severe deficiency of folic acid. The primary outcome measure was ultrasound diagnosis of fetal spina bifida in pregnant women. The secondary outcome measure was serum folate levels in pregnant women. The control groups were obtained from allowable therapeutic abortions or pregnancy test. In case group, induced abortions were performed when fetus was found to be spina bifida or anencephaly by B-mode ultrasound. After approval of the patient consent, urine was collected and frozen in −20°C refrigerator. This study was approved by the Ethics Committee of National Research Institute for Family Planning. The collection of fetal tissues followed the procedures that are in accordance with the ethical standards as formulated in the Helsinki Declaration.
The detection of BBP in maternal urine by GC-MS
The urine samples (100 μL) were extracted by hexane and shaken on the concentrator. Then organic phase was taken from the upper layer. The process of extraction is repeated for three times. The organic phase was adsorbed by N2 and dissolved by hexane. After the pretreatment of urine, the detection of BBP and its metabolites MBZP was performed by GC-MS assay, and recovery (%), retention time (R.T.), characteristic ion, and limits of quantification (LOQs) of BBP and MBZP were shown in Table S1, respectively.
Embryo treatment
White Leghorn chicken eggs (Bovan strain) were purchased from Merial Vital Laboratory Animal Technology Co., Ltd (Beijing, China). To begin embryonic development, chick eggs were placed in automatic tilting racks in an incubator (Grumpatch, Savannah, GA, USA) and incubated at 38°C and 60% humidity. According to our preliminary experiments, chick embryos were treated with BBP 31.1 μg/mL with/without 104 μg/mL folic acid (FA), 1312.9 μg/mL ferrous sulfate (FeSO4, Fe), or 105 μg/mL choline (CHO) at Hamburger-Hamilton (HH) stages 6, 8, and 12 [18]. BBP, FA, FeSO4, and CHO were directly injected into the center of the egg yolk via a small hole at the blunt end of the egg using an established protocol [19] at HH stages 6, 8, and 12, respectively. Embryos were harvested for analysis after incubation for 72 h (HH stage 20).
The detection of ROS in chick embryos
Live chick embryos, harvested after 72 h, were taken into the 24-well cell culture plate. 10 μM DCFH-DA 500 μL was added into the plate at 37°C for 30 min. Samples without the addition of DCFH-DA were used as the negative control. The whole-mount images were visualized using 20× objective under Zeiss lumar V12 fluorescence stereomicroscope (Zeiss, Jena, Germany). Fluorescence intensity was analyzed using Axiovision Rel.4.8 software. Quantification of fluorescence in each treatment group should substract the background from the negative control values.
The detection of oxidative stress-related indicators in chick embryos
The detection of malondialdehyde (MDA) was performed by Lipid Peroxidation (MDA) Assay Kit (Beyotime, China). The absorbance of samples was recorded at 532 nm with a 96-well plate reader (Bio-Rad 3550). The content of MDA could be calculated according to the standard curve.
The detection of total superoxide dismutase (SOD) and CuZn/Mn-SOD was carried out by Cu/Zn-SOD and Mn-SOD Assay Kit with WST-1 (Beyotime, China). The absorbance of samples was recorded at 450 nm with a 96-well plate reader (Bio-Rad 3550). The inhibition ratio is shown by the following equation: The inhibition ratio = [(A blank 1-A blank2)-(Asample-Ablank3)]/(A blank 1-A blank 2) × 100%. The definition of the unit of SOD activity is that SOD activity is one unit when the inhibition ratio is 50% at the reaction system described above.
The detection of glutathione peroxidase (GPX) was performed by Cellular Glutathione Peroxidase Assay Kit (Beyotime, China). The absorbance of samples was recorded at 340 nm with a 96-well plate reader (Bio-Rad 3550). The recording was performed every 30s a time for at least 3 min. The definition of the unit of GPX activity is that one unit can catalyze 1 μm nicotinamide adenine dinucleotide phosphate (NADPH) to nicotinamide adenine dinucleotide phosphate (NADP+) within 1 min in the existence of glutathione (GSH), glutathione reductase (GR), and t-Bu-OOH at 25°C and PH 8.0.
Terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end labeling (TUNEL) staining
In whole-mount TUNEL staining, chicken embryos were fixed, dehydrated, and rehydrated through graded methanol concentrations into PBS containing 0.1% Tween-20 (PBST). DeadEnd Colorimetric TUNEL System (Promega, Madison, WI, USA) was used for in situ visualization of DNA fragmentation in whole-mount embryos. Embryos were incubated with a solution consisting of equilibration buffer, nucleotide mix, and TdT enzyme at 4 °C overnight and then incubated with streptavidin-horseradish peroxidase and stained with diaminobenzidine. Embryos were examined and recorded using 20× objective under the bright field of a Zeiss lumar V12 fluorescence stereomicroscope (Zeiss, Jena, Germany). At least three embryos were analyzed in each treatment group in whole-mount TUNEL staining. Due to the limit in the magnification of the stereomicroscope (Zeiss, Jena, Germany) in our laboratory, so far, it is not ideal to use this scope to observe individual apoptotic cells in the whole-mount photographs. So the mean optical densities (MOD) of apoptotic signals were analyzed using NIS-Elements Br 3.0 software (Nikon, Japan) in five different optical fields selected in a random manner for each sample. The percentage of MOD of apoptotic signals were expressed as ratio of MOD of apoptotic signals vs total signals of five different optical fields selected in a random manner.
Data analysis
The analyses were performed using the SPSS 25.0 statistical software package. All data were analyzed using analysis of variance (ANOVA), in which results were presented as mean values (±SD) in at least three independent experiments. A value of P < 0.05 was considered statistically significant.
Results
The distribution of BBP in urine in NTD population
To investigate whether NTDs are associated with BBP, the distribution of BBP and its metabolites MBZP was detected in urine from 44 pregnant women whose babies had NTDs and 40 age-matched controls by GC-MS. Four cases are excluded in this study and the percentage was about 10%. As shown in Table 1 and Fig. S1, the detection ratio of positive BBP and MBZP in the NTD population was about 36.59 and 4.88%, respectively. In control group, we only detected BBP, and the detection ratio of positive samples was about 10.00%. These results indicated that the incidence of NTDs in humans might associate with the distribution of BBP.
Table 1.
Detection of benzyl butyl phthalate (BBP) and its metabolites in urine
| Metabolites | Controls | Cases | ||||
|---|---|---|---|---|---|---|
| N | N of positive samples | Positive ratio | N | N of positive samples | Positive ratio (%) | |
| BBP | 40 | 4 | 10.00% | 41 | 15 | 36.59** |
| MBZP | 40 | 0 | 0% | 41 | 2 | 4.88* |
MBZP: monobenzyl phthalate
**Compared with controls, P < 0.01
*Compared with controls, P < 0.05
BBP induces ROS and alters the level of oxidative stress indicators in chick embryos
Since the results from the NTD population indicate that higher detection for BBP may be observed in women with NTD affected fetuses, we decided to use chick embryos as models to further our investigations on BBP exposure. The ROS level and oxidative stress indicators were identified in BBP-treated chick embryos. As shown in Fig. 1, the fluorescence signals of ROS were increased 2.1-fold in BBP-treated chick embryos compared with the control (administration of saline, P < 0.01), suggesting that BBP could induce the production of oxidative stress in chick embryos. The result was similar from the analysis of the fluorescence signals of ROS in the brain, heart, and spine of chick embryos, respectively (Fig. 1B2–B4).
Figure 1.
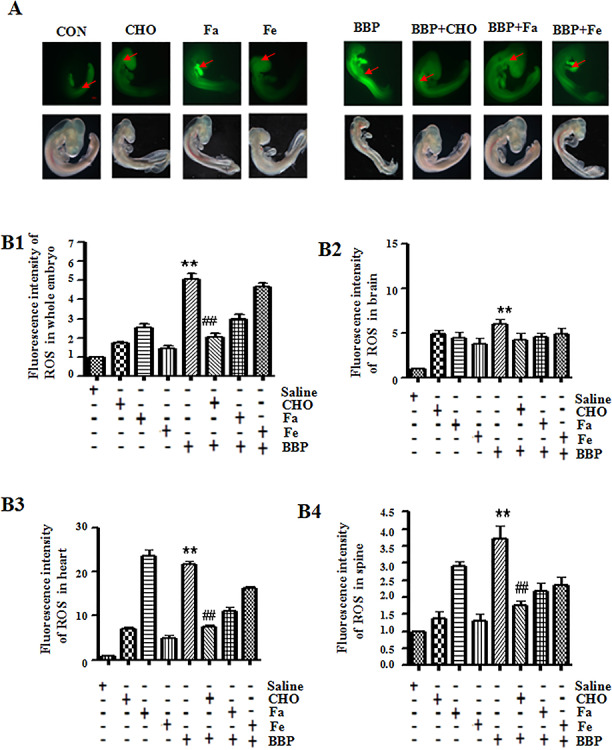
Effects of benzyl butyl phthalate (BBP) on reactive oxygen species (ROS) in chick embryos. (A) The ROS activity was analyzed in chick embryos treated with BBP and/or nutrients for 72 h. Scale bar = 500 μm. Red arrow indicated positive signals. (B1-B4) The histogram represents the mean optical densities (MOD) of positively signals of ROS in whole embryos, brain, heart, and spine, respectively. *P < 0.05, or **P < 0.01, compared with controls (administration of saline, folic acid (FA, 104 μg/mL), FeSO4 (Fe, 1312.9 μg/mL), or choline (CHO, 105 μg/mL) alone); #P < 0.05, or ##P < 0.01, compared with administration of BBP (31.1 μg/mL) alone
Oxidative stress indicators, including SOD, MDA, and GPX, were altered after BBP treatment. As shown in Fig. 2, the total SOD activity was significantly decreased in BBP -treated chick embryos (P < 0.05) as compared with the control (administration of saline). Cu-Zn SOD activity was also dramatically decreased (P < 0.05). BBP significantly reduced the level of MDA (P < 0.01) but did not obviously change the activity of GPX. All these results further implied that BBP-induced abnormal embryonic development may be closely associated with oxidative stress response.
Figure 2.
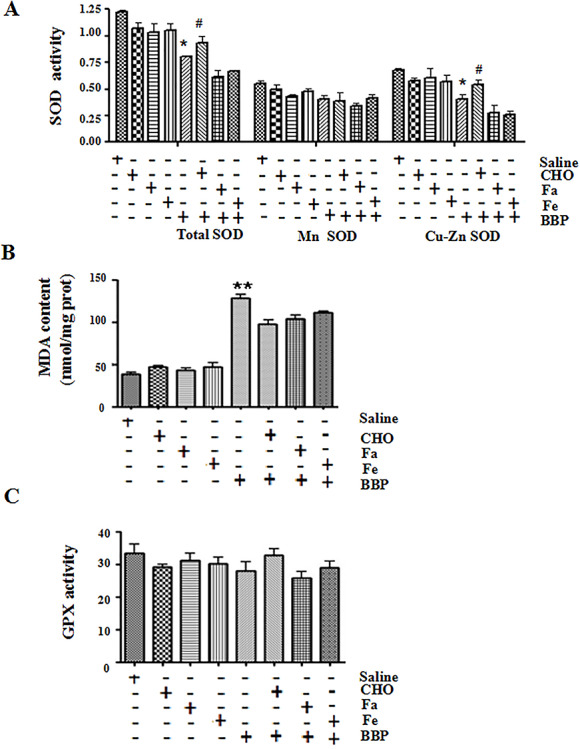
effects of benzyl butyl phthalate (BBP) on the oxidative stress-related indicators in chick embryos. (A) The activity of total superoxide dismutase (SOD), Mn SOD, and Cu-Zn SOD was analyzed in chick embryos treated with BBP and/or nutrients for 72 h. (B and C) The malondialdehyde (MDA) content and glutathione peroxidase (GPX) activity were detected in chick embryos treated with BBP and/or nutrients. All data correspond to mean ± S.D (n = 3). *P < 0.05, or **P < 0.01, compared with control (administration of saline, folic acid (FA, 104 μg/mL), FeSO4 (Fe, 1312.9 μg/mL), or choline (CHO, 105 μg/mL) alone); #P < 0.05, or ##P < 0.01, compared with administration of BBP (31.1 μg/mL) alone
BBP affects the apoptosis in chick embryos
It has been reported that oxidative stress can induce cellular apoptotic responses [17]. Therefore, we analyzed the change of cell apoptosis in BBP-treated chick embryos by whole-mount TUNEL assay. The results showed that TUNEL-positive signals were significantly increased in whole embryo after BBP treatment compared with controls (Fig. 3A and B1, P < 0.01), which indicated that BBP could promote apoptosis. The result was similar from the analysis of TUNEL-positive signals in the brain, heart, and spine of chick embryos, respectively (Fig. 3B2–B4).
Figure 3.
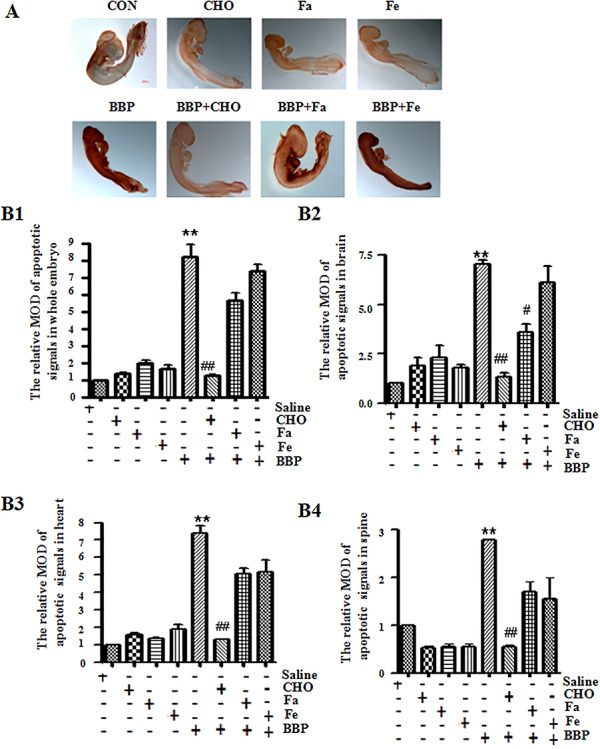
effects of benzyl butyl phthalate (BBP) on the apoptosis in chick embryos. (A) Apoptosis signals were detected by whole-mount TUNEL staining in chick embryos treated with BBP and/or nutrients for 72 h. Scale bar = 500 μm. (B1-B4) The histogram represents the relative mean optical densities (MOD) of apoptotic signals in whole embryos, brain, heart, and spine, respectively. The percentage of MOD of apoptotic signals were expressed as ratio of MOD of apoptotic signals vs total signals of five different optical fields selected in a random manner. *P < 0.05, or **P < 0.01, compared with control (administration of saline, folic acid (FA, 104 μg/mL), FeSO4 (Fe, 1312.9 μg/mL), or choline (CHO, 105 μg/mL) alone); #P < 0.05, or ##P < 0.01, compared with administration of BBP (31.1 μg/mL) alone
High dose of CHO protects chick embryos from BBP-induced teratogenic effects
In order to seek the nutrients which can antagonize toxicity caused by BBP, recovery experiments by nutritional supplements were performed to observe the changes of phenotype (Figs 1–3). The supplement of FeSO4 (Fe, 1312.9 μg/mL), folic acid (FA, 104 μg/mL), or CHO (105 μg/mL) was administrated into chick embryos treated with or without BBP (31.1 μg/mL), respectively.
The ROS level was evidently restrained in BBP-treated chick embryos by CHO (105 μg/mL, Fig. 1A and B1, Table 2). Similar results were from the analysis of the fluorescence signals of ROS in the heart and spine of chick embryos, respectively (Fig. 1B3–B4).
Table 2.
The effect of supplement of nutritive elements on BBP-induced teratogenic effects in chick embryos
| Risk factor | BBP | ||
|---|---|---|---|
| Nutritive element | Fa | CHO | Fe |
| Concentration | 104 μg/mL | 105 μg/mL | 1312.9 μg/mL |
| Apoptosis | No | Yes | No |
| ROS | No | Yes | No |
| Total SOD | No | Yes | No |
| Cu-Zn SOD | No | Yes | No |
| Mn SOD | No | No | No |
| GPX | No | No | No |
| MDA | No | Yes | No |
BBP, benzyl butyl phthalate; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; GPX, glutathione peroxidase; MOD, mean optical densities; Fe, FeSO4; FA, folic acid; CHO, choline
Yes means that the supplement of nutritive element can partially restore teratogenic effects in chick embryos. No means that this kind of nutritive element has little effect.
CHO (105 μg/mL) supplement increased total SOD and Cu-Zn SOD activity in BBP-treated chick embryos (Fig. 2A, P < 0.05, Table 2). However, CHO (105 μg/mL) had little effect on the level of MDA (Fig. 2B, Table 2). Moreover, Fe and FA had no obvious effect on ROS level, SOD activity, and MDA content. Moreover, the GPX activity had almost no significant changes after the treatment of BBP and other nutrients.
In addition, CHO (105 μg/mL) significantly restrained apoptosis of BBP-treated chick embryos (Fig. 3A and B1, P < 0.01,Table 2). However, there were no significant changes in BBP-induced apoptosis in chick embryos with supplement of Fe and FA. The result was similar from analysis of TUNEL-positive signals in the brain, heart, and spine of chick embryos, respectively (Fig. 3B2–B4). All these results indicated that high dose of CHO protected chick embryos from BBP-induced abnormal molecular changes.
Discussion
The study firstly reported exposure conditions of BBP in maternal body liquid in pregnant women whose babies had NTDs, revealing that BBP had adverse effects on human pregnancy and early embryo development.
BBP, which is commonly added during the manufacturing of plastics to increase flexibility and elasticity, can cause developmental toxicity [20]. Nicole et al. reported that BBP induces caudal defects during embryonic development [20]. BBP markedly inhibited the outgrowth of nerve fibers and glial cells from cerebellar explants of newborn rat in primary culture at concentrations of 7.0 × 10−4 M [11]. However, the possible effects of BBP on human NTD remain elusive. In this study, the detection ratio of BBP and its metabolites MBZP in maternal urine in NTD population was higher than that in controls (P < 0.01, P < 0.05, respectively), which indicated that BBP might be associated with the NTDs to some extent.
To further identify the effects of BBP on early embryo development and investigate the possible pathogenic mechanism, the study chose chick embryos as an animal model for further investigation. There are various advantages of chicken embryo, such as its transparent appearance and faster time course. Moreover, since there is no maternal influence in chicken embryos, it can reflect the direct action of exogenous high BBP on the nervous system. It seems that the model of chick embryo has been widely used in many researches focusing on NTDs [21–23].
Some reports indicated that the occurrence of NTDs associated with oxidative stress [13–15]. Our research indicates that the level of ROS was significantly enhanced in BBP-treated chick embryos (P < 0.01). Additionally, the alteration of oxidative stress-related indicators shows that BBP enhanced lipid peroxidation and inhibited the antioxidation in chick embryos. These results imply that BBP induced abnormal development of neural via boosting the oxidative stress response.
Studies revealed that oxidative stress can induce the response of cellular apoptosis [24, 25]. Emerging evidence indicate that oxidative stress closely associates with induction of programmed cell death in neurodevelopment in both animal models and human nerve biopsies [24–26]. In our research, the signal of apoptosis was significantly more obvious in BBP-treated chick embryos than control (P < 0.01), which was consistent with the results of oxidative stress level, implying that oxidative stress was concerned with BBP-induced abnormal development of neural tube by accelerating cell apoptosis.
In order to further analyze the effects of BBP on embryo development, the recovery experiment by nutritional supplements was performed to seek the nutrients which can antagonize embryo toxicity of BBP. The results showed that CHO (105 μg/mL) supplementation could partially restore the level of ROS and oxidative stress indicators and restrained apoptosis. Studies indicate that CHO has critical and diverse roles in both cellular maintenance and growth across all life stages of the human, including roles in membrane synthesis, neurotransmission, one-carbon metabolism, and lipid transport [27]. CHO closely associates with adverse health outcomes, including birth defects, hepatic steatosis, neurodevelopment and cognition alterations, cancer, and cardiovascular disease (CVD) [27]. In our research, high-dose CHO may inhibit the occurrence of oxidative stress by increasing the SOD activity and reducing MDA content to antagonize toxic damage induced by BBP.
It’s known that FA is essential for health from early life to old age, which is vital for rapidly growing tissues and proliferating cells [28]. Beneficial effects of folate fortification and supplementation in preventing neural tube defects (NTD) have been well-documented [29–31]. However, FA supplement, which did not show significant effect in protecting embryos in the study, may be associated with its little effect on oxidative stress response. These findings may provide clues to neural tube defects which can be not protected from FA supplementation. However, the molecular mechanism of CHO protective effect still needs further research.
Conclusion
The study established the first association between BBP and the incidence of human NTDs by analyzing the detection rate of positive BBP in NTD population. The results were then further confirmed by experiments from chick embryos. Additionally, CHO (105 μg/mL), not FA supplementation, could partially reverse the embryo toxicity induced by BBP. The molecular mechanism of CHO protective effect still needs research in the future. All these findings may give insight into understanding of NTD etiology and create an opportunity to approach the prevention and diagnosis of NTDs.
Competing interests
The authors declare that they have no competing interests.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2016YFC1000307) and CAMS Innovation Fund for Medical Sciences (CIFMS, 2018-I2M-1-004).
References
- 1. Au K-S, Northrup H, Kirkpatrick TJ et al. Promotor genotype of the platelet-derived growth factor receptor-alpha gene shows population stratification but not association with Spina bifida Meningomyelocele. Am J Med Genet A 2005;139:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liao Y, Wang J, Li X et al. Identifying environmental risk factors for human neural tube defects before and after folic acid supplementation. BMC Public Health 2009;9:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren A, Qiu X, Jin L et al. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc Natl Acad Sci U S A 2011;108:12770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol 2010;88:653–69. [DOI] [PubMed] [Google Scholar]
- 5. Hook EB, Czeizel AE. Can terathanasia explain the protective effect of folic-acid supplementation on birth defects? Lancet 1997;350:513–5. [DOI] [PubMed] [Google Scholar]
- 6. Brender JD, Zhan FB, Suarez L et al. Linking environmental hazards and birth defects data. Int J Occup Environ Health 2006;12:126–33. [DOI] [PubMed] [Google Scholar]
- 7. Brender JD, Suarez L, Felkner M et al. Maternal exposure to arsenic, cadmium, lead, and mercury and neural tube defects in offspring. Environ Res 2006;101:132–9. [DOI] [PubMed] [Google Scholar]
- 8. Gilboa SM, Mendola P, Olshan AF et al. Comparison of residential geocoding methods in population-based study of air quality and birth defects. Environ Res 2006;101:256–62. [DOI] [PubMed] [Google Scholar]
- 9. Main KM. Phthalate monoesters and infant reproductive health. Gesundheitswesen 2008;1:S46–8. [DOI] [PubMed] [Google Scholar]
- 10. Teranishi H, Kasuya M. The effects of phthalate esters on fibroblasts in primary culture. Toxicol Lett 1980;6:11–5. [DOI] [PubMed] [Google Scholar]
- 11. Kasuya M. Toxicity of butylbenzyl phthalate (BBP) and other phthalate esters to nervous tissue in culture. Toxicol Lett 1980;6:373–8. [DOI] [PubMed] [Google Scholar]
- 12. Ahmad R, Verma Y, Gautam AK et al. Assessment of estrogenic potential of di-n-butyl phthalate and butyl benzyl phthalate in vivo. Toxicol Ind Health 2015;31:1296–303. [DOI] [PubMed] [Google Scholar]
- 13. Siman CM, Eriksson UJ. Vitamin E decreases the occurrence of malformations in the offspring of diabetic rats. Diabetes 1997a;46:1054–61. [DOI] [PubMed] [Google Scholar]
- 14. Siman CM, Eriksson UJ. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia 1997b;40:1416–24. [DOI] [PubMed] [Google Scholar]
- 15. Chang TI, Horal M, Jain SK et al. Oxidant regulation of gene expression and neural tube development: Insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia 2003;46:538–45. [DOI] [PubMed] [Google Scholar]
- 16. McGinnis WR. Could oxidative stress from psychosocial stress affect neurodevelopment in autism? J Autism Dev Disord 2007;37:993–4. [DOI] [PubMed] [Google Scholar]
- 17. Zhou J, Cai ZH, Xing KZ. Potential mechanisms of phthalate ester embryotoxicity in the abalone Haliotis diversicolor supertexta. Environ Pollut 2011;159:1114–22. [DOI] [PubMed] [Google Scholar]
- 18. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Developmental Dynamics: An Official Publication of the American Association of Anatomists 1992;195:231–72. [DOI] [PubMed] [Google Scholar]
- 19. Rosenquist TH, Ratashak SA, Selhub J. Homocysteine induces congenital defects of the heart and neural tube: Effect of folic acid. Proc Natl Acad Sci U S A 1996;93:15227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy NM, Zambrzycka E, Santangelo J. Butyl benzyl phthalate (BBP) induces caudal defects during embryonic development. Environ Toxicol Pharmacol 2017;56:129–35. [DOI] [PubMed] [Google Scholar]
- 21. Chen XL, An Y, Gao YH et al. Rare deleterious PARD3 variants in the aPKC-binding region are implicated in the pathogenesis of human cranial neural tube defects via disrupting apical tight junction formation. Hum Mutat 2017;38:378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kobus-Bianchini K, Bourckhardt GF, Ammar D. Homocysteine-induced changes in cell proliferation and differentiation in the Chick embryo spinal cord: Implications for mechanisms of neural tube defects (NTD). Reprod Toxicol 2017;69:167–73. [DOI] [PubMed] [Google Scholar]
- 23. Uriarte S, Barrena MJ, López de Torre B et al. Amniotic and serum Alphafetoprotein in the Chick embryo with neural tube defect. Cirugía Pediátrica: Organo Oficial de la Sociedad Española de Cirugía Pediátrica 1993;6:137–40. [PubMed] [Google Scholar]
- 24. Youn CK, Song PI, Kim MH et al. Human 8-oxoguanine DNA glycosylase suppresses the oxidative stress induced apoptosis through a p53-mediated signaling pathway in human fibroblasts. Mol Cancer Res 2007;5:1083–98. [DOI] [PubMed] [Google Scholar]
- 25. Takeda K, Matsuzawa A, Nishitoh H et al. Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct 2003;28:23–9. [DOI] [PubMed] [Google Scholar]
- 26. Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci 2002;959:368–83. [DOI] [PubMed] [Google Scholar]
- 27. Wiedeman AM, Barr SI, Green TJ. Dietary choline intake: Current state of knowledge across the life cycle. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kao TT, Chu CY, Lee GH et al. Folate deficiency-induced oxidative stress contributes to neuropathy in young and aged zebrafish — Implication in neural tube defects and Alzheimer's diseases. Neurobiol Dis 2014;71:234–44. [DOI] [PubMed] [Google Scholar]
- 29. Czeizel AE, Dudás I, Vereczkey A et al. Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 2013;5:4760–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang HC, De SH, Chen G et al. Effectiveness of folic acid fortified flour for prevention of neural tube defects in a high risk region. Nutrients 2016;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zaganjor I, Sekkarie A, Tsang BL et al. Describing the prevalence of neural tube defects worldwide: A systematic literature review. PLoS One 2016;11:e0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


