Abstract
The aim of this study was to evaluate the impact of creatine supplementation (CS) on renal function in young, healthy, and active subjects. We used a randomized, double-blind, placebo-controlled clinical trial as the study design. Thirty-six healthy male university students were recruited and divided into three groups: group placebo, group G3 (3 g/day of CS), and group G5 (5 g/day of CS). To assess renal function, new kidney biomarkers, kidney injury molecule-1 (KIM-1) and monocyte chemoattractant protein-1 (MCP-1), were quantified. Serum albumin, serum creatinine, serum urea, estimated glomerular filtration rate (eGFR), proteinuria, and albuminuria were also measured. All groups were evaluated at two times: prior CS or placebo (pre) and after 35 days on CS or placebo (post). After 35 days of intervention, all characteristics were maintained without significant difference (P > 0.05) between the groups, including serum creatinine, eGFR, and more sensitive kidney biomarker concentrations (KIM-1 and MCP-1). The paired analysis showed that the supplemented groups (G3 and 5G) had increased serum creatinine and decreased eGFR levels (P < 0.05). However, the values were still within the normal reference range. In conclusion, the results of renal function evaluation did not show any difference between the evaluated groups. Increased serum creatinine and decreased eGFR levels in CS groups can be explained by increased creatine stores and metabolism, since creatinine is a by-product of creatine metabolism. These findings indicate that the use of CS at doses of 3 g and 5 g/day for a short period (35 days) is safe and did not impair the kidneys or renal function in young healthy subjects.
Keywords: sports supplementation, kidney injury, creatinine, KIM-1, MCP-1
Introduction
Creatine monohydrate is one of the most consumed dietary supplements in the world [1]. Creatine supplementation (CS) has been well known by researchers and professional athletes for at least half a century [2]. CS began to gain popularity at the 1992 Olympics in Barcelona, when British sprinter Linford Christie won the 100-meter race and attributed his gold medal to CS [3].
The first doubts concerning the CS safety occurred in 1998. In this year, three young wrestler fighters died in preparation for a competition while consuming CS [4]. Also in 1998, Kuehl et al. reported a case where CS was correlated with symptoms such as weight loss, fatigue, and dyspnea in a college football athlete [5].
Specifically regarding renal function, worries and questions began to appear after British nephrologists Pritchard and Kalra published a study reporting significant renal function loss in subjects who used 2 g of creatine per day for 14 days [6]. Studies in animal models also have demonstrated severe renal function loss in rats supplemented with creatine [7–9]. Despite these findings, several more recent studies have demonstrated that CS is perfectly safe for humans [10–17]. However, virtually all of these studies are limited by the capability to detect renal function decline at an early stage.
Therefore, although it has already been the subject of numerous studies, the impact of CS on renal function has not yet been fully clarified and promotes intense debates in the medical and academic field. This lack of consensus is mainly linked to the inherent limitations in evaluating the renal function decline based on markers commonly used in clinical practice [18].
Serum creatinine and eGFR based on creatinine values are routinely used as kidney function biomarkers. However, they are not optimal to specifically detect injury or dysfunction early enough to allow prompt therapeutic intervention. Moreover, creatinine is also secreted by the proximal tubules and overestimates eGFR [19].
Additional candidate biomarkers, such as urinary monocyte chemoattractant protein-1 (MCP-1) (glomerular and tubulointerstitial damage) and urinary kidney injury molecule-1 (KIM-1) (specifically for the proximal tubules), have been reported as being a more sensitive and specific kidney biomarker [20]. However, none of them met the criterion for use in specific clinical contexts. For the first time, these biomarkers were evaluated in this present study, aiming to detect clinical or subclinical kidney injury associated with creatine supplementation. Moreover, the present study is relevant as a new evidence of urinary MCP-1 and KIM-1 use as a renal biomarker.
Thus, this study, by means of a randomized, double-blind, placebo-controlled clinical trial, aims to investigate the impact of CS on renal function through traditional clinical evaluation and through more sensitive kidney biomarkers: KIM-1 and MCP-1.
Methods
Study design and participants
This is a randomized, double-blind, placebo-controlled clinical trial involving 36 healthy male university students engaged in resistance training. Inclusion criteria included the following: not using any kind of medicine; no personal history of cardiovascular, kidney, or liver disease; and not consuming any supplements that contained creatine in the last 60 days. Also, they were strictly advised to avoid alcohol consumption and not to change their food intake habits. More details about the study design can be found in the flow diagram (Fig. 1).
Figure 1.
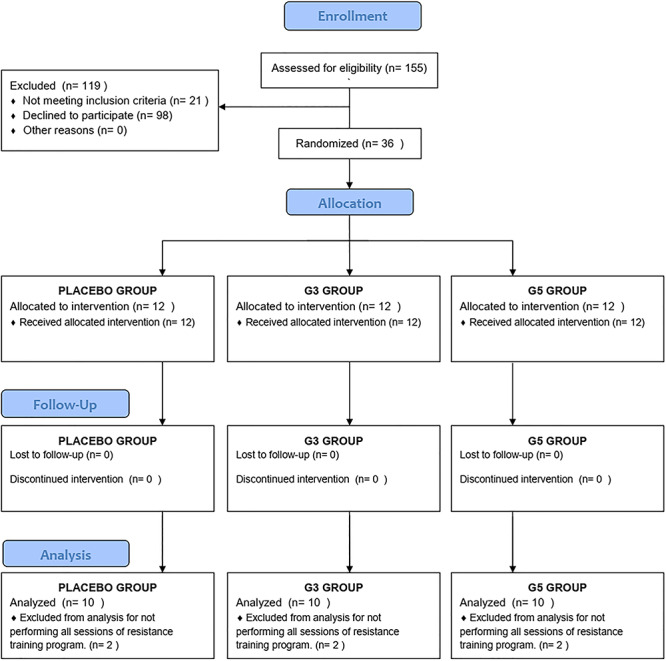
Flow diagram protocol based on CONSORT standards [21].
Creatine supplementation (CS) protocol and experimental approach
Pure micronized creatine monohydrate powder, supplied by Midway Labs, a reliable company in Brazil, was used in the study. The supplier guarantees the product purity based on a high-performance liquid chromatography test. In total, 36 kits with 35 sachets each of creatine powder or placebo (microcrystalline cellulose) were prepared. The kits and sachets were identical, and the kits were randomly coded. The association between the code and the composition of the sachets in each kit were known only by the Pharmacy School professionals at Universidade Federal do Ceará. The subjects and the researchers were blinded. Only after the study was finished, they had access to the codes, and it was possible to identify the correlation between codes and groups.
The participants used one sachet per day and were instructed to mix and dissolve the contents of the sachet in 200 ml of water before drinking the solution. They were divided into three groups in a randomized, double-blind fashion as follows: group placebo (supplementation with placebo, a compound of inert substance with color, solubility, and taste similar to those of creatine, using a dose of 5 g/day), group G3 (3 g/day of CS), and group G5 (5 g/day of CS). Moreover, all groups were evaluated at two times: prior to CS (pre) and after 35 days on CS (post). All subjects had to perform a standardized resistance training program three times a week for 5 weeks, according to the study protocol.
Regular consumption of creatine and attendance at training sessions were strictly monitored during the 35 days of the study. A diary was used to monitor the frequency of training sessions and creatine consumption. Six participants, two from each group, were excluded for not meeting the minimum number of weekly physical training sessions.
Renal function evaluation and novel biomarkers
To assess the glomerular function, proteinuria and albuminuria were investigated and determined as the ratio of urinary creatinine and expressed as “mg/g-Cr.” The estimated glomerular filtration rate (eGFR) was determined based on serum creatinine and using the CKD-EPI formula [22]. The urinary levels of novel biomarkers KIM-1 (uKIM-1) and MCP-1 (uMCP-1) were quantified by enzyme-linked immunosorbent assay (ELISA) kits for human beings obtained from R&S systems (Minneapolis, MN) and determined as “pg/ml” and “pg/mg-Cr” to eliminate the bias of the urinary difference between the subjects [23]. Urinary MCP-1: the coefficient range of intra-assay variances was 4.2 to 5.9%; and for inter-assay range, of 4.5 to 5.9%. Urinary KIM-1: the coefficient range of intra-assay variances was 3.9 to 4.4%; and for inter-assay range, of 6.0 to 7.8%. All analyses were performed at the Nephrology Tropical Diseases Laboratory at Universidade Federal do Ceará.
Statistical analysis
All variables were tested for normal or non-normal distribution using Shapiro–Wilk test. Normal data were expressed as mean ± standard deviation and non-normal data as median and interquartile range. To compare the three groups (placebo, G3, and G5), ANOVA or Kruskal–Wallis test was used as appropriate. Moreover, to compare the renal function between two time points in each group (before CS and after 35 days of CS), the paired t-test or Wilcoxon rank test was applied.
The sample size was determined by the recruitment feasibility. Cohen’s d value was calculated to estimate the effect size for paired samples through a standard formula [24]. Regarding all paired variables, the mean ± standard deviation of Cohen’s d was −0.22 ± 0.77 and minimum to maximum values were − 1.76–1.82. All tests were two-tailed, with P values <0.05 considered as statistically significant. The analyses were performed using SPSS version 20.0 for Windows (Chicago, IL,USA).
Ethical aspects
All participants signed the written informed consent form. This study was filed with the certificate of presentation for ethical appreciation (no. 52825816.9.0000.5045) and was approved by the Research Ethics Committee (appraisement report number 1690479). All the determinations of Resolution 466/12 of the National Health Council, which deals with the guidelines and norms regulating research involving human beings in Brazil, were followed.
More information about the obedience of ethical commandments can be obtained using the ethical appreciation and the appraisement report numbers on the website http://plataformabrasil.saude.gov.br/login.jsf.
Results
Among the 36 subjects involved in this study, 6 participants who did not fully observe the study protocol were excluded. In total, 30 male students finished the study. All groups were similar regarding age, weight, height, and BMI (Table 1). Traditional laboratory parameters and new kidney biomarkers levels were also homogenous for the three groups at baseline (Table 2).
Table 1.
Group characterization at the pre-intervention moment
| Placebo (N = 10) | G3 (N = 10) | G5 (N = 10) | P* | |
|---|---|---|---|---|
| Age (years) | 21.6 ± 3.0 | 24.4 ± 6.1 | 21.4 ± 2.8 | 0.32 |
| Weight (kg) | 77.4 ± 10.4 | 79.4 ± 13.3 | 74.1 ± 9.5 | 0.55 |
| Height(m) | 1.78 ± 0.1 | 1.75 ± 0.1 | 1.76 ± 0.1 | 0.45 |
| BMI (kg/m2) | 24.2 ± 1.5 | 25.7 ± 3.0 | 24.0 ± 2.4 | 0.08 |
Data are presented as mean ± standard deviation. BMI, body mass index. (ANOVA).
*Significant P < 0.05. (P < 0.05 shows that all groups are homogeneous).
Table 2.
Laboratory data and novel renal biomarkers prior to creatine supplementation or placebo (baseline—pre)
| Parameter | Placebo (N = 10) | G3 (N = 10) | G5 (N = 10) | P* |
|---|---|---|---|---|
| Serum albumin (g/dl) | 3.9 ± 1.1 | 3.8 ± 1.3 | 4 ± 0.8 | 0.103 |
| Serum creatinine (mg/dl) | 0.9 ± 0.2 | 0.7 ± 0.12 | 0.9 ± 0.21 | 0.170 |
| Serum urea (mg/dl) | 21.3 ± 7.9 | 24.6 ± 4.2 | 27.1 ± 8.4 | 0.938 |
| eGFR (ml/min 1.73 m2) | 128 ± 25 | 121 ± 16 | 124 ± 19 | 0.787 |
| Proteinuria (mg/dl) | 3.2 ± 1.4 | 2.9 ± 1.9 | 3.8 ± 1.8 | 0.101 |
| Proteinuria (mg/g-Cr) | 54.9 ± 42.8 | 75.9 ± 78.1 | 36.9 ± 23.1 | 0.901 |
| Albuminuria (mg/L) | 6.9 ± 0.65 | 6.6 ± 0.5 | 6.1 ± 0.5 | 0.319 |
| uKIM-1 (pg/ml) | 43 (22–157) | 63 (26–140) | 68 (20–169) | 0.432 |
| uKIM-1 (pg/mg-Cr) | 85 (69–117) | 117 (81–298) | 87 (25–177) | 0.827 |
| MCP-1 (pg/ml) | 55 (31–108) | 35 (23–43) | 42 (29–100) | 0.575 |
| MCP-1 (pg/mg-Cr) | 69 (28–112) | 94 (40–147) | 52 (29–122) | 0.794 |
Data are presented as mean ± standard deviation or as median and interquartile range in parenthesis. eGFR, estimated glomerular filtration rate using the CKD-EPI formula; uKIM-1, urinary kidney injury molecule-1; uMCP-1, urinary monocyte chemotactic protein-1. ANOVA for normal data and Kruskal–Wallis test for non-normal data.
*Significant P < 0.05. (P > 0.05 shows that all groups are homogeneous).
Moreover, after 35 days of creatine supplementation, there was no significant difference in the parameters evaluated among the groups, including serum creatinine, eGFR, and more sensitive kidney biomarker levels (KIM-1 and MCP-1) (Table 4).
Table 4.
Paired analysis (before and after 35 days of creatine supplementation or placebo)
| Placebo | G3 | G5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post 35 days | P * | Pre | Post 35 days | P * | Pre | Post 35 days | P * | |
| Serum albumin (g/dl) | 3.9 ± 1.1 | 3.9 ± 0.4 | 0.657 | 3.8 ± 1.3 | 3.6 ± 0.7 | 0.270 | 4 ± 0.8 | 4 ± 0.3 | 0.754 |
| Serum creatinine (mg/dl) | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.605 | 0.7 ± 0.12 | 1.1 ± 0.2 | 0.025* | 0.9 ± 0.21 | 1.3 ± 0.3 | 0.001* |
| Serum urea (mg/dl) | 21.3 ± 7.9 | 28 ± 7.8 | 0.338 | 24.6 ± 4.2 | 27 ± 9.6 | 0.467 | 27.1 ± 8.4 | 28.5 ± 10.2 | 0.386 |
| eGFR (ml/min 1.73 m2) | 128 ± 25 | 119 ± 21 | 0.055 | 121 ± 16 | 96 ± 14 | 0.010* | 124 ± 19 | 99 ± 12 | 0.025* |
| Proteinuria (mg/dl) | 3.2 ± 1.4 | 3 ± 1.5 | 0.690 | 2.9 ± 1.9 | 3.1 ± 1.6 | 0.767 | 3.8 ± 1.8 | 5.5 ± 4.4 | 0.231 |
| Proteinuria (mg/g-Cr) | 54.9 ± 42.8 | 39.4 ± 19.9 | 0.356 | 75.9 ± 78.1 | 43.2 ± 28.7 | 0.302 | 36.9 ± 23.1 | 45.1 ± 33.9 | 0.440 |
| Albuminuria (mg/l) | 6.9 ± 0.65 | 7.9 ± 0.61 | 0.283 | 6.6 ± 0.5 | 7.1 ± 0.6 | 0.093 | 6.1 ± 0.5 | 7.1 ± 0.3 | 0.210 |
| uKIM-1 (pg/ml) | 43 (22–157) | 68 (22–181) | 0.744 | 63 (26–140) | 84 (48–243) | 1.000 | 68 (20–169) | 130 (96–250) | 0.374 |
| uKIM-1 (pg/mg-Cr) | 85 (69–117) | 111 (29–166) | 0.479 | 117 (81–298) | 142 (84–171) | 0.173 | 87 (25–177) | 144 (68–188) | 0.173 |
| MCP-1 (pg/ml) | 55 (31–108) | 40 (0–96) | 0.369 | 35 (23–43) | 34 (14–57) | 0.441 | 42 (29–100) | 54 (21–105) | 0.767 |
| MCP-1 (pg/mg-Cr) | 69 (28–112) | 60 (0–77) | 0.466 | 94 (40–147) | 41 (23–57) | 0.069 | 52 (29–122) | 48 (20–74) | 0.484 |
Data are presented as mean ± standard deviation or as median and interquartile range in parenthesis. eGFR, estimated glomerular filtration rate using the CKD-EPI formula; uKIM-1, urinary kidney injury molecule-1; uMCP-1, urinary monocyte chemotactic protein-1. Paired t-test for normal data and Wilcoxon test for non-normaldata.
*Significant P < 0.05, after 35 days (post-intervention) vs. pre (baseline).
When comparing the values found at baseline (Table 2) and post-supplementation (Table 4) against normal reference values (Table 3), one can conclude that renal function in all groups was normal at both times, before and after 35 days of CS or placebo.
Table 3.
Reference values for the investigated parameters
| Renal parameter | Reference value |
|---|---|
| Serum albumin (g/dl) | 3.5 to 5 g/dl* |
| Serum creatinine (mg/dl) | 0.7 to 1.3 mg/dl* |
| Serum urea (mg/dl) | 16 to 40 mg/dl* |
| eGFR (ml/min 1.73 m2) | 90–138 (Male)* |
| Proteinuria (mg/dl) | 1.0 to 15.0 mg/dl* |
| Proteinuria (mg/g-Cr) | <150 mg/g-Cr* |
| Albuminuria (mg/L) | <30 mg/l* |
| uKIM-1 (pg/ml) | N/D |
| uKIM-1 (pg/mg-Cr) | N/D |
| MCP-1 (pg/ml) | N/D |
| MCP-1 (pg/mg-Cr) | N/D |
eGFR, estimated glomerular filtration rate using CKD-EPI formula; uKIM-1, urinary kidney injury molecule-1; uMCP-1, urinary monocyte chemotactic protein-1; N/D, not defined value.
*The data is based on the Brazilian consensus for standardization.
The paired analysis (Table 4) investigated the intragroup difference, for the three groups, between different times pre- and post-CS or placebo. A significant increase in serum creatinine levels was found for the groups supplemented with creatine (G3 and G5) (P < 0.05). As the eGFR was estimated based on serum creatinine, these groups also showed a significant decrease in the estimated glomerular filtration rate after 35 days of intervention. However, it is important to note that even with significant changes between pre- and post-supplementation moments, serum creatinine and eGFR still remained within the normal reference values (serum creatinine between 0.7–1.3 mg/dl and eGFR > 90 ml/min 1.73 m2. Also in the supplemented groups, the paired analysis did not show any change in KIM-1 and MCP-1 levels when evaluated as total excreted value, as well as urinary creatinine ratio. The placebo group did not show any changes.
Discussion
Creatine is a natural nutrient, which is endogenously synthesized by humans in small amounts (1 g/day) by the liver and kidneys from the amino acids glycine, methionine, and arginine [9, 25]. The phosphorylated form plays an important role in increasing the turnover of adenosine triphosphate (ATP) [15]. Thus, creatine has great influence in providing rapid energy (anaerobic alactic metabolism) for muscle contraction, since the phosphocreatine compound is able to donate its phosphate group to adenosine diphosphate (ADP) and, therefore, rapidly resynthesize adenosine triphosphate (ATP) [9, 26]. A previous study by our research group also confirmed this fact and demonstrated that creatine supplementation, even at low doses and without the use of the loading phase, is effective for increasing maximal strength and endurance of upper limbs [27].
Several studies have shown that CS might be safe for renal function [10–17], including a recent systematic review and meta-analysis [28]. However, in addition to the work of Kuehl [5] and Pritchard and Kalra [6], studies in animal models have demonstrated severe renal function loss in rats supplemented with creatine [7–9]. Edmund et al. observed the exacerbation of disease progression in an animal model of cystic renal disease, using a creatine daily dose of 0.30 g/kg of body weight during the loading phase (week 1) and 0.03–0.05 g/kg in the remaining weeks of the study. This protocol simulating typical supplemented human intake level is based on body weight (21 g/day loading dose and 3 g/day maintenance dose for a 70-kg man) [7]. On the other hand, Souza et al. found evidence of impaired kidney function in healthy animals; however, the dose used, considering a body weight basis, was about 15 to 20 times higher than the creatine dose normally used by humans [9].
The major limitation of all these studies is associated with the use of creatinine as a key marker of renal function deterioration. Unfortunately, despite being the standard renal biomarker in clinical practice, serum creatinine has a few limitations. Its concentration may vary considerably according to gender, age, lean mass, physical exercise, muscle metabolism, body weight, nutritional status, and hydration status [18]. Another problem is that renal disease is normally diagnosed only when it is already fully established, with clear clinical signs, symptoms, and/or laboratory abnormalities [18, 29, 30]. Serum creatinine, e.g., will only be outside the reference values when renal function is already severely reduced, with at least 50% loss of glomerular function [30]. Thus, creatine supplementation can alter serum creatinine levels and may contribute as a false indicator of kidney injury. Serum creatinine levels most likely do not indicate kidney injury following creatine supplementation [28].
Thus, in an attempt to overcome all of these limitations, our study investigated the standard markers of renal function and, unprecedented to the best of our knowledge for subjects on CS, also investigated two new biomolecules recognized as reliable, early, and sensitive markers of renal function decline, i.e. KIM-1 and MCP-1 [31–33].
Dose–response relationship and proposal mechanism of toxicity or kidney injury
It has been demonstrated that oral supplementation with creatine monohydrate increases the total amount of muscle creatine (TCr), as well as free creatine (FCr) and phosphocreatine (PCr) [2, 34]. Creatinine is a residual metabolite of creatine, and the transformation of creatine into creatinine occurs in muscle tissue, where between 1 and 2% of free creatine spontaneously and irreversibly converts into creatinine daily [30, 35]. Regarding renal physiology, it is known that the main route of creatine and creatinine excretion is through urine [36, 37] and that creatinine needs to be filtered in the glomerulus, being actively secreted by the renal tubules [30, 35].
Glomerular hyperfiltration has been defined either as an atypically high glomerular filtration rate (GFR), increased filtration fraction, or as increased filtration per nephron. Increased filtration per nephron occurs as an adaptive response to nephron loss and leads to glomerular hypertension and subsequent glomerulosclerosis, with progressive renal function decline [38]. Therefore, it should be noted that eventually, even when there is a decrease in the number of nephrons, the GFR may remain clinically stable. This occurs because of a compensatory mechanism in each nephron due to increased pressure filtration or glomerular hypertrophy, which invariably leads to glomerular hyperfiltration [39].
Regarding tubular damage, interstitial nephritis and acute tubular necrosis have been reported in a few case reports. Ardalan et al. reported the case of a healthy man with no personal or family history of kidney disease and a history of creatine use for only 3 weeks (dose of 20 g/day for 3 days and maintenance dose of 1 g/day for 3 weeks). The author associated the creatine consumption with development of renal dysfunction and interstitial nephritis with acute tubular necrosis. The renal function was recovered after stopping the creatine supplement and starting treatment with corticosteroids [40]. Similarly, Koshy et al. presented a case of a healthy 20-year-old man with a 4-day history of nausea, vomiting, and bilateral flank pain that started after ~4 weeks on creatine supplementation (5 g/day). A renal biopsy showed acute focal interstitial nephritis and focal tubular injury. After being hospitalized and treated with intravenous fluid and pain medication, the patient’s blood pressure, serum creatinine concentration, and urinalysis were normalized [41].
Thus, it is possible to propose that, in theory, creatine supplementation might induce increased filtration (hyperfiltration) of creatinine by the glomeruli and increased secretion (hypersecretion) of creatinine through the renal tubules. Both mechanisms have a strong tendency to develop into kidney injury and failure and would be dose-dependent, i.e. the higher the dose of creatine administered, the greater the need for hyperfiltration and hypersecretion.
Traditional renal evaluation
Our results did not show any significant difference between the placebo group or supplemented groups (G3 and G5). The three groups were homogeneous for all traditional renal parameters (serum albumin, serum creatinine, serum urea, eGFR, proteinuria, and albuminuria) at both times: pre- (baseline) and post-supplementation (after 35 days of CS or placebo).
However, when analyzing each group individually (paired analysis), a significant increase in serum creatinine and a decrease in eGFR were observed after 35 days of intervention (Table 4), exclusively in the CS groups (G3 and G5). Other experiments with human beings also found similar results and concluded that there was no renal function impairment, despite increased serum creatinine and decreased eGFR [13, 15, 16].
These findings can be explained by the fact that serum creatinine concentrations vary considerably due to gender, age, muscle mass, muscle metabolism, body weight, nutritional status, and hydration status [18]. Moreover, it is important to note that in our study, despite a significant increase in serum creatinine and a decrease in eGFR, the values found after 35 days of CS (G3 and G5 groups) still were within the normality reference range (serum creatinine between 0.7 and 1.3 mg/dl and eGFR between 90 and 138 ml/min 1.73 m2).
However, when considering the assessment of renal function, we cannot disregard creatinine clearance. It is a fact that creatinine clearance, as it assesses the association between serum and urinary creatinine, is capable of providing an excellent estimate of GFR and, thus, constitutes an important indicator of kidney overload or injury [42]. Unfortunately, our study was unable to assess creatinine clearance.
Novel biomarkers of kidney injury
Considering the limitations in early diagnosis of renal function decline, the study of and the search for sensitive molecules capable of early detection of inflammation and renal function loss became the focus of doctors and researchers worldwide [43]. For this purpose, two molecules seem very promising: urinary KIM-1 and MCP-1. KIM-1 is a type I transmembrane glycoprotein. It is a 104 kDa protein in its complete form, presenting a cytoplasmic and extracellular portion [32, 44, 45]. This molecule is undetectable in healthy kidneys, but highly expressed in proximal tubular cells in kidney injury. In urine samples, KIM-1 values increase in just 24 h after coronary epithelium injury-induced AKI, while estimated glomerular filtration rate (eGFR) takes 48 h to increase [46]. KIM-1 concentration is also absent in other body cells, including other renal cells. These features make this biomarker particularly reliable and highly specific for renal tubular damage [31, 32].
Regarding KIM-1, our findings showed that the urinary concentrations of KIM-1 in the creatine supplemented groups (G3 and G5) did not differ from the results found in the placebo group (Tables 2 and 3). Even the paired analysis (pre- versus post-supplementation) did not show any significant difference for KIM-1 values after 35 days of creatine supplementation with 3 g or 5 g/day.
It is important to note that KIM-1 is still an experimental and investigational biomarker and therefore there are no established reference values for this molecule. However, several experiments, performed in different contexts, have been successful in linking/associating urinary KIM-1 concentration to numerous clinical conditions of renal impairment, need for dialysis, and death. The high specificity and sensitivity of KIM-1 in the early diagnosis of acute tubular kidney injury was also clearly evidenced [47–50].
Our study also investigates MCP-1 levels. This molecule was the first human CC chemokine described in the literature and is probably one of the most researched chemokines in this family. MCP-1 is a protein comprising 76 amino acids and also belongs to the class of inflammatory chemokines [33]. As an inflammatory chemokine, MCP-1 has the ability to attract monocytes in response to various pathological conditions. It is also able to promote secretion of enzymes such as histamine and the expression of vascular adhesion molecules from natural killer cells, basophils, and T lymphocytes. Therefore, MCP-1 constitutes an expressive pro-inflammatory factor [33] and has been shown that can be synthesized by many kinds of kidney tissue cells, including tubular epithelial, endothelial, mesangial cells, and podocytes [51]. The presence of MCP-1 in urine was related to early diagnosis of AKI, being related to values of NGAL in an AKI-induced protocol. The values changed only 4 h after the injury, demonstrating an important role in the early diagnosis of AKI [52].
As occurred with KIM-1, our data did not show any difference between the urinary concentration of MCP-1 in the CS groups (G3 and G5) and the results found in the placebo group (Tables 2 and 3). Even the paired analysis (pre- versus post-supplementation) did not show any significant difference for MCP-1 values after 35 days of CS with 3 g or 5 g/day.
Similar to KIM-1, MCP-1 is still an experimental and investigational biomarker, and therefore there are no established reference values for this molecule. However, several experiments have been successful in demonstrating the association between MCP-1 urinary levels and the development and progression of renal inflammation and disease [53]. Therefore, the presence of MCP-1 in urine and renal tissue has been detected and repeatedly associated with diabetic nephropathy [54], crescentic glomerulonephritis [55], nephrotic lupus nephritis [56], and membranoproliferative glomerulonephritis [57, 58].
Conclusion
The results of the present study indicate that the use of CS at dose of 3 g and 5 g/day for a short period (35 days) did not impair renal function or kidney health. The renal parameters in supplemented groups did not show any differences when compared with the placebo group. Even novel and sensitive renal decline biomarkers (KIM- and MCP-1) did not show any evidence of kidney injury or renal function decline.
Our data also shows that supplemented groups had increased serum creatinine and decreased eGFR levels. However, the values are still within the normal reference range. Therefore, in the absence of renal function loss or kidney injury indicators, these findings could be explained by increased creatine intake through supplementation. Thus, it is possible to conclude that CS for a short time and with doses between 3 g and 5 g/day seems to be safe for healthy and active young male subjects.
Statement of Ethics
Human and animal rights
All participants signed a written informed consent. This research was approved in all instances required for research involving human beings in Brazil. The study was registered with the certificate of presentation for ethical appreciation (no. 52825816.9.0000.5045) and was approved by the Ethics and Research Committee (appraisement report number 1690479). All the determinations of resolution 466/12 of the National Health Council, which deals with the guidelines and norms regulating research involving humans in Brazil, were verified.
Disclosure Statement
The authors declare that there is no conflict of interest regarding the study or results presented in this study.
Author Contributions
José de Oliveira Vilar Neto provided the main idea and helped in data collection, data analysis, and preparation of the manuscript. Carlos Alberto da Silva, Gdayllon Cavalcante Meneses, and Elizabeth de Francesco Daher contributed in data analysis and preparation of the manuscript. Daniel Vieira Pinto helped in data collection and preparation of the manuscript. Luciana Catunda Brito, Said Goncalves da Cruz Fonseca, Renata de Sousa Alves, Alice Maria Costa Martins, and Cláudio de Oliveira Assumpção gave assistance in data analysis.
Acknowledgement
The authors thank the Institute of Physical Education and Sports and the Centers of Pharmacy and Medicine of the Federal University of Ceará.
Research Conducted: Federal University of Ceará, Fortaleza, Ceará, Brazil
Congresses
None.
Funding Sources
The authors declare that they have not used financial resources of any institution.
Funding
No funding was received for the study.
Conflict of interest statement. No conflicts of interest are declared by the authors.
References
- 1. Gualano B, Artioli GG, Lancha Junior AH. Suplementação de creatina e metabolismo de glicose: efeitos terapêuticos ou adversos? Revista Brasileira de Medicina do Esporte 2008;14:478. [Google Scholar]
- 2. Williams MH, Branch JD. Creatine supplementation and exercise performance: an update. J Am Coll Nutr 1998;17:216–34. [DOI] [PubMed] [Google Scholar]
- 3. Peralta J, Amancio OMS. A creatina como suplemento ergogênico para atletas. Rev Nutr Campinas 2002;15:83–93. [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC). Hyperthermia and dehydration-related deaths associated with intentional rapid weight loss in three collegiate wrestlers--North Carolina, Wisconsin, and Michigan, November–December 1997 MMWR. 1998;47:105–8. [PubMed] [Google Scholar]
- 5. Kuehl K, Goldberg L, Elliot D. Renal insufficiency after creatine supplementation in a college football athlete. Med Sci Sports Exerc 1998;30:235. [Google Scholar]
- 6. Pritchard NR, Kalra PA. Renal dysfunction accompanying oral creatine supplements. Lancet 1998;351:1252–3. [DOI] [PubMed] [Google Scholar]
- 7. Edmunds JW, Jayapalan S, DiMarco NM et al. Creatine supplementation increases renal disease progression in Han:SPRD-cy rats. Am J Kidney Dis 2001;37:73–8. [DOI] [PubMed] [Google Scholar]
- 8. Souza WM, Heck TG, Wronski EC et al. Effects of creatine supplementation on biomarkers of hepatic and renal function in young trained rats. Toxicol Mech Methods 2013;23:697–701. [DOI] [PubMed] [Google Scholar]
- 9. Souza RA, Miranda H, Xavier M et al. Effects of high-dose creatine supplementation on kidney and liver responses in sedentary and exercised rats. J Sports Sci Med 2009;8:672–81. [PMC free article] [PubMed] [Google Scholar]
- 10. Gualano B, Salles PV, Roschel H et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Eur J Appl Physiol 2011;111:749–56. [DOI] [PubMed] [Google Scholar]
- 11. Lugaresi R, Leme M, Salles PV et al. Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet? J Int Soc Sports Nutr 2013;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gualano B, Ferreira DC, Sapienza MT et al. Effect of short-term high-dose creatine supplementation on measured GFR in a young man with a single kidney. Am J Kidney Dis 2010;55:e7–9. [DOI] [PubMed] [Google Scholar]
- 13. Gualano B, Ugrinowitsch C, Novaes RB et al. Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Eur J Appl Physiol 2008;103:33–40. [DOI] [PubMed] [Google Scholar]
- 14. Robinson TM, Sewell DA, Casey A et al. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br J Sports Med 2000;34:284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poortmans JR, Auquier H, Renaut V et al. Effect of short-term creatine supplementation on renal responses in men. Eur J Appl Physiol Occup Physiol 1997;76:566–7. [DOI] [PubMed] [Google Scholar]
- 16. Poortmans JR, Francaux M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc 1999;31:1108–10. [DOI] [PubMed] [Google Scholar]
- 17. Mayhew DL, Mayhew JL, Ware JS. Effects of long-term creatine supplementation on liver and kidney functions in American college football players. Int J Sport Nutr Exerc Metab 2002;12:453–60. [DOI] [PubMed] [Google Scholar]
- 18. Kirsztajn GM. Avaliação do ritmo de filtração glomerular. J Bras Patol Med Lab 2007;4:257–64. [Google Scholar]
- 19. Ronco C, Rizo-Topete L, Serrano-Soto M et al. Pro: prevention of acute kidney injury: time for teamwork and new biomarkers. Nephrol Dial Transplant 2017;32:408–13. [DOI] [PubMed] [Google Scholar]
- 20. Urbschat A, Obermuller N, Haferkamp A. Biomarkers of kidney injury. Biomarkers 2011;16:S22–30. [DOI] [PubMed] [Google Scholar]
- 21. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int 2010;78:486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people's published data: general procedures for research consumers. Psychol Methods 1996;1:331–40. [Google Scholar]
- 25. Andres S, Ziegenhagen R, Trefflich I et al. Creatine and creatine forms intended for sports nutrition. Mol Nutr Food Res 2017;61. [DOI] [PubMed] [Google Scholar]
- 26. Chilibeck PD, Kaviani M, Candow DG et al. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med 2017;8:213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliveira Vilar Neto J, Silva CA, Lima AB et al. Effects of low-dose creatine monohydrate on muscle strength and endurance. Asian J Sports Med 2018;9:1e+. [Google Scholar]
- 28. Souza ESA, Pertille A, Reis Barbosa CG et al. Effects of creatine supplementation on renal function: a systematic review and meta-analysis. J Ren Nutr 2019;29:480–9. [DOI] [PubMed] [Google Scholar]
- 29. Lopes MB, Araujo LQ, Passos MT et al. Estimation of glomerular filtration rate from serum creatinine and cystatin C in octogenarians and nonagenarians. BMC Nephrol 2013;14:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirsztajn GM. Avaliação da função renal. J Bras Nefrol 2009;31:14–20. [Google Scholar]
- 31. Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci 2009;5:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc 2014;125:293–9; discussion 9. [PMC free article] [PubMed] [Google Scholar]
- 33. Kim MJ, Tam FW. Urinary monocyte chemoattractant protein-1 in renal disease. Clin Chim Acta 2011;412:2022–30. [DOI] [PubMed] [Google Scholar]
- 34. Persky AM, Rawson ES. Safety of creatine supplementation. Subcell Biochem 2007;46:275–89. [DOI] [PubMed] [Google Scholar]
- 35. Sodré FL, Costa JCB, Lima JC. Avaliação da função e da lesão renal: Um desafio laboratorial. J Bras Pat Med Lab 2007;43:329–37. [Google Scholar]
- 36. Kim HJ, Kim CK, Carpentier A et al. Studies on the safety of creatine supplementation. Amino Acids 2011;40:1409–18. [DOI] [PubMed] [Google Scholar]
- 37. Hultman E, Soderlund K, Timmons JA et al. Muscle creatine loading in men. J Appl Physiol 1996;81:232–7. [DOI] [PubMed] [Google Scholar]
- 38. Helal I, Fick-Brosnahan GM, Reed-Gitomer B et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012;8:293. [DOI] [PubMed] [Google Scholar]
- 39. Parving HH. Diabetic nephropathy: prevention and treatment. Kidney Int 2001;60:2041–55. [DOI] [PubMed] [Google Scholar]
- 40. Ardalan M, Samadifar Z, Vahedi A. Creatine monohydrate supplement induced interstitial nephritis. J Nephropathol 2012;1:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koshy KM, Griswold E, Schneeberger EE. Interstitial nephritis in a patient taking creatine. N Engl J Med 1999;340:814–5. [DOI] [PubMed] [Google Scholar]
- 42. Pline KA, Smith CL. The effect of creatine intake on renal function. Ann Pharmacother 2005;39:1093–6. [DOI] [PubMed] [Google Scholar]
- 43. McCullough PA, Bouchard J, Waikar SS et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: executive summary from the tenth consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol 2013;182:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonventre JV. Kidney injury Molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl 2008;241:78–83. [DOI] [PubMed] [Google Scholar]
- 45. Ichimura T, Asseldonk EJ, Humphreys BD et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 2008;118:1657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vijayasimha M, Padma V, Mujumdar SK et al. Kidney injury molecule-1: a urinary biomarker for contrast-induced acute kidney injury. Med J DY Patil Univ 2014;7:321–5. [Google Scholar]
- 47. Zhang PL, Rothblum LI, Han WK et al. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int 2008;73:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carvalho Pedrosa D, Macedo de Oliveira Neves F, Cavalcante Meneses G et al. Urinary KIM-1 in children undergoing nephrotoxic antineoplastic treatment: a prospective cohort study. Pediatr Nephrol 2015;30:2207–13. [DOI] [PubMed] [Google Scholar]
- 49. Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med 2017;55:1074–89. [DOI] [PubMed] [Google Scholar]
- 50. Xu PC, Zhang JJ, Chen M et al. Urinary kidney injury molecule-1 in patients with IgA nephropathy is closely associated with disease severity. Nephrol Dial Transplant 2011;26:3229–36. [DOI] [PubMed] [Google Scholar]
- 51. Haller H, Bertram A, Nadrowitz F et al. Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens 2016;25:42–9. [DOI] [PubMed] [Google Scholar]
- 52. Munshi R, Johnson A, Siew ED et al. MCP-1 gene activation marks acute kidney injury. JASN 2011;22:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shoukry A, Bdeer Sel A, El-Sokkary RH. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol Cell Biochem 2015;408:25–35. [DOI] [PubMed] [Google Scholar]
- 54. Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol 2008;294:F697–701. [DOI] [PubMed] [Google Scholar]
- 55. Viedt C, Orth SR. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: does it more than simply attract monocytes? Nephrol Dial Transplant 2002;17:2043–7. [DOI] [PubMed] [Google Scholar]
- 56. Zoja C, Donadelli R, Colleoni S et al. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int 1998;53:1608–15. [DOI] [PubMed] [Google Scholar]
- 57. Grandaliano G, Gesualdo L, Ranieri E et al. Monocyte chemotactic peptide-1 expression and monocyte infiltration in acute renal transplant rejection. Transplantation 1997;63:414–20. [DOI] [PubMed] [Google Scholar]
- 58. Rovin BH, Doe N, Tan LC. Monocyte chemoattractant protein-1 levels in patients with glomerular disease. Am J Kidney Dis 1996;27:640–6. [DOI] [PubMed] [Google Scholar]


