In this study, we observed an elevated fracture rate in children with anxiety disorders initiating benzodiazepine treatment compared to SSRI treatment, with no increase in young adults.
Abstract
BACKGROUND:
Benzodiazepines are commonly prescribed to treat anxiety disorders and have been associated with falls and fractures in older adults. It is unknown whether benzodiazepines increase fracture risk in youth. We examined whether youth with anxiety disorders initiating benzodiazepine treatment have an increased risk of fractures compared with youth initiating selective serotonin reuptake inhibitors (SSRIs).
METHODS:
We used claims from commercially insured children (6–17 years) and young adults (18–24) with a recent anxiety disorder diagnosis, initiating benzodiazepines or SSRIs (2008–2016). Youth were followed until fracture, treatment discontinuation or switching, disenrollment, 3 months, or December 31, 2016. The primary end point was diagnostic codes for upper and lower limb fractures. Incident fracture rates, incident rate ratios (IRRs), and incident rate differences (IRDs) were estimated with propensity score inverse probability of treatment weighting.
RESULTS:
The cohort included 120 715 children and 179 768 young adults. In children, crude fracture rates during treatment were 33.1 per 1000 person-years (PYs) for benzodiazepine initiators and 25.1 per 1000 PYs for SSRI initiators. Adjusted IRR and IRD were 1.53 (95% confidence interval [CI]: 0.94–2.50) and 13.4 per 1000 PYs. Risk was heightened in children initiating long-acting benzodiazepines versus SSRIs (adjusted IRR = 2.30 [95% CI: 1.08–4.91]). Fracture rates were lower in young adults, with minimal differences between treatments (adjusted IRR = 0.85 [95% CI: 0.57–1.27]; adjusted IRD = −1.3 per 1000 PYs).
CONCLUSIONS:
An increased rate of fractures in children, but not young adults, with anxiety disorders initiating benzodiazepine treatment compared to SSRI treatment suggests a need for increased caution in the weeks after benzodiazepine initiation in children.
What’s Known on This Subject:
Benzodiazepine side effects include dizziness and slowed reaction time. Benzodiazepine treatment has been associated with an increased risk of fractures in older adults. It is unknown whether benzodiazepine treatment increases fracture risk in younger populations compared to alternative treatments.
What This Study Adds:
We observed a heightened fracture rate in children with anxiety disorders initiating benzodiazepine treatment compared to selective serotonin reuptake inhibitor treatment, with no heightened rate in young adults. Increased caution after benzodiazepine initiation may be warranted in children.
Benzodiazepines are a commonly prescribed medication class in the United States,1–3 including in younger populations, with 2.8% of adolescents (12–17 years) and 6.3% of young adults (18–25 years) reporting a past-year prescription of benzodiazepine or tranquilizer use.4 In mental health–related visits, 9% of adolescents and 21% of young adults were prescribed a benzodiazepine or other sedative-hypnotic medication.5 Benzodiazepines are prescribed for psychiatric and nonpsychiatric conditions and are commonly used for anxiety disorders among youth6,7 despite not being a recommended or Food and Drug Administration–approved pharmacotherapy for pediatric anxiety disorders.8–10
Side effects of benzodiazepine treatment can include slowed reaction time, drowsiness, dizziness, and weakness,11,12 which can result in injury. Benzodiazepine treatment is associated with an increased risk of motor vehicle crashes, falls, and fractures.13–18 Recent meta-analyses and studies support earlier findings that adult benzodiazepine users have an increased fracture risk compared with adults who do not use benzodiazepine, with most studies being focused on hip fractures in older adults.15,19–23 The greatest risk of hip fracture was observed with short-term benzodiazepine use,19,24,25 suggesting new users may be unaccustomed to benzodiazepine effects. In a case control study with stratified analyses of individuals ≤40 years of age, anxiolytic use remained associated with an increased fracture risk compared with never users.26 We are unaware of a previous study that has been focused on benzodiazepines and fracture risk in young persons.
If benzodiazepine side effects contribute to unintentional falls, young persons may be susceptible to heightened fracture risk during benzodiazepine treatment. Pediatric fractures are most often caused by falls.27,28 Falls account for one-third of nonfatal injuries in children, with ∼2.8 million children presenting to the emergency department (ED) for fall-related injuries annually.29 Fractures can limit daily activities, negatively impact quality of life, and require medical procedures.30–32
We evaluated fracture risk in children and young adults with anxiety disorders initiating benzodiazepine treatment compared to selective serotonin reuptake inhibitors (SSRIs), a guideline-recommended pharmacotherapy for anxiety disorders.10 Benzodiazepines and SSRIs are the most commonly prescribed medications for anxiety disorders, with similar prevalence in adults and with SSRIs prescribed more frequently than benzodiazepines in children.33–35 By focusing on youth with anxiety disorders and employing an active comparator, we sought to directly inform treatment decisions while reducing confounding by indication.
Evaluating the association between benzodiazepine treatment and fracture risk compared to therapeutic alternatives (ie, SSRIs) in young people can inform clinicians, parents, and patients in treating anxiety disorders. We therefore aimed to determine if children and young adults with anxiety disorders initiating benzodiazepine treatment have a short-term increased risk of fractures compared with SSRI initiators. Children (6–17 years) and young adults (18–24 years) were examined separately given differences in injury and fracture rates36–38 and prescribing guidelines.10,39–41
Methods
Data Source and Study Population
We used MarketScan Commercial Claims and Encounters data from January 1, 2007, through December 31, 2016. The database covers individuals with employer-sponsored health insurance and their dependents across the United States. Patient-level details were available on insurance enrollment, outpatient and inpatient services, and outpatient dispensed prescriptions. Service visits contained information on the date, provider type, diagnostic codes, and procedure codes.
We identified privately insured children (6–17 years) and young adults (18–24) newly initiating benzodiazepine or SSRI treatment (Table 1 footnotes). We used records of dispensed prescriptions to identify new benzodiazepine and SSRI treatment episodes, defined as no benzodiazepine or SSRI prescriptions in the year before treatment initiation with continuous insurance enrollment during that year (Supplemental Fig 2). We required an anxiety disorder diagnosis within the 30 days before or the day of treatment initiation. Anxiety disorders were defined with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes (Supplemental Table 4).
TABLE 1.
Children and Young Adults Initiating Benzodiazepine or SSRI Treatment of an Anxiety Disorder, 2008–2016: Selected Patient Characteristics
| Selected Patient Characteristic | Children (6–17 y) | Young Adults (18–24 y) | ||
|---|---|---|---|---|
| Benzodiazepinea | SSRIb | Benzodiazepinea | SSRIb | |
| n = 12 840 | n = 107 875 | n = 57 684 | n = 122 084 | |
| Male sex, n (%) | 4774 (37.2) | 41 311 (38.3) | 19 836 (34.4) | 39 939 (32.7) |
| Age, mean (SD) | 14.6 (2.63) | 13.6 (3.03) | 21.3 (1.94) | 20.7 (1.97) |
| Anxiety disorder (previous 30 d), n (%) | ||||
| Unspecified anxiety | 5997 (46.7) | 45 929 (42.6) | 31 986 (55.5) | 63 050 (51.6) |
| Generalized anxiety disorder | 2097 (16.3) | 29 604 (27.4) | 8446 (14.6) | 29 010 (23.8) |
| Panic disorder | 1614 (12.6) | 3885 (3.6) | 7413 (12.9) | 6092 (5.0) |
| Adjustment disorder with anxiety | 1360 (10.6) | 13 326 (12.4) | 2664 (4.6) | 9097 (7.5) |
| Acute stress disorder | 406 (3.2) | 1293 (1.2) | 2207 (3.8) | 2811 (2.3) |
| Phobic disorder | 288 (2.2) | 686 (0.6) | 1032 (1.8) | 689 (0.6) |
| Social phobia | 110 (0.9) | 2794 (2.6) | 432 (0.7) | 2451 (2.0) |
| Other anxiety | 399 (3.1) | 3425 (3.2) | 1624 (2.8) | 4463 (3.7) |
| Multiple diagnoses | 569 (4.4) | 6933 (6.4) | 1880 (3.3) | 4421 (3.6) |
| Provider on recent anxiety diagnosis, n (%) | ||||
| Psychiatry | 1419 (11.1) | 24 611 (22.8) | 3907 (6.8) | 12 405 (10.2) |
| Psychology | 1872 (14.6) | 15 988 (14.8) | 2589 (4.5) | 8784 (7.2) |
| Pediatrician | 1992 (15.5) | 21 536 (20.0) | 1346 (2.3) | 5456 (4.5) |
| Family practitioner | 2248 (17.5) | 15 811 (14.7) | 19 595 (34.0) | 46 877 (38.4) |
| Other general (nurse practitioner, internal medicine) | 1136 (8.8) | 9159 (8.5) | 10 830 (18.8) | 22 831 (18.7) |
| Other provider type | 2168 (16.9) | 12 663 (11.7) | 10 022 (17.4) | 14 576 (11.9) |
| Unknown or multiple provide types | 2005 (15.6) | 8107 (7.5) | 9395 (16.3) | 11 155 (9.1) |
| Recent psychiatric diagnoses (0–30 d), n (%) | ||||
| ADHD | 1006 (7.8) | 16 775 (15.6) | 3142 (5.4) | 7751 (6.3) |
| Depression | 1286 (10.0) | 29 418 (27.3) | 5698 (9.9) | 30 622 (25.1) |
| Sleep disorder | 519 (4.0) | 3234 (3.0) | 3130 (5.4) | 5944 (4.9) |
| Conduct disorder | 151 (1.2) | 3310 (3.1) | 100 (0.2) | 366 (0.3) |
| OCD | 144 (1.1) | 3085 (2.9) | 218 (0.4) | 1356 (1.1) |
| PTSD | 87 (0.7) | 1036 (1.0) | 372 (0.6) | 945 (0.8) |
| Any psychiatric comorbidity (1 y), n (%) | 4829 (37.6) | 65 353 (60.6) | 17 719 (30.7) | 53 090 (43.5) |
| Nonpsychiatric diagnoses (1 y), n (%) | ||||
| Asthma | 1656 (12.9) | 11 044 (10.2) | 4608 (8.0) | 8368 (6.9) |
| Chest pain, palpitations | 1908 (14.9) | 6886 (6.4) | 10 893 (18.9) | 12 645 (10.4) |
| Dizziness, fainting | 1098 (8.6) | 5565 (5.2) | 4504 (7.8) | 6991 (5.7) |
| Muscle spasm | 420 (3.3) | 1964 (1.8) | 1844 (3.2) | 2933 (2.4) |
| Injury-related diagnoses (1 y unless specified), n (%) | ||||
| Fracture (91 d–1 y) | 500 (3.9) | 3462 (3.2) | 1089 (1.9) | 2075 (1.7) |
| Injury diagnosis (excluding fracture) | 3242 (25.2) | 22 896 (21.2) | 10 918 (18.9) | 20 830 (17.1) |
| Fall diagnosis | 168 (1.3) | 1422 (1.3) | 453 (0.8) | 959 (0.8) |
| Joint disorder, nontraumatic | 2143 (16.7) | 14 316 (13.3) | 6903 (12.0) | 13 929 (11.4) |
| Recent prescriptions (0–90 d), n (%) | ||||
| Stimulant | 1266 (9.9) | 15 714 (14.6) | 4445 (7.7) | 8576 (7.0) |
| Antidepressant (baseline SSRI use excluded) | 1085 (8.5) | 5204 (4.8) | 6677 (11.6) | 8163 (6.7) |
| Anticonvulsant | 426 (3.3) | 1599 (1.5) | 1319 (2.3) | 1997 (1.6) |
| Antipsychotic, lithium | 364 (2.8) | 2411 (2.2) | 659 (1.1) | 1054 (0.9) |
| Hypnotic | 110 (0.9) | 361 (0.3) | 1390 (2.4) | 2196 (1.8) |
| Opioid | 1422 (11.1) | 4770 (4.4) | 7590 (13.2) | 10 555 (8.6) |
| Psychotherapy claims (recent, 0–30 d), n (%) | 3796 (29.6) | 46 604 (43.2) | 6359 (11.0) | 21 226 (17.4) |
| ED visits (recent, 0–30 d), n (%) | 3627 (28.2) | 10 191 (9.4) | 14 766 (25.6) | 12 462 (10.2) |
| Preventive outpatient visit (1 y), n (%) | 6527 (50.8) | 59 937 (55.6) | 23 394 (40.6) | 53 053 (43.5) |
OCD, obsessive compulsive disorder; PTSD, posttraumatic stress disorder.
Benzodiazepine treatment included prescriptions for alprazolam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, estazolam, flurazepam, halazepam, lorazepam, midazolam, oxazepam, prazepam, quazepam, temazepam, or triazolam.
SSRI treatment included dispensed prescription for citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, or vilazodone.
We excluded new users with diagnoses for bipolar disorder, schizophrenia, personality disorder, other psychosis, autistic disorder, substance use disorder, or epilepsy and/or convulsions in the previous year and an inpatient admission in the previous 30 days. We excluded persons with any fracture diagnosis in the 3 months before treatment initiation to reduce misclassification of follow-up fracture visits as an outcome. For youth with >1 new benzodiazepine or SSRI treatment episode meeting inclusion criteria, we selected the first episode. Lastly, we excluded youth initiating benzodiazepine and SSRI treatment on the same day.
Measures
Incident Fracture Event
We identified fracture events using ICD-9-CM or ICD-10-CM diagnostic codes from inpatient and outpatient records beginning the day after benzodiazepine or SSRI initiation and ending at 3 months. The primary outcome was incidence of upper or lower limb factures (shoulder, upper arm, forearm, wrist, femur, lower leg, ankle) because these fractures are more likely than other fracture locations to be caused by falls.27,28,42–45 For a secondary outcome, we included all fracture locations. Fracture definitions are presented in Supplemental Table 5.
Treatment Duration
We estimated benzodiazepine and SSRI treatment duration with prescription refill dates and days’ supply. If there was no prescription refill within 20 days (grace period) after the days’ supply ended for the previous prescription, the individual was considered to have discontinued treatment. In sensitivity analyses, 10- and 30-day grace periods were used.
Additional Measures
Patient characteristics were identified from inpatient and outpatient records (ICD-9-CM, ICD-10-CM, or Current Procedural Terminology codes) and dispensed prescription records in the year before or day of treatment initiation. Broadly, these included age, sex, anxiety disorder diagnosis, provider type of anxiety diagnosis, psychotherapy claims, psychiatric diagnoses, health care use measures, nonpsychiatric medical diagnoses, pediatric chronic complex condition scale,46 injuries, and psychotropic and other medications dispensed in the previous 90 days.
Analysis
We described children and young adults with anxiety disorders initiating benzodiazepine or SSRI treatment and estimated treatment duration and switching between therapies (eg, SSRI initiator filling a benzodiazepine). Primary analyses were as treated given investigation of a safety concern with hypothesized effect during drug use.47 Youth contributed person-time beginning the day after treatment initiation until the first of the following: incident fracture, treatment discontinuation, treatment switching, insurance disenrollment, 3 months, or the end of data.
Our primary measures of adverse effect included crude and propensity score–adjusted incident fracture rates, incident rate differences (IRDs), and incident rate ratios (IRRs). We estimated propensity scores of benzodiazepine treatment separately in children and young adults; variables included potential confounders or predictors of fracture, including year of treatment initiation to account for temporal shifts in prescribing and fracture diagnoses. We used inverse probability of treatment weighting (IPTW) and applied asymmetric trimming,48 excluding those with a propensity score below the first percentile of benzodiazepine initiators and above the 99th percentile of SSRI initiators, to select youth with greater treatment equipoise. In secondary analyses, we estimated results using stabilized inverse probability of treatment weighting (SIPTW) without trimming, restricting follow-up to 30 days, and by duration of action for the initial benzodiazepine (long acting, short acting).
Sensitivity Analyses
We completed intention-to-treat analyses with no censoring at treatment discontinuation or switching and as-treated estimates using 10- and 30-day grace periods to define treatment discontinuation. We examined results in youth with an initial benzodiazepine or SSRI days’ supply value ≥3, excluding youth with baseline antidepressant use, and censoring at September 30, 2015, given the shift to ICD-10-CM codes. We stratified results by sex and common comorbid psychiatric diagnoses (attention-deficit/hyperactivity disorder [ADHD], depression) and restricted the data to youth without previous injury diagnostic codes given hypothesized differing baseline fracture rates in these subgroups. We additionally examined results in youth without recent psychotropic prescriptions and we examined results stratified by any psychiatric comorbidity diagnosis. Propensity scores were re-created with trimming applied to each subgroup. For exploratory analyses in benzodiazepine initiators, we examined fracture rates by a high versus lower initial benzodiazepine dose per day. A high dose per day was defined empirically as a dose greater than the third quartile on the basis of the distributions in children (diazepam >10 mg/day, alprazolam >1 mg/day, clonazepam >1 mg/day, lorazepam >2 mg/day) and in young adults (diazepam >15 mg/day, alprazolam >1.25 mg/day, clonazepam >1.1 mg/day, lorazepam >2.1 mg/day). The exploratory analyses included 92% of children and 94% of young adults initiating benzodiazepine treatment; expanded details are in the Supplemental Information.
Results
The final child cohort included 12 840 benzodiazepine initiators and 107 875 SSRI initiators with anxiety disorders, and the young adult cohort included 57 684 benzodiazepine initiators and 122 084 SSRI initiators. Unspecified anxiety disorder and generalized anxiety disorder were the most common anxiety diagnoses, and benzodiazepine initiators were older, more likely to have panic disorder and recent ED visits, and less likely to have comorbid mental health diagnoses (Table 1). Alprazolam and lorazepam were the most common initial benzodiazepine agents (Supplemental Table 6).
Treatment Duration and Switching
Most children (82%) and young adults (77%) initiating benzodiazepine treatment did not have a second prescription fill before discontinuation. Three months after initiation, 8% of child and 11% of young adult benzodiazepine initiators remained on treatment, compared with more than half of SSRI initiators (Supplemental Table 6). In addition, 22% of child and 17% of young adult benzodiazepine initiators filled an SSRI prescription in the 3 months after, and fewer SSRI initiators filled a benzodiazepine during follow-up.
Children: Incident Fractures
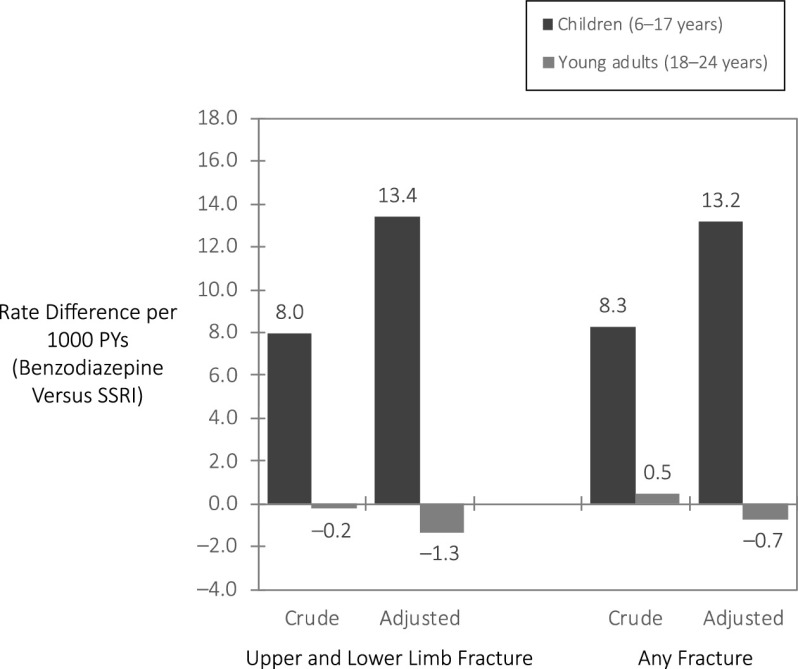
In children, the incident upper and lower limb fracture rate during the first months of treatment was 25.5 per 1000 person-years (PYs), with 33.1 per 1000 PYs in benzodiazepine initiators and 25.1 per 1000 PYs in SSRI initiators (Table 2). After weighting and trimming, benzodiazepine and SSRI initiators were well balanced (Supplemental Table 7). The adjusted fracture rate was elevated in benzodiazepine initiators compared with SSRI initiators: adjusted IRR = 1.53 (95% confidence interval [CI]: 0.94–2.50) and IRD = 13.4 per 1000 PYs (Table 2, Fig 1). The association was stronger for long-acting benzodiazepine initiators versus SSRI initiators: adjusted IRD = 32.9 and IRR = 2.30 (CI: 1.08–4.91). Considering initial benzodiazepine dose, the crude fracture rate was 52.7 (CI: 25.2–96.8) per 1000 PYs in initiators of high dose-per-day benzodiazepine compared to 30.3 (CI: 19.8–44.5) per 1000 PYs in initiators of lower dose-per-day benzodiazepine.
TABLE 2.
Incident Fracture Rates in Children and Young Adults Initiating Benzodiazepine or SSRI Treatment Over a Maximum of 3 Months on Treatment
| No. Persons | Primary Fracture (Upper and Lower Limb)a | Any Fractureb | ||||||
|---|---|---|---|---|---|---|---|---|
| No. Fracture Events | PY | Rate per 1000 PYs | IRR (95% CI) | No. Fracture Events | Rate per 1000 PYs | IRR (95% CI) | ||
| Children (6–17 y) | ||||||||
| Crude | 120 715 | 598 | 23 441 | 25.5 | — | 1162 | 49.7 | — |
| Benzodiazepine initiators | 12 840 | 38 | 1148 | 33.1 | 1.32 (0.95–1.83) | 66 | 57.6 | 1.17 (0.91–1.50) |
| SSRI initiators | 107 875 | 560 | 22 293 | 25.1 | Referent | 1096 | 49.3 | Referent |
| Adjustedc | ||||||||
| Benzodiazepine | 10 864 | 380 | 9871 | 38.5 | 1.53 (0.94–2.50) | 618 | 62.7 | 1.27 (0.88–1.82) |
| SSRI | 95 991 | 553 | 22 046 | 25.1 | Referent | 1088 | 49.5 | Referent |
| Stratified analysis, adjustedc | ||||||||
| Long-actingd benzodiazepine | 3480 | 562 | 9634 | 58.3 | 2.30 (1.08–4.91) | 884 | 92.0 | 1.84 (1.09–3.13) |
| SSRIe | 98 596 | 535 | 21 071 | 25.4 | Referent | 1049 | 49.9 | Referent |
| Short-acting benzodiazepine | 7184 | 279 | 9399 | 29.7 | 1.21 (0.54–2.70) | 443 | 47.2 | 0.96 (0.53–1.75) |
| SSRIe | 94 565 | 517 | 21 009 | 24.6 | Referent | 1029 | 49.1 | Referent |
| Young adults (18–24 y) | ||||||||
| Crude | 179 768 | 254 | 28 827 | 8.8 | — | 775 | 26.9 | — |
| Benzodiazepine initiators | 57 684 | 51 | 5896 | 8.7 | 0.98 (0.72–1.33) | 161 | 27.3 | 1.02 (0.86–1.21) |
| SSRI initiators | 122 084 | 203 | 22 931 | 8.9 | Referent | 614 | 26.8 | Referent |
| Adjustedc | ||||||||
| Benzodiazepine | 51 912 | 130 | 17 007 | 7.6 | 0.85 (0.57–1.27) | 447 | 26.4 | 0.97 (0.78–1.21) |
| SSRI | 109 690 | 269 | 30 084 | 8.9 | Referent | 814 | 27.1 | Referent |
| Stratified analysis, adjustedc | ||||||||
| Long-actingd benzodiazepine | 11 288 | 116 | 13 845 | 8.4 | 0.95 (0.49–1.84) | 372 | 26.9 | 0.99 (0.67–1.46) |
| SSRIe | 110 569 | 202 | 22 766 | 8.9 | Referent | 620 | 27.3 | Referent |
| Short-acting benzodiazepine | 39 974 | 111 | 15 255 | 7.3 | 0.80 (0.52–1.25) | 388 | 25.5 | 0.94 (0.73–1.23) |
| SSRIe | 108 959 | 252 | 27 779 | 9.1 | Referent | 748 | 27.0 | Referent |
—, not applicable.
Upper and lower limb fracture: fractures of the shoulder, upper arm, forearm, wrist, femur, lower leg, and/or ankle.
PYs under any fracture definition are not displayed and are similar to PYs under primary fracture definition.
Adjusted results: IPTW applied in trimmed cohort; total person count displayed is unweighted, and remaining displayed results are weighted.
Long-acting benzodiazepine is defined as initial prescription for chlordiazepoxide, clobazam, clorazepate, clonazepam, diazepam, or flurazepam.
SSRI reference group varies given reweighting or trimming in each stratified cohort.
FIGURE 1.
Incident fracture rate difference in benzodiazepine versus SSRI initiators in children and young adults. Subjects were followed for a maximum of 3 months; upper and lower limb fractures include fractures of the shoulder, upper arm, forearm, wrist, femur, lower leg, and/or ankle.
Under the any fracture outcome definition, the incident fracture rate approximately doubled (49.7 per 1000 PYs) and the IRR approached null, whereas the IRD remained similar to our primary fracture definition (Table 2). Results were consistent when restricting follow-up to a maximum of 1 month, in children without a previous injury, and in children without recent psychotropic prescriptions (Table 3). We observed a heightened fracture rate in benzodiazepine initiators when using a stricter 10-day grace period to define treatment discontinuation (IRR = 1.83 [CI: 1.07–3.14]). Fracture rates were higher in boys and children with ADHD; the association between benzodiazepine versus SSRI treatment and fractures was stronger in children with ADHD, although estimates are less precise.
TABLE 3.
Secondary and Sensitivity Analyses: Benzodiazepine and SSRI (Referent) Initiators and Rate of Fractures (Primary Definition: Upper and Lower Limb)
| Crude | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Benzodiazepine Users | SSRI Users | IRR (95% CI) | IRD per 1000 PYs | IRR (95% CI) | IRD per 1000 PYs | |
| Fracture Rate per 1000 PYs | Fracture Rate per 1000 PYs | |||||
| Children (6–17 y), % of cohort | 33.1 | 25.1 | 1.32 (0.95–1.83) | 8.0 | 1.53 (0.94–2.50) | 13.4 |
| SIPTW estimate | — | — | — | — | 1.36 (0.84–2.19) | 9.0 |
| Maximum of 30 d follow-up | 35.3 | 25.7 | 1.37 (0.94–2.00) | 9.6 | 1.54 (0.88–2.69) | 14.0 |
| Intention-to-treat analysis | 23.9 | 24.4 | 0.98 (0.77–1.25) | −0.5 | 1.16 (0.81–1.66) | 3.9 |
| Treatment duration grace period | ||||||
| 10 d | 36.0 | 25.0 | 1.44 (1.00–2.08) | 11.0 | 1.83 (1.07–3.14) | 20.5 |
| 30 d | 30.0 | 24.9 | 1.21 (0.89–1.65) | 5.2 | 1.35 (0.84–2.15) | 8.5 |
| Initial d supply 3+ d (98%) | 30.5 | 25.1 | 1.21 (0.85–1.74) | 5.4 | 1.34 (0.79–2.27) | 8.4 |
| Fracture outcomes by ICD-9-CM only (81%) | 31.3 | 26.0 | 1.20 (0.84–1.72) | 5.3 | 1.32 (0.84–2.89) | 8.1 |
| No previous injury (67%) | 21.1 | 19.7 | 1.07 (0.64–1.80) | 1.4 | 1.58 (0.79–3.15) | 11.3 |
| No baseline antidepressant use (95%) | 33.3 | 25.0 | 1.33 (0.94–1.89) | 8.3 | 1.43 (0.83–2.44) | 10.6 |
| No psychotropic use (78%) | 27.9 | 24.6 | 1.14 (0.75–1.71) | 3.3 | 1.44 (0.69–3.00) | 10.4 |
| No depression diagnosisb (70%) | 34.0 | 27.0 | 1.26 (0.88–1.80) | 7.0 | 1.44 (0.83–2.50) | 11.6 |
| Stratification by ADHD diagnosis | ||||||
| ADHD (21%) | 58.8 | 27.2 | 2.16 (1.14–4.11) | 31.6 | 2.00 (0.86–4.66) | 27.2 |
| No ADHD (79%) | 28.6 | 24.5 | 1.17 (0.80–1.71) | 4.1 | 1.56 (0.84–2.89) | 13.6 |
| Stratification by psychiatric comorbidity | ||||||
| Psychiatric comorbidity (58%) | 39.7 | 25.6 | 1.55 (0.97–2.50) | 14.2 | 1.89 (1.03–3.46) | 22.8 |
| No comorbidity (42%) | 28.8 | 24.4 | 1.18 (0.75–1.86) | 4.4 | 0.86 (0.45–1.66) | −3.2 |
| Stratification by sex | ||||||
| Male (38%) | 45.0 | 33.4 | 1.35 (0.86–2.12) | 11.7 | 1.40 (0.76–2.58) | 13.5 |
| Female (62%) | 25.6 | 20.0 | 1.28 (0.79–2.09) | 5.5 | 1.34 (0.66–2.69) | 6.5 |
| Young adults (18–24 y), % of cohort | 8.7 | 8.9 | 0.98 (0.72–1.33) | −0.2 | 0.85 (0.57–1.27) | −1.3 |
| SIPTW estimate | — | — | — | — | 0.90 (0.61–1.35) | −0.9 |
| Maximum of 30 d follow-up | 7.9 | 8.9 | 0.88 (0.59–1.32) | −1.0 | 0.79 (0.47–1.33) | −2.0 |
| Intention-to-treat analysis | 8.6 | 8.7 | 0.99 (0.79–1.23) | −0.1 | 0.83 (0.64–1.08) | −1.5 |
| Treatment duration grace period | ||||||
| 10 d | 8.9 | 8.6 | 1.03 (0.73–1.46) | 0.3 | 0.97 (0.62–1.52) | −0.3 |
| 30 d | 8.9 | 8.9 | 1.00 (0.76–1.32) | 0.0 | 0.84 (0.59–1.20) | −1.4 |
| Initial d supply 3+ d (98%) | 8.6 | 8.8 | 0.98 (0.72–1.34) | −0.2 | 0.86 (0.57–1.28) | −1.3 |
| Fracture outcomes by ICD-9-CM only (80%) | 8.0 | 9.3 | 0.87 (0.61–1.22) | −1.2 | 0.68 (0.45–1.03) | −2.9 |
| No previous injury (72%) | 6.0 | 7.1 | 0.85 (0.55–1.31) | −1.0 | 0.68 (0.41–1.12) | −2.3 |
| No baseline antidepressant use (92%) | 8.8 | 9.1 | 0.98 (0.71–1.35) | −0.2 | 0.85 (0.55–1.31) | −1.4 |
| No psychotropic use (82%) | 8.2 | 8.7 | 0.93 (0.65–1.34) | −0.6 | 0.75 (0.48–1.18) | −2.3 |
| No depression diagnosisb (76%) | 9.0 | 9.0 | 1.00 (0.72–1.39) | 0.0 | 0.85 (0.58–1.26) | −1.3 |
| No ADHD diagnosisb (90%) | 8.5 | 8.6 | 1.00 (0.72–1.38) | 0.0 | 0.83 (0.56–1.24) | −1.4 |
| Stratification by psychiatric comorbidity | ||||||
| Psychiatric comorbidity (39%) | 8.8 | 9.0 | 0.98 (0.58–1.65) | −0.2 | 0.98 (0.49–1.95) | −0.2 |
| No comorbidity (61%) | 8.6 | 8.8 | 0.98 (0.67–1.44) | −0.2 | 0.85 (0.55–1.32) | −1.2 |
| Stratification by sex | ||||||
| Male (33%) | 10.8 | 12.2 | 0.89 (0.56–1.40) | −1.4 | 0.62 (0.37–1.06) | −4.6 |
| Female (67%) | 7.4 | 7.3 | 1.02 (0.68–1.55) | 0.2 | 1.04 (0.61–1.77) | 0.3 |
—, not applicable.
Adjusted results: IPTW applied in trimmed cohort (except when noted for SIPTW results).
Results from strata with <10 fracture events in a treatment group are not displayed.
Young Adults: Incident Fractures
In young adults, the incident upper and lower limb fracture rate was 8.8 per 1000 PYs, approximately one-third of the rate in children. The fracture rate was similar in benzodiazepine (8.7 per 1000 PYs) and SSRI initiators (8.9 per 1000 PYs) (Table 2). After weighting and trimming, benzodiazepine and SSRI initiators were well balanced (Supplemental Table 8). The adjusted fracture rate was 7.6 per 1000 PYs in benzodiazepine initiators and 8.9 per 1000 PYs in SSRI initiators, with an adjusted IRR of 0.85 (CI: 0.57–1.27) and IRD of −1.3 per 1000 PYs (Table 2, Fig 1). The crude fracture rate was similar in young adults initiating benzodiazepine treatment on a high or low dose per day, that of 8.3 (CI: 4.0–15.3) and 8.8 (CI: 6.2–12.1) per 1000 PYs, respectively.
Under the any fracture definition, the young adult incident fracture rate tripled to 26.9 per 1000 PYs with no association between treatment type and fracture (Table 2). Most findings from additional analyses were similar to the primary analysis (Table 3). Incident upper and lower limb fracture rates were lower in male benzodiazepine versus SSRI initiators; however, under the any fracture definition (results not shown), the association was null (IRR = 0.94). Similarly, when restricting follow-up to outcomes under ICD-9-CM fracture codes, the estimate was null under the any fracture definition.
Discussion
With our findings, we provide novel comparative estimates of fracture rates during benzodiazepine and SSRI treatment in children and young adults with anxiety disorders. In children initiating benzodiazepines, we observed a heightened rate of upper and lower limb fractures compared to SSRI treatment, with no heightened rate in young adult benzodiazepine initiators. Increased caution may be warranted at benzodiazepine initiation in children with anxiety disorders, particularly with initiation of long-acting benzodiazepines. These findings add to the limited understanding of benzodiazepine treatment safety in youth.
The elevated upper and lower limb fracture rate in children initiating benzodiazepine treatment compared to SSRI treatment (IRR = 1.53) is similar to estimates from older adult benzodiazepine users compared with nonusers with pooled meta-analysis effect estimates of risk ratios of 1.25 to 1.52.15,19,21,22 We focused on short-term fracture risk because benzodiazepine treatment is typically for short durations in youth with anxiety disorders and authors of literature regarding older adults have observed greater fall and fracture risk in the first weeks after initiation.19,24,25,49 However, not all study authors observed this short-term effect.20
Fracture rates were higher in children initiating long-acting benzodiazepines. In adults, there is evidence suggesting benzodiazepines with longer half-lives have a higher relative risk of fractures and motor vehicle crashes than benzodiazepines with shorter half-lives13,50,51; however, findings are mixed.18,24,25,52 In meta-analyses, authors observed similar fracture risk in short-acting and long-acting adult benzodiazepine users.15,21 Preferential prescribing of short-acting benzodiazepines to adults at increased fall or fracture risk may have contributed to observations of no elevated risk with long-acting benzodiazepines.25 Relatedly, children prescribed long-acting benzodiazepines for anxiety could have a baseline elevated fracture risk beyond what we controlled for and may also have greater benzodiazepine exposure if prescribed for regular, daily use versus as-needed use. In our exploratory analysis, we observed an elevated fracture rate in children initiating a high benzodiazepine dose per day versus lower dose per day. In older adults, higher benzodiazepine doses are associated with a heightened fracture risk.25,26,50 It is suggested in our crude estimates that a closer examination of benzodiazepine dose on fracture risk and the interaction of dose and half-life will further inform prescribing decisions.
The estimated rate difference for fractures in children receiving benzodiazepine versus SSRI treatment was moderately small (13 per 1000 PYs), yet notable because alternative treatments are available; these fractures thus represent potentially preventable adverse outcomes. The heightened fracture rate difference in boys and children with ADHD may be related to greater activity levels and thus increased opportunities for treatment-related adverse effects that result in fracture-causing falls. Most falls in children and young adults are without incident; however, falls can result in fracture including severe, life-threatening injuries, particularly if falls occur from elevated heights.29,53 Clinicians could advise caution in activities that increase the likelihood of a severe injury until it is clear how benzodiazepine treatment will affect the child.
In young adults with anxiety disorders, we did not observe an elevated fracture rate in benzodiazepine initiators versus SSRI initiators. Several factors may contribute to this finding. Young adults are generally less active and experience fewer fall-related injuries than do children.54,55 Therefore, there may be less opportunity for benzodiazepine adverse effects to cause a fall that could result in fracture. Young adults may receive increased warnings surrounding benzodiazepine side effects given this age group drives and benzodiazepine drug labels state that individuals should not drive or engage in dangerous activities until they know how the medication will affect them. Relatedly, young adults likely have more awareness of symptoms and side effects and autonomy over when medication is taken. Benzodiazepines are more commonly prescribed to young adults than children56; children who receive benzodiazepines may, on average, have higher anxiety severity than young adults, a potential source of residual confounding. Subthreshold or undiagnosed conduct disorder is another potential source of residual confounding more prevalent in children. Young adults may be more likely to have previous medical or nonmedical exposure to benzodiazepine4,57 that lessens their risk of benzodiazepine-related fractures. Alcohol and drug use are potential unmeasured confounders that can increase accidental injury and fall risk58–60 and could increase the likelihood of receiving SSRIs given concerns related to benzodiazepine misuse. This could lead to attenuated fracture risk effect, particularly in young adults for whom substance use is more prevalent; however, there is uncertainty because patient treatment preferences could differ by substance use. In addition, our primary fracture outcome was uncommon in young adults and perhaps less clinically relevant than for children.
As previously recognized, the observed association between benzodiazepines and fractures in older adults could be biased by confounding by indication.15,18,22 Our restriction to youth with anxiety disorders, active comparator design, and implementation of IPTW sought to reduce the potential for confounding by indication while addressing a clinically relevant question. Despite our study design, unmeasured confounding may persist. Antidepressants (including SSRIs, our active comparator) have been associated with an elevated fracture risk compared to no antidepressant use.22,61,62 The proposed mechanism of fracture risk with benzodiazepine treatment is related to adverse effects such as sedation or dizziness leading to a fall, whereas for SSRIs, the hypothesized mechanisms are an increased fall risk or decreased bone mineral density.63,64 However, findings are inconsistent on the association between SSRIs and fractures26,65 and studies may be susceptible to confounding by indication.62,66 Whether our observed associations between benzodiazepines and fractures hold across benzodiazepine indications and compared with other alternative pharmacotherapies remains unclear.
Limitations of the work should be considered. We cannot be certain the primary treatment indication was an anxiety disorder and whether, and when, filled prescriptions were consumed. We lack clinical severity measures; it is unclear how much unmeasured differences in psychiatric condition severity exist between youth initiating a benzodiazepine versus SSRI and how anxiety severity impacts fracture risk. Further research is needed to evaluate whether elevated fracture risk with benzodiazepine treatment is concentrated in children with a psychiatric comorbidity or if this observation is a result of differences in prescribing and/or patient characteristics between children with and without psychiatric comorbidities. Benzodiazepine treatment is typically shorter than SSRI treatment; however, our results restricting to 30 days of treatment compare outcomes over a similar denominator. Many subgroup analyses were limited by sample size; future research will determine if findings remain across populations. Although the primary fracture definition intended to capture fractures more likely to be caused by a fall, fractures could be caused by any mechanism and be unrelated to treatment. We do not expect differential misclassification of fracture coding by treatment; however, fracture events were based on diagnostic codes and may not represent true events. The validity of fracture diagnostic codes varies by fracture type, with positive predictive values typically estimated at >80%.67–69 Our data overlapped the transition to ICD-10-CM codes; authors of future analyses could examine this research question strictly under ICD-10-CM codes.
Conclusions
We observed a heightened rate of fractures in children with anxiety disorders initiating benzodiazepine treatment compared to SSRI treatment but not in young adults. Increased caution in the weeks after benzodiazepine initiation in children with anxiety disorders may be warranted. Moreover, given the lack of efficacy evidence and limited safety data for benzodiazepine treatment of pediatric anxiety disorders, this safety signal is an important consideration in the benefit-harm evaluation for prescribing benzodiazepines to youth with anxiety disorders.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- CI
confidence interval
- ED
emergency department
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICD-10-CM
International Classification of Diseases, 10th Revision, Clinical Modification
- IPTW
inverse probability of treatment weighting
- IRD
incident rate difference
- IRR
incident rate ratio
- PY
person-year
- SIPTW
stabilized inverse probability of treatment weighting
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Dr Bushnell made substantial contributions to the conception and design, analysis, and interpretation of data, drafted the initial manuscript, and revised the manuscript; Drs Crystal, Gerhard, and Olfson made substantial contributions to the conception and design and interpretation of data and critically revised and reviewed the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
FINANCIAL DISCLOSURE: Dr Bushnell received support from the National Institute of Mental Health under award T32MH013043. Dr Gerhard reports grants and personal fees from Bristol-Myers Squibb and personal fees from Merck, Pfizer, Eli Lilly, and Intra-Cellular Therapies outside the submitted work.
FUNDING: Supported by the National Institute of Mental Health under award T32MH013043. Dr Crystal’s work is supported by awards from the Agency for Healthcare Research and Quality (1R01HS026001-01A1), Patient-Centered Outcomes Research Institute (IHS-1409-23194), and National Institutes of Health (1R01DA047347-01 and UL1TR003017). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health. 2016;106(4):686–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142 [DOI] [PubMed] [Google Scholar]
- 3.Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCabe SE, Wilens TE, Boyd CJ, Chua K-P, Voepel-Lewis T, Schepis TS. Age-specific risk of substance use disorders associated with controlled medication use and misuse subtypes in the United States. Addict Behav. 2019;90:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortuna RJ, Robbins BW, Caiola E, Joynt M, Halterman JS. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics. 2010;126(6):1108–1116 [DOI] [PubMed] [Google Scholar]
- 6.Sidorchuk A, Isomura K, Molero Y, et al. Benzodiazepine prescribing for children, adolescents, and young adults from 2006 through 2013: a total population register-linkage study. PLoS Med. 2018;15(8):e1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bushnell GA, Crystal S, Olfson M. Prescription benzodiazepine use in privately insured US children and adolescents. Am J Prev Med. 2019;57(6):775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Whiteside SPH, Sim L, et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: a systematic review and meta-analysis. JAMA Pediatr. 2017;171(11):1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ipser JC, Stein DJ, Hawkridge S, Hoppe L. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev. 2009;(3):CD005170. [DOI] [PubMed] [Google Scholar]
- 10.Connolly SD, Bernstein GA; Work Group on Quality Issues . Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–283 [DOI] [PubMed] [Google Scholar]
- 11.Brunton LL, Chabner BA, Knollman BC. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12th ed New York, NY: McGraw-Hill, Inc; 2011 [Google Scholar]
- 12.US Food and Drug Administration FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-serious-risks-and-death-when-combining-opioid-pain-or. Accessed May 7, 2018
- 13.Smink BE, Egberts ACG, Lusthof KJ, Uges DRA, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systematic literature review. CNS Drugs. 2010;24(8):639–653 [DOI] [PubMed] [Google Scholar]
- 14.Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663–673 [DOI] [PubMed] [Google Scholar]
- 15.Xing D, Ma XL, Ma JX, Wang J, Yang Y, Chen Y. Association between use of benzodiazepines and risk of fractures: a meta-analysis. Osteoporos Int. 2014;25(1):105–120 [DOI] [PubMed] [Google Scholar]
- 16.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960 [DOI] [PubMed] [Google Scholar]
- 17.Park H, Satoh H, Miki A, Urushihara H, Sawada Y. Medications associated with falls in older people: systematic review of publications from a recent 5-year period. Eur J Clin Pharmacol. 2015;71(12):1429–1440 [DOI] [PubMed] [Google Scholar]
- 18.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001;158(6):892–898 [DOI] [PubMed] [Google Scholar]
- 19.Donnelly K, Bracchi R, Hewitt J, Routledge PA, Carter B. Benzodiazepines, Z-drugs and the risk of hip fracture: a systematic review and meta-analysis. PLoS One. 2017;12(4):e0174730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Requena G, Huerta C, Gardarsdottir H, et al. Hip/femur fractures associated with the use of benzodiazepines (anxiolytics, hypnotics and related drugs): a methodological approach to assess consistencies across databases from the PROTECT-EU project. Pharmacoepidemiol Drug Saf. 2016;25(suppl 1):66–78 [DOI] [PubMed] [Google Scholar]
- 21.Khong TP, de Vries F, Goldenberg JSB, et al. Potential impact of benzodiazepine use on the rate of hip fractures in five large European countries and the United States. Calcif Tissue Int. 2012;91(1):24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takkouche B, Montes-Martínez A, Gill SS, Etminan M. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171–184 [DOI] [PubMed] [Google Scholar]
- 23.Requena G, Logie J, Martin E, et al. Do case-only designs yield consistent results across design and different databases? A case study of hip fractures and benzodiazepines. Pharmacoepidemiol Drug Saf. 2016;25(suppl 1):79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner AK, Zhang F, Soumerai SB, et al. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164(14):1567–1572 [DOI] [PubMed] [Google Scholar]
- 25.Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vestergaard P, Prieto-Alhambra D, Javaid MK, Cooper C. Fractures in users of antidepressants and anxiolytics and sedatives: effects of age and dose. Osteoporos Int. 2013;24(2):671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedström EM, Svensson O, Bergström U, Michno P. Epidemiology of fractures in children and adolescents. Acta Orthop. 2010;81(1):148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rennie L, Court-Brown CM, Mok JYQ, Beattie TF. The epidemiology of fractures in children. Injury. 2007;38(8):913–922 [DOI] [PubMed] [Google Scholar]
- 29.Borse N, Sleet DA. CDC Childhood Injury Report: patterns of unintentional injuries among 0- to 19-year olds in the United States, 2000-2006. Fam Community Health. 2009;32(2):189. [DOI] [PubMed] [Google Scholar]
- 30.Kopjar B, Wickizer TM. Fractures among children: incidence and impact on daily activities. Inj Prev. 1998;4(3):194–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding R, McCarthy ML, Houseknecht E, et al. ; CHAT Study Group . The health-related quality of life of children with an extremity fracture: a one-year follow-up study. J Pediatr Orthop. 2006;26(2):157–163 [DOI] [PubMed] [Google Scholar]
- 32.Moorin RE, Hendrie D. The epidemiology and cost of falls requiring hospitalisation in children in Western Australia: a study using linked administrative data. Accid Anal Prev. 2008;40(1):216–222 [DOI] [PubMed] [Google Scholar]
- 33.Wu C-H, Wang C-C, Katz AJ, Farley J. National trends of psychotropic medication use among patients diagnosed with anxiety disorders: results from medical expenditure panel survey 2004–2009. J Anxiety Disord. 2013;27(2):163–170 [DOI] [PubMed] [Google Scholar]
- 34.Olfson M, Marcus SC, Wan GJ, Geissler EC. National trends in the outpatient treatment of anxiety disorders. J Clin Psychiatry. 2004;65(9):1166–1173 [DOI] [PubMed] [Google Scholar]
- 35.Bushnell GA, Compton SN, Dusetzina SB, et al. Treating pediatric anxiety: initial use of SSRIs and other antianxiety prescription medications. J Clin Psychiatry. 2018;79(1):16m11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheu Y, Chen L-H, Hedegaard H. Sports- and recreation-related injury episodes in the United States, 2011–2014. Natl Health Stat Rep. 2016;(99):1–12 [PubMed] [Google Scholar]
- 37.Karl JW, Olson PR, Rosenwasser MP. The epidemiology of upper extremity fractures in the United States, 2009. J Orthop Trauma. 2015;29(8):e242–e244 [DOI] [PubMed] [Google Scholar]
- 38.Immerman I, Livermore MS, Szabo RM. Use of emergency department services for hand, wrist, and forearm fractures in the United States in 2008. J Surg Orthop Adv. 2014;23(2):98–104 [DOI] [PubMed] [Google Scholar]
- 39.Slee A, Nazareth I, Bondaronek P, Liu Y, Cheng Z, Freemantle N. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019;393(10173):768–777 [DOI] [PubMed] [Google Scholar]
- 40.Williams T, Hattingh CJ, Kariuki CM, et al. Pharmacotherapy for social anxiety disorder (SAnD). Cochrane Database Syst Rev. 2017;(10):CD001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Psychiatric Association Practice Guideline for the Treatment of Patients With Panic Disorder, 2nd ed Washington, DC: American Psychiatric Association; 2009 [Google Scholar]
- 42.Ryan LM, Teach SJ, Searcy K, et al. Epidemiology of pediatric forearm fractures in Washington, DC. J Trauma. 2010;69(suppl 4):S200–S205 [DOI] [PubMed] [Google Scholar]
- 43.Liu EH, Alqahtani S, Alsaaran RN, Ho ES, Zuker RM, Borschel GH. A prospective study of pediatric hand fractures and review of the literature. Pediatr Emerg Care. 2014;30(5):299–304 [DOI] [PubMed] [Google Scholar]
- 44.Allareddy V, Itty A, Maiorini E, et al. Emergency department visits with facial fractures among children and adolescents: an analysis of profile and predictors of causes of injuries. J Oral Maxillofac Surg. 2014;72(9):1756–1765 [DOI] [PubMed] [Google Scholar]
- 45.Arora R, Fichadia U, Hartwig E, Kannikeswaran N. Pediatric upper-extremity fractures. Pediatr Ann. 2014;43(5):196–204 [DOI] [PubMed] [Google Scholar]
- 46.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernán MA, Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9(1):48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glynn RJ, Lunt M, Rothman KJ, Poole C, Schneeweiss S, Stürmer T. Comparison of alternative approaches to trim subjects in the tails of the propensity score distribution. Pharmacoepidemiol Drug Saf. 2019;28(10):1290–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neutel CI, Hirdes JP, Maxwell CJ, Patten SB. New evidence on benzodiazepine use and falls: the time factor. Age Ageing. 1996;25(4):273–278 [DOI] [PubMed] [Google Scholar]
- 50.Vestergaard P, Rejnmark L, Mosekilde L. Anxiolytics and sedatives and risk of fractures: effects of half-life. Calcif Tissue Int. 2008;82(1):34–43 [DOI] [PubMed] [Google Scholar]
- 51.Hebert C, Delaney JAC, Hemmelgarn B, Lévesque LE, Suissa S. Benzodiazepines and elderly drivers: a comparison of pharmacoepidemiological study designs. Pharmacoepidemiol Drug Saf. 2007;16(8):845–849 [DOI] [PubMed] [Google Scholar]
- 52.Brandt J, Leong C. Benzodiazepines and Z-drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17(4):493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang MY, Kim KA, Griffith PM, et al. Injuries from falls in the pediatric population: an analysis of 729 cases. J Pediatr Surg. 2001;36(10):1528–1534 [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention; National Center for Injury Prevention and Control. Nonfatal injury data. 2019. Available at: https://www.cdc.gov/injury/wisqars/nonfatal.html. Accessed September 9, 2019
- 55.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188 [DOI] [PubMed] [Google Scholar]
- 56.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM; Centers for Disease Control and Prevention (CDC) . Controlled substance prescribing patterns–Prescription Behavior Surveillance System, eight states, 2013. MMWR Surveill Summ. 2015;64(9):1–14 [DOI] [PubMed] [Google Scholar]
- 57.Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70(2):97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen CM, Yoon Y-H. Usual alcohol consumption and risks for nonfatal fall injuries in the United States: results from the 2004–2013 National Health Interview Survey. Subst Use Misuse. 2017;52(9):1120–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherpitel CJ, Ye Y, Andreuccetti G, et al. Risk of injury from alcohol, marijuana and other drug use among emergency department patients. Drug Alcohol Depend. 2017;174:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitale S, van de Mheen D. Illicit drug use and injuries: a review of emergency room studies. Drug Alcohol Depend. 2006;82(1):1–9 [DOI] [PubMed] [Google Scholar]
- 61.Coupland C, Hill T, Morriss R, Moore M, Arthur A, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in people aged 20-64 years: cohort study using a primary care database. BMC Med. 2018;16(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Q, Bencaz AF, Hentz JG, Crowell MD. Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies. Osteoporos Int. 2012;23(1):365–375 [DOI] [PubMed] [Google Scholar]
- 63.Wadhwa R, Kumar M, Talegaonkar S, Vohora D. Serotonin reuptake inhibitors and bone health: a review of clinical studies and plausible mechanisms. Osteoporos Sarcopenia. 2017;3(2):75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feuer AJ, Demmer RT, Thai A, Vogiatzi MG. Use of selective serotonin reuptake inhibitors and bone mass in adolescents: an NHANES study. Bone. 2015;78:28–33 [DOI] [PubMed] [Google Scholar]
- 65.Gracious BL, Fontanella CA, Phillips GS, Bridge JA, Marcus SC, Campo JV. Antidepressant exposure and risk of fracture among Medicaid-covered youth. J Clin Psychiatry. 2016;77(7):e950–e956 [DOI] [PubMed] [Google Scholar]
- 66.Andrade C. Antidepressant drugs and the risk of hip fracture in the elderly: is there more to it than confounding by indication? J Clin Psychiatry. 2019;80(4):19f12999. [DOI] [PubMed] [Google Scholar]
- 67.Curtis JR, Mudano AS, Solomon DH, Xi J, Melton ME, Saag KG. Identification and validation of vertebral compression fractures using administrative claims data. Med Care. 2009;47(1):69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sing C-W, Woo Y-C, Lee ACH, et al. Validity of major osteoporotic fracture diagnosis codes in the Clinical Data Analysis and Reporting System in Hong Kong. Pharmacoepidemiol Drug Saf. 2017;26(8):973–976 [DOI] [PubMed] [Google Scholar]
- 69.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high–a systematic review. J Clin Epidemiol. 2013;66(3):278–285 [DOI] [PubMed] [Google Scholar]