Abstract
Lead (Pb) is an environmental and industrial contaminant that still represents a public health problem. In this paper, we investigated the effect of Pb on proliferation, lipid peroxidation and the number of micronucleated cells in exponentially growing 3T3-L1 fibroblasts, a cell line previously used to evaluate different environmental contaminants.
We found that Pb (10 μM or higher) was able to inhibit proliferation of exponentially growing cells after 24-h treatment, which was evaluated by the MTT assay and cell counting in Neubauer chamber, but cell survival was not affected according to the trypan blue exclusion assay. On the other hand, Pb was able to increase lipid peroxidation and the number of micronucleated cells, which are indicative of oxidative stress and genotoxic damage respectively. We also found that removal of Pb after 24-h treatment allowed cells to recover proliferation. Our results indicate that Pb was able to induce oxidative stress and genotoxicity in this cell line under standardized conditions, which supports the involvement of Pb in similar effects observed in human exposed to this heavy metal. In addition, Pb inhibits proliferation of exponentially growing fibroblasts but cells resume proliferation after removal of this metal, which suggests that it is important to move away Pb-exposed individuals from the source of contamination.
Keywords: lead, 3T3-L1 fibroblasts, proliferation, lipid peroxidation, micronucleated cells
Introduction
Lead (Pb) is a heavy metal and an environmental contaminant that still represents a public health problem. World Health Organization [1] has identified Pb as 1 of 10 chemicals of major public health concern, which requires action by Member States to protect the health of workers, children and women of reproductive age.
Pb enters the organism by ingestion of Pb-contaminated dust, water or food and/or by inhalation of particles containing Pb [2] and is found in the body in different organs such as brain, liver, kidney and bones but it is mainly stored in bones. Consistently, an increase in blood Pb was found in women after menopause when a decrease in bone density usually occurs [3].
Pb produces biochemical, physiological and behavioral alterations, which are usually greater in children [4, 5]. It can affect children’s brain development resulting in reduced intelligence quotient (IQ), behavioral changes such as reduced attention span and increased antisocial behavior and reduced educational attainment.
In 2006, the Scientific Committee on Neurotoxicology and Psychophysiology and the Scientific Committee on the Toxicology of Metals of the International Commission on Occupational Health recommended to reduce blood Pb levels to 30 μg/dl for industrial workers and 5 μg/dl for children. Nevertheless, Murata et al. [6], reported that the neurotoxic effects of Pb in workers appear to be initiated at levels <18 μg/dl, which are somewhat higher than the critical level of Pb neurotoxicity in children. At present, according to the Centers for Disease Control and Prevention, Pb blood levels <5 μg/dl are not considered to be indicative of Pb poisoning in children and chelation therapy is recommended when a child blood Pb test results ≥45 μg/dl.
Although Pb blood levels have decreased mainly because of the removal of Pb from gasoline [4, 7], exposure to Pb still remains in many different activities such as: soldering, plumbing work, working with metals or alloys containing Pb, mining, welding leaded steel [8]. In keeping with this, it was found that pottery-glaze workers had increased Pb blood levels and genotoxic damage in peripheral blood lymphocytes [9].
The delivery of drinking water through Pb pipes, the use of leaded-paints and Pb-acid batteries for motor vehicles still continues in some countries. In the last decade, exposure to Pb-contaminated soil and dust resulting from mining caused mass Pb poisoning and multiple deaths of children in Nigeria [10]. Recently [11], blood Pb levels were evaluated in 554 children and teenagers from 5 to 16 years old in the Colombian Caribbean and the higher average value of 8.9 ± 0.8 μg/dl was found in a poor fishing community, Tasajera. In this report, Pb exposure was associated with several alterations including physical development, IQ and the transcription of genes associated with inflammation, heme synthesis and oxidative stress.
Although most of Pb poisoning occurs in low- and middle-income countries, increase in blood Pb in children was also found in the USA, in the postindustrial city of Flint, Michigan, which changed its water supply to the Flint River as a temporary measure. Thus, a more corrosive water source was introduced into an aging water distribution system that contains a high percentage of Pb pipes and Pb plumbing, which favored Pb leaching into drinking water [12].
Elevated Pb exposure has been inversely correlated with femoral bone density and associated with osteoporosis [3, 13]. Consistently, it was found that rats exposed to Pb have decreased bone mass that resulted in bones that were more susceptible to fracture and that Pb promoted enhanced adipogenesis and decreased osteoblastogenesis [14, 15].
Pb exposure disturbs the balance of oxidants and antioxidant defenses, causing oxidative damage [16], studies in vivo have suggested that Pb increases the production of reactive oxygen species in fish [17]. Also, oxidative DNA damage and genotoxic effects of Pb in subjects occupationally exposed to this heavy metal has been reported [18].
3T3-L1 fibroblasts are a useful tool in the study of adipocyte differentiation. After addition of a mixture containing insulin, dexamethasone and 3-isobutyl-1-methylxanthine (MIX), post-confluent 3T3-L1 fibroblasts differentiate to adipocytes. In a previous paper, we found that Pb in concentrations up to 10 μM also improve adipogenesis in this cell line [19].
3T3-L1 fibroblasts have been used to evaluate the effects on cell survival and differentiation of arsenic trioxide, hexavalent chromium, as well as, a commercial preparation of the herbicide glyphosate [20–22].
In the present investigation, we evaluated the effect of Pb on proliferation, lipid peroxidation and the number of micronucleated cells in exponentially growing 3T3-L1 fibroblasts.
Materials and methods
Chemicals
Dulbecco’s Modified Eagle Medium (DMEM) and trypsin were obtained from Thermo Fischer Scientific (Waltham, MA), Hoechst 33258 was purchased from Sigma Chemical Co. (St. Louis, MO). Lead acetate trihydrate (Pb(CH3CO2)2·3H2O, CAS 6080-56-4) was purchased from Mallinckrodt (Argentina). 3T3-L1 fibroblasts were obtained from Asociación Banco Argentino de Células (origin: ATCC).
Cell culture and treatment of 3T3-L1 fibroblasts
3T3-L1 fibroblasts were cultured in DMEM +10% fetal bovine serum (FBS) with 100 μg/ml streptomycin, 100 U/ml penicillin and 250 ng/ml fungizone (DMEM +10% FBS). When indicated, Pb acetate was added to the plate in DMEM +10% FBS as vehicle, to obtain the appropriate final concentration.
Cell counting in exponentially growing cells
3T3-L1 fibroblasts were cultured in 24-well plates until they reached 30–40% confluence. At that moment, some wells were treated for 24 or 48 h with different μM concentrations of Pb, as indicated in each case, others were treated with DMEM +10% FBS alone (control) or sodium acetate (NaAc). At the end of these treatments, cells were either trypsinized, resuspended in phosphate-buffered saline (PBS) and an aliquot was counted using a Neubauer chamber after addition of trypan blue for analysis of cell survival or were incubated for 1 h at 37°C with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml), which was added to each well to reach a final concentration of 1 mg/ml MTT, and then, the supernatants were carefully aspirated and 200 μl of ice-cold ethanol was added to each well to dissolve the crystal product, which is measured by the absorbance at 570 nm.
Quantification of lipid peroxidation based on Fe(III)–xylenol orange complex Determination
3T3-L1 fibroblasts were cultured in 6-well plates until they reached 50% confluence. At that time, cells were treated for 24 h with medium (C) or with H2O2 250 μM or Pb 10 μM. After these treatments, cells were trypsinized, washed twice with PBS and resuspended in PBS. Then cells were centrifuged, the supernatants were removed and the pellets of cells were resuspended in methanol and used to determine lipid peroxidation by oxidation of FeSO4 to Fe(III)–xylenol orange complex by a modification of the method of Hermes-Lima et al. [23] as previously described [24].
Quantification of micronucleated cells
Fibroblasts were cultured on coverslips in 24-well plates until they reached 50% confluence. At this time, cells were treated for 24 h with medium (C), or with different μM concentrations of Pb. At the end of these treatments, cells were fixed in 4% formaldehyde and then stained with Hoechst 33258 for microscopic visualization of the nuclei.
Statistical analysis
The experiments were carried out three times unless otherwise stated. All data were expressed as mean ± SE Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test or Student’s t-test as indicated in each case, P values < 0.05 were considered significant.
Results
Effect of Pb exposure on exponentially growing 3T3-L1 fibroblasts
To evaluate the effect of Pb exposure on the viability of 3T3-L1 fibroblasts, we first investigated the effect of the addition of different concentrations of Pb acetate to exponentially growing cells using the MTT colorimetric assay (Fig. 1A). We found an increase in the absorbance of control cells after 24 h with respect to that obtained at the beginning of the experiment (time zero control, C0), as expected for proliferating cells. However, Pb concentrations 10 μM or higher inhibited this increase in absorbance while concentrations of 5 μM or lower not significantly differ from samples not treated with Pb or treated with sodium acetate 50 μM (C24 or NaAc, respectively). We further analyzed the dose-dependent effect of Pb on 3T3-L1 fibroblasts proliferation by cell counting in Neubauer chamber at 24 h and found similar results (Fig. 1B). Although cell number was decreased in the presence of 10 or 50 μM Pb, floating cells detached from the plates were not observed and analysis of trypan blue exclusion indicated that the number of dead cells was not increased with this treatment (data not shown), which suggests that Pb was inhibiting proliferation rather than cell viability.
Figure 1.
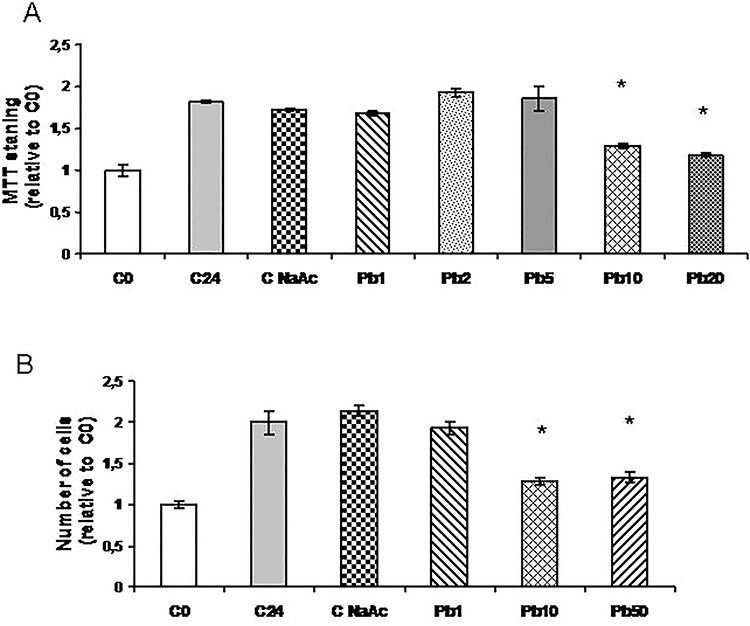
Dose-dependent effect of Pb on exponentially growing 3T3-L1 fibroblasts. Cells were cultured in 24-well plates until they reached 30–40% confluence. At that time, two plates were counted as time zero control (C0). Others were treated for 24 h with medium (C24), NaAc or different μM concentrations of Pb acetate (Pb) as indicated in each case. At the end of these treatments, cells were either incubated with MTT solution (A) or trypsinized and counted in Neubauer chamber (B). Both results are expressed relative to C0, which is set to 1. Results represent mean ± SE of three independent experiments. Asterisk represents significantly different from C24, P < 0.05 (ANOVA)
Effect of Pb exposure on lipid peroxidation
As several cytotoxic agents induce oxidative stress and it has been reported an increase of oxidative stress in Pb-exposed workers [25], we investigated the effect of the treatment of exponentially growing 3T3-L1 fibroblasts with 10 μM Pb for 24 h on lipid peroxidation that was used as an indicator of oxidative stress. H2O2 250 μM was used as a positive control for induction of oxidative stress. We found that Pb, as well as H2O2,were able to increase lipid peroxidation measured by the oxidation of Fe (II) and the appearance of Fe(III)–xylenol orange complex (Fig. 2).
Figure 2.
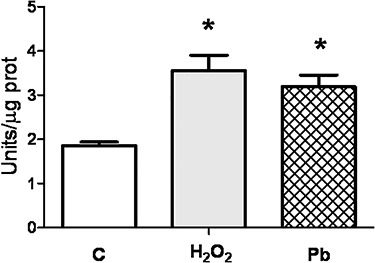
Effect of Pb on lipid peroxidation. 3T3-L1 fibroblasts were cultured in 6-well plates until they reached 50% confluence. At this time, cells were treated for 24 h with medium (C) or with H2O2 250 μM or Pb 10 μM and then lipid peroxidation was determined as indicated in Methods. Results represent mean ± SE of two independent experiments. Asterisk represents significantly different from C24, P < 0.05 (Student’s t-test).
Effect of Pb exposure on the number of micronucleated cells
On the other hand, an increase in micronucleus, which is associated with genotoxicity, was found in Pb-treated human lymphocytes [26] and lymphocytes from Pb-exposed workers [27]. Taking this into account, we analyzed the number of micronucleated cells in exponentially growing 3T3-L1 fibroblasts by microscopic observation after nuclei staining with Hoechst. In cells exposed to Pb concentrations of 10 μM or higher, which inhibit proliferation, the number of micronucleated cells significantly increased (Fig. 3).
Figure 3.
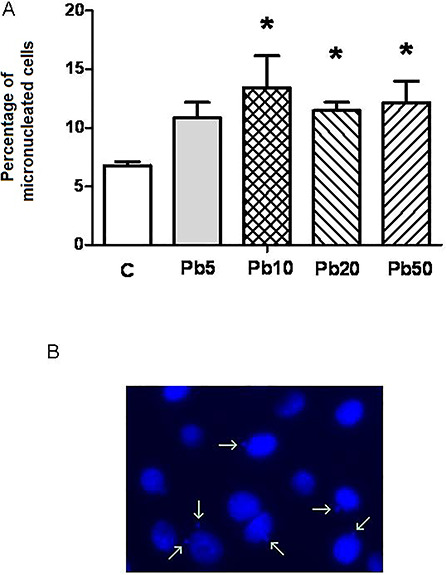
Effect of Pb on the number of micronucleated cells. 3T3-L1 fibroblasts were cultured in 24-well plates until they reached 50% confluence. At this time, cells were treated for 24 h with medium (C), or with different μM concentrations of Pb: 5 μM (Pb5), 10 μM (Pb10), 20 μM (Pb20) and 50 μM (Pb50). At the end of these treatments, cells were fixed in 4% formaldehyde and nuclei were stained with Hoechst 33258. At least 300 cells were analyzed to calculate the percentage of micronucleated cells for each treatment. Results represent mean ± SE of three independent experiments. Asterisk represents significantly different from C24, P < 0.05 (Student’s t-test) (A). A representative image of 3T3-L1 fibroblasts nuclei is shown where micronuclei are indicated by arrows (B).
Effect of Pb removal from exponentially growing 3T3-L1 fibroblasts
On the other hand, we wanted to evaluate if cells were able to recover their ability to proliferate after the removal of Pb. Thus, we treated 3T3-L1 fibroblasts with Pb for 24, 48 or 72 h with two concentrations that inhibit proliferation (10 and 50 μM). As shown in Fig. 4, although the number of cells increased with time in untreated cells, in cells treated with 10 μM Pb for 24, 48 or 72 h, the number of cells was significantly decreased with respect to those in the corresponding control (C24, C48 or C72, respectively). However, if Pb was removed after 24 h of treatment and replaced with medium between 24 and 48 h, proliferation was improved with respect to cells continuously exposed to Pb for 48 h. In addition, if recovery period was extended to 48 h, proliferation was almost completely recovered (Fig. 4). This is in keeping with the fact that cell viability was not affected by this treatment. Similar results were found with 50 μM Pb, which indicates that proliferation could be recovered after removal of the heavy metal.
Figure 4.
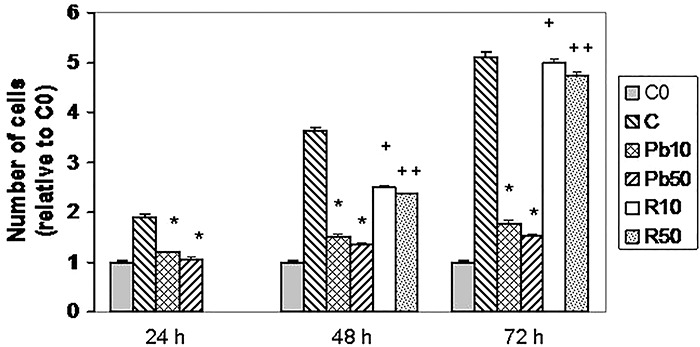
Effect of Pb removal from exponentially growing cells. Cells were cultured in 24-well plates until they reached 30–40% confluence. At that time, two plates were counted as time zero control (C0) and other plates were treated, in duplicates, for 24, 48 or 72 h with medium alone (C) or two concentrations of Pb (10 μM or 50 μM) for 24, 48 or 72 h. To analyze recovery, in other plates after 24 h treatment with 10 or 50 μM Pb, the medium was changed to a medium free of Pb for another 24 h or 48 h (R10 or R50) as indicated in this figure. At the end of these treatments, cells were trypsinized and counted in Neubauer chamber as indicated in methods. Results are expressed relative to C0, which is set to 1 and represent mean ± SE of three independent experiments. Asterisk significantly different from C at the corresponding time, P < 0.05; plus sign represents significantly different from 10 μM Pb for 48 or 72 h, P < 0.05 and double plus sign represents significantly different from 50 μM Pb for 48 h or 72 h, P < 0.05 (ANOVA).
Discussion
In this paper, we found that Pb was able to inhibit proliferation of exponentially growing 3T3-L1 fibroblasts at concentrations of 10 μM or higher after treatments for 24 h (Fig. 1) 48 and 72 h (Fig. 4). On the other hand, after these treatments with Pb floating cells were not observed and the number of dead cells was not increased according to the trypan blue exclusion assay, which suggests that Pb was not affecting cell viability. Consistently we found that removal of Pb after 24 h treatment, allowed cells to recover proliferation with respect to cells continuously exposed to Pb (Fig. 4). These results indicate that Pb effect on exponential cell proliferation is not irreversible under these conditions, which may be relevant from the environmental and toxicological point of view.
On the other hand, we found that treatment of 3T3-L1 fibroblasts with 10 μM Pb for 24 h was able to induce oxidative stress, which was evaluated by enhancement of lipid peroxidation. These results are in keeping with previous reports in Pb-exposed workers where lipid peroxidation was evaluated by the malondialdehyde assay [18, 25, 28, 29]
Genotoxic effects of Pb were previously reported in subjects exposed to Pb [18, 25], some of them evaluated the presence of micronucleus [26–28]. We confirmed the ability of Pb to induce genotoxic effects in 3T3-L1 fibroblasts after 24 h exposure. This is in agreement with previous results in other cell lines [30, 31].
The study with cell lines grow in culture in the laboratory under standardized conditions help to clarify the ability of Pb to induce specific effects since it is difficult to be certain that the differences observed among different Pb-exposed individuals are due to increase levels of Pb in circulation taking into account the likelihood of different background and exposition to a variety of different environmental contaminants among the individuals analyzed.
In our assays, we showed cytotoxic and genotoxic effects of Pb with concentrations of 10 μM. A concentration of Pb in blood of 30 μg/dl, which corresponds to 1.45 μM Pb, is recommended as acceptable for industrial workers by the Scientific Committee on Neurotoxicology and Psychophysiology and the Scientific Committee on the Toxicology of Metals of the International Commission on Occupational Health. Although blood concentrations recommended for Pb are lower than those used in our assays, higher local concentrations in certain tissues such as bone in Pb-exposed individuals could not be discarded. In addition, we evaluated acute treatments with Pb (24-72 h), however as Pb exposure is usually chronic in Pb-exposed individuals, similar effects are likely to be obtained with lower concentrations of Pb than the ones used in our assays [25].
In conclusion, according to our results, Pb was able to induce oxidative stress and genotoxicity in this cell line under standardized conditions, which supports the involvement of Pb in similar effects observed in human exposed to this heavy metal. In addition, Pb inhibits proliferation of exponentially growing fibroblasts but cells resumed proliferation after removal of this metal, which suggests that it is important to move away Pb-exposed individuals from the source of contamination.
Acknowledgements
This work was supported by research grants from Consejo Nacional de Investigaciones Cientıficas y Técnicas of Argentina (CONICET, PIP/0291) and Universidad de Buenos Aires. The funders had no role in study design, data and analysis, decision to publish or preparation of the manuscript.
Conflicts of interest
None declared.
References
- 1. World Health Organization Lead poisoning and health. Report, World Health Organization; 2018. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health [Google Scholar]
- 2. Kastury F, Smith E, Lombi E et al. Dynamics of lead bioavailability and speciation in indoor dust and x-ray spectroscopic investigation of the link between ingestion and inhalation pathways. Environ Sci Technol 2019. doi: 10.1021/acs.est.9b03249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nash D, Magder LS, Sherwin R et al. Bone density-related predictors of blood lead level among peri- and postmenopausal women in the United States: The third National Health and nutrition examination survey, 1988-1994. Am J Epidemiol 2004;160:901–11. [DOI] [PubMed] [Google Scholar]
- 4. Needleman H. Lead poisoning. Annu Rev Med 2004;55:209–22. [DOI] [PubMed] [Google Scholar]
- 5. Liu J, Ai Y, McCauley L et al. Blood lead levels and associated sociodemographic factors among preschool children in the south eastern region of China. Paediatr Perinat Epidemiol 2012;26:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murata K, Iwata T, Dakeishi M et al. Lead toxicity: does the critical level of lead resulting in adverse effects differ between adults and children? J Occup Health 2009;51:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Martínez SA, Simonella L, Hansen C et al. Blood lead levels and enzymatic biomarkers of environmental lead exposure in children in Cordoba, Argentina, after the ban of leaded gasoline. Hum Exp Toxicol 2013;32:449–63. [DOI] [PubMed] [Google Scholar]
- 8. Driscoll TR, Carey RN, Peters S et al. The Australian work exposures study: occupational exposure to lead and lead compounds. Ann Occup Hyg Aug 2015;312015. doi: 10.1093/annhyg/mev056. [DOI] [PubMed] [Google Scholar]
- 9. Kašuba V, Rozgaj R, Milić M et al. Evaluation of genotoxic effects of lead in pottery-glaze workers using micronucleus assay, alkaline comet assay and DNA diffusion assay. Int Arch Occup Environ Health 2012;85:807–18. [DOI] [PubMed] [Google Scholar]
- 10. Tirima S, Bartrem C, von Lindern I et al. Environmental remediation to address childhood lead poisoning epidemic due to artisanal gold mining in Zamfara, Nigeria. Environ Health Perspect 2016;124:1471–8. doi: 10.1289/ehp.1510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alvarez-Ortega N, Caballero-Gallardo K, Olivero-Verbel J. Toxicological effects in children exposed to lead: a cross-sectional study at the Colombian Caribbean coast. Environ Int 2019. doi: 10.1016/j.envint.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 12. Hanna-Attisha M, LaChance J, Sadler RC et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health 2016. doi: 10.2105/AJPH.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell JR, Auinger P. The association between blood lead levels and osteoporosis among adults--results from the third national health and nutrition examination survey (NHANES III). Environ Health Perspect 2007;115:1018–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beier EE, Maher JR, Sheu TJ et al. Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ Health Perspect 2013;121:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beier EE, Inzana JA, Sheu TJ et al. Effects of combined exposure to lead and high-fat diet on bone quality in juvenile male mice. Environ Health Perspect 2015;123:935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan Y, Zhao X, Yu J et al. Lead-induced oxidative damage in rats/mice: a meta-analysis. J Trace Elem Med Biol 2020;58:126443. doi: 10.1016/j.jtemb.2019.126443Epub 2019 Dec 4. [DOI] [PubMed] [Google Scholar]
- 17. Lee JW, Choi H, Hwang UK et al. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: a review. Environ Toxicol Pharmacol 2019;68:101–8. doi: 10.1016/j.etap.2019.03.010Epub 2019 Mar 8. [DOI] [PubMed] [Google Scholar]
- 18. Pawlas N, Olewińska E, Markiewicz-Górka I et al. Oxidative damage of DNA in subjects occupationally exposed to lead. Adv Clin Exp Med 2017;26:939–45. doi: 10.17219/acem/64682. [DOI] [PubMed] [Google Scholar]
- 19. Martini CN, Gabrielli M, Bonifacino G et al. Lead enhancement of 3T3-L1 fibroblasts differentiation to adipocytes involves ERK, C/EBPβ and PPARγ activation. Mol Cell Biochem 2018;437:37–44. doi: 10.1007/s11010-017-3093-y. [DOI] [PubMed] [Google Scholar]
- 20. Wang ZX, Jiang CS, Liu L et al. The role of AKT on arsenic trioxide suppression of 3T3-L1 preadipocyte differentiation. Cell Res 2005;15:379–86. [DOI] [PubMed] [Google Scholar]
- 21. Martini CN, Gabrielli M, Vila MC. A commercial formulation of glyphosate inhibits proliferation and differentiation to adipocytes and induces apoptosis in 3T3-L1 fibroblasts. Toxicol In Vitro 2012;26:1007–13. [DOI] [PubMed] [Google Scholar]
- 22. Martini CN, Brandani JN, Gabrielli M et al. Effect of hexavalent chromium on proliferation and differentiation to adipocytes of 3T3-L1 fibroblasts. Toxicol In Vitro 2014;28:700–6. [DOI] [PubMed] [Google Scholar]
- 23. Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III) xylenol orange complex formation. Free Radic Biol Med 1995;19:271–80. [DOI] [PubMed] [Google Scholar]
- 24. Martini CN, Gabrielli M, Brandani JN et al. Glyphosate inhibits PPAR gamma induction and differentiation of Preadipocytes and is able to induce oxidative stress. J Biochem Mol Toxicol 2016;30:404–13. doi: 10.1002/jbt.21804. [DOI] [PubMed] [Google Scholar]
- 25. Dobrakowski M, Pawlas N, Kasperczyk A et al. Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum Exp Toxicol 2017;36:744–54. doi: 10.1177/0960327116665674. [DOI] [PubMed] [Google Scholar]
- 26. Shah AJ, Lakkad BC, Rao MV. Genotoxicity in lead treated human lymphocytes evaluated by micronucleus and comet assays. Indian J Exp Biol 2016;54:502–8. [PubMed] [Google Scholar]
- 27. Nersesyan A, Kundi M, Waldherr M et al. Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat Res 2016;770:119–39. doi: 10.1016/j.mrrev.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 28. Singh Z, Chadha P, Sharma S. Evaluation of oxidative stress and genotoxicity in battery manufacturing workers occupationally exposed to lead. Toxicol Int 2013;20:95–100. doi: 10.4103/0971-6580.111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Čabarkapa A, Borozan S, Živković L et al. CaNa2EDTA chelation attenuates cell damage in workers exposed to lead-a pilot study. Chem Biol Interact 2015;242:171–8. doi: 10.1016/j.cbi.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 30. Liu X, Wu J, Shi W et al. Lead induces Genotoxicity via oxidative stress and promoter methylation of DNA repair genes in human Lymphoblastoid TK6 cells. Med Sci Monit 2018;24:4295–304. doi: 10.12659/MSM.908425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanders T, Liu YM, Tchounwou PB. Cytotoxic, genotoxic, and neurotoxic effects of mg, Pb, and Fe on pheochromocytoma (PC-12) cells. Environ Toxicol 2015;30:1445–58. doi: 10.1002/tox.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]


