We present the successful management of a critically ill, previously healthy child with COVID-19 presenting with respiratory failure and severe thrombocytopenia.
Abstract
The novel severe acute respiratory syndrome coronavirus 2 is a worldwide pandemic. The severe morbidity and mortality associated with coronavirus disease 2019 has mostly affected the elderly or those with underlying medical conditions. We present a case of a 12-year-old girl with no past medical history who presented with fever, cough, and vomiting. Laboratory evaluation revealed severe thrombocytopenia and elevated markers of inflammation. The patient progressed to respiratory failure, and testing results for the severe acute respiratory syndrome coronavirus 2 returned positive. Because of the severity of her thrombocytopenia, she was treated with intravenous immunoglobulin and steroids with prompt improvement in platelets. The patient’s severe acute respiratory distress syndrome was managed with mechanical ventilation, inhaled nitric oxide, and then airway pressure release ventilation. After azithromycin and hydroxychloroquine were given without improvement, our patient received tocilizumab, an anti–interleukin-6 receptor antibody, and remdesivir, a broad antiviral agent, with significant clinical benefit soon afterward. Given that severe pediatric coronavirus disease 2019 is rare, we hope to inform pediatric providers on the clinical course and management considerations as this pandemic continues to spread.
As of April 22, 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been responsible for >2.4 million infections and >150 000 deaths worldwide, with the United States now having the largest number of reported cases.1 It is suggested in available data that most children have mild disease and that children with severe disease appear to be younger (usually <1 years of age)2 or have preexisting medical conditions.3 Mild thrombocytopenia has been seen in adult patients with severe coronavirus disease 2019 (COVID-19),4 and there is 1 report of immune thrombocytopenia (ITP) associated with COVID-19 in an adult patient with underlying autoimmune hypothyroidism.5 We report a case of severe COVID-19 in a previously healthy 12-year-old child presenting with respiratory failure and severe thrombocytopenia.
Clinical Presentation
A previously healthy 12-year-old girl presented with 5 days of fever, nonproductive cough, 2 days of nonbloody emesis, worsening shortness of breath, and hematuria. Her temperature was 39.6°C, pulse was 129 beats per minute, respiratory rate was 26 breaths per minute, and oxygen saturation was 89% on room air. Her weight was 60 kg and her BMI was 25. On physical examination, she had dyspnea, diminished breath sounds diffusely, and petechiae. The rest of her examination was unremarkable. Chest radiograph revealed bilateral diffuse airspace opacities and small pleural effusion.
Laboratory findings on admission were remarkable for severe thrombocytopenia, lymphopenia, and elevated inflammatory markers (C-reactive protein [CRP], procalcitonin, and ferritin) (Table 1). The only abnormality on peripheral blood smear was severe macrothrombocytopenia. The results for nasopharyngeal swab respiratory viral panel by multiplex polymerase chain reaction (PCR) for 16 common pathogens such as rhinovirus and influenza were negative.
Table 1.
Admission and Hyperinflammation Laboratory Results
| Laboratory Measures | Value | Reference Range |
|---|---|---|
| Admission (HD 0) | ||
| White blood cell count, per μL | 5470 | 4500–13500 |
| Absolute lymphocyte count, per μL | 711 (L) | 1485–6480 |
| Hemoglobin, g/dL | 12.3 | 12–16 |
| Platelet count, ×103/μL | <10 (L) | 150–450 |
| Prothrombin time, s | 15.3 | 12.6–15.9 |
| Activated partial thromboplastin time, s | 53.6 (H) | 26–38 |
| Fibrinogen, mg/dL | 424 (H) | 200–400 |
| Sodium, mmol/L | 132 (L) | 134–143 |
| Creatinine, mg/dL | 0.69 | 0.30–0.80 |
| Total bilirubin, mg/dL | 0.8 | 0.2–1.0 |
| Aspartate aminotransferase, U/L | 37 (H) | 17–33 |
| Alanine aminotransferase, U/L | 25 | 11–33 |
| CRP, mg/dL | 11.5 (H) | <1.0 |
| Procalcitonin, ng/mL | 0.83 (H) | <0.10 |
| Ferritin, ng/mL | 481 (H) | 14–79 |
| HD 4a | ||
| CRP, mg/dL | 8.3 (H) | <1.0 |
| Ferritin, ng/mL | 600 (H) | 14–79 |
| IL-2 receptor, pg/mL | 910 | <1033 |
| IL-6, pg/mL | 10 (H) | <5 |
| Interferon-γ, pg/mL | <5 | <5 |
| IL-10, pg/mL | <5 | <18 |
| HD 7b | ||
| CRP, mg/dL | 10.3 (H) | <1.0 |
| Ferritin, ng/mL | 436 (H) | 14–79 |
| IL-2 receptor, pg/mL | 1486 (H) | <1033 |
| IL-6, pg/mL | 34 (H) | <5 |
| Interferon-γ, pg/mL | 10 (H) | <5 |
| IL-10, pg/mL | 9 | <18 |
| CXCL9, pg/ml | 248(H) | <121 |
| IL-18, pg/mL | 1184 (H) | 89–540 |
CXCL9, C-X-C motif chemokine 9; H, high; IL-2, interleukin-2; IL-10, interleukin-10; IL-18, interleukin-18; L, low.
Drawn on HD 4.
Drawn before administration of tocilizumab on HD 7.
Hospitalization Course
The patient was admitted to the ICU on high-flow nasal cannula but subsequently required intubation and mechanical ventilation on 100% oxygen on hospital day (HD) 1. She had continued desaturations and so was started on inhaled nitric oxide (iNO) with improvement in PaO2 and oxygen saturations (oxygen index was 30 before and 9.7 after iNO). Empirical antibiotics for presumed sepsis were initiated. Because of the risk for bleeding with concern for ITP, intravenous immunoglobulin (IVIg) was given on HDs 1 and 2 (1 g/kg per dose) along with methylprednisolone (1.5 mg/kg) on HD 2 with good recovery of platelets (143 × 109/L on HD 4). Azithromycin was started on HD 2 for 3 days as an antiinflammatory agent in the setting of acute respiratory distress syndrome (ARDS).
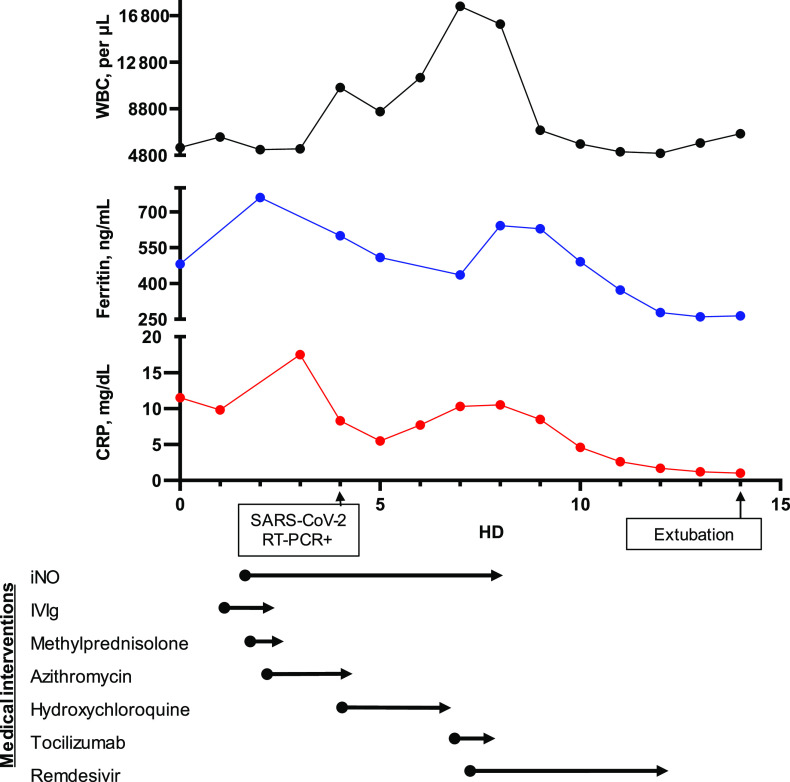
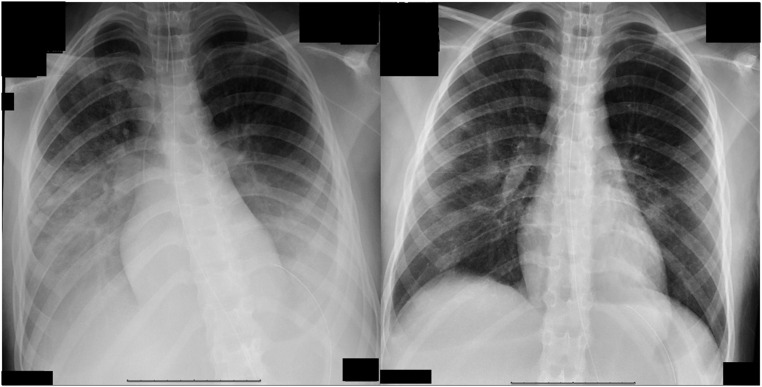
A summary of the hospital course and treatments is shown in Fig 1. A nasopharyngeal SARS-CoV-2 real-time reverse transcription PCR test was sent after admission and returned with positive results on HD 4. The patient did not have any known exposure to patients with COVID-19 or recent travel. Hydroxychloroquine was started (400 mg twice daily on HD 4 followed by 200 mg twice daily until HD 7) for off-label treatment of severe COVID-19 infection.6,7 A hyperinflammation workup was performed after the SARS-CoV-2 testing resulted positive to guide additional immunomodulatory therapy (Table 1). Attempts were made to wean ventilator support and iNO but were unsuccessful. On HD 7, because of continued fever, ARDS and elevated inflammatory markers including interleukin-6 (IL-6), 2 doses of tocilizumab (8 mg/kg 12 hours apart), a humanized monoclonal IL-6 receptor antibody were given, and she was changed to airway pressure release ventilation for enhanced ARDS management. We obtained permission for compassionate use of remdesivir, which was also started on HD 7 (200 mg on HD 7 followed by 100 mg daily). On HD 8, the patient had significant clinical (oxygen index improved from 7.9 to 5.5) and radiographic (Fig 2) improvement and was weaned off iNO. We discontinued remdesivir on HD 12 because of mildly elevated transaminases per compassionate use guidelines, and after a cautious wean of ventilatory support to avoid risk of reintubation per available guidance at the time,8 the patient was extubated on HD 14. On HD 24, the patient was discharged from the hospital after undergoing a short inpatient rehabilitation stay.
FIGURE 1.
Timeline of medical interventions and inflammatory laboratories: white blood cell (WBC) count (black), ferritin (blue), and CRP (red). RT-PCR+, positive result for reverse transcription polymerase chain reaction.
FIGURE 2.
Chest radiograph findings HD 7 (left) and HD 8 (right).
Discussion
We report the successful management of a critically ill child with COVID-19 in the United States. In contrast to children with severe COVID-19 from the People’s Republic of China,3 our patient was older and had no previous medical history. Her presentation with severe thrombocytopenia raised the concern for acute ITP given the degree of thrombocytopenia, peripheral blood smear, and lack of other physical examination findings such as organomegaly. Other causes for thrombocytopenia were considered in the differential including thrombosis, microangiopathic hemolytic anemia, hemophagocytic lymphohistiocytosis, hypersplenism, ARDS, the coronavirus infection itself, and medications. ITP is a diagnosis of exclusion, and although the other causes of thrombocytopenia could not be ruled out with complete certainty, the clinical and laboratory findings were not supportive of these alternative diagnoses. Therefore, her thrombocytopenia was treated as ITP with standard first-line treatments, IVIg, and corticosteroids,9 with good recovery of her platelets. Although mild thrombocytopenia has been reported in older patients with COVID-19,4 our patient’s presentation with profound thrombocytopenia was atypical. Similar to adult patients, our patient’s thrombocytopenia was associated with a more severe disease course.10
After our patient’s SARS-CoV-2 reverse transcription PCR test results returned positive, hydroxychloroquine was initiated on the basis of preliminary reports available at the time suggesting enhanced viral clearance in vitro6 and in a small case series of adult patients with COVID-19.7 However, it is suggested in recent evidence that hydroxychloroquine provides no benefit,11 and our patient showed no improvement with its use.
Remdesivir is an adenosine analog that inhibits viral replication with broad antiviral activity including against SARS-CoV-2 in vitro.12 Although there are currently no approved therapies for SARS-CoV-2, preliminary evidence from a case series of adult patients seems to suggest some clinical improvement with the use of remdesivir,13 but results from placebo-controlled randomized trials are still pending. Our patient received remdesivir under compassionate use and tolerated it well aside from mildly elevated transaminases, which were also reported in adults. The patient had clear signs of clinical improvement after remdesivir; however, its role as a single agent is unclear because it was given in combination with tocilizumab.
Because of the severity of the patient’s severe acute respiratory syndrome in an otherwise healthy child, a hyperinflammation workup was initiated to guide additional immunomodulatory therapy. It is suggested in adult data that cytokine storm syndrome may play a role in the morbidity and mortality in a subset of patients with COVID-19.14 IL-6 is a proinflammatory cytokine implicated in cytokine release syndrome (CRS), which is sometimes seen after chimeric antigen T-cell therapies for malignancies.15 In a retrospective review of 150 hospitalized adult patients with COVID-19, CRP, another marker of acute inflammation, and IL-6 were significantly higher in those patients who died than in those who were discharged from the hospital.16 Elevated IL-6 levels were also reported in 2 of the 3 critically ill children with COVID-19 from Wuhan, China.17
Tocilizumab is a humanized IL-6 receptor monoclonal antibody approved by the Food and Drug Administration to treat rheumatologic disease18 as well as CRS.19 Reports in adults have revealed a potential benefit of tocilizumab in COVID-19,20,21 and clinical trials in adult patients with COVID-19 pneumonia are ongoing.22,23 Our patient’s inflammatory laboratories before administration of tocilizumab were elevated (Table 1) but not to the extent in other literature.17 Because our patient received other immunomodulatory medications (IVIg and corticosteroids) early in her disease course for treatment of presumed ITP, this may have reduced the inflammation to some degree as evidenced by declining markers of inflammation from HDs 3 to 5 (Fig 1). Although our patient was treated with multiple SARS-CoV-2–directed (hydroxychloroquine and remdesivir) as well as ARDS-directed therapies (iNO and airway pressure release ventilation), her sustained improvement after the administration of tocilizumab with normalization of inflammatory markers and extubation within 7 days is consistent with results of its use in CRS.19 Our patient tolerated it well without any significant toxicities aside from mildly elevated transaminases, which may have been precipitated or exacerbated by concomitant administration of remdesivir.
In addition to tocilizumab, other cytokine-directed therapies, such as sarilumab, another IL-6 receptor antibody, anakinra, an IL-1 receptor antagonist, and emapalumab, an interferon-γ antibody, are all under investigation in clinical trials for the management of COVID-19–associated hyperinflammation and acute lung injury.24,25 We acknowledge the limitations of drawing treatment conclusions from our single case, but given that severe COVID-19 is uncommon in children and current clinical trials for immunomodulatory drugs are being conducted only in adults, our report serves to inform the pediatric community about the potential use of antiviral agents such as remdesivir and immunomodulatory agents such as tocilizumab in severe pediatric cases.
Conclusions
We report a case of severe pediatric COVID-19 in the United States in an otherwise healthy child presenting with severe thrombocytopenia and ARDS. Consistent with adult literature on COVID-19, our patient’s severe disease course was associated with thrombocytopenia and elevated inflammatory markers. The patient’s severe respiratory disease did not improve on IVIg, steroids, azithromycin, and hydroxychloroquine. We did observe a temporal clinical improvement after the administration of tocilizumab and remdesivir. To the best of our knowledge, there have been no reports to date involving the use of either remdesivir or tocilizumab in pediatric patients with severe COVID-19. Our report contributes to the evolving literature on COVID-19 revealing that although rare, severe COVID-19 does occur in the pediatric age group even in previously healthy children. In addition, with our case, we illustrate that hyperinflammation may be important in the pathophysiology of COVID-19 severe acute respiratory syndrome and that treatment with cytokine-directed agents such as tocilizumab could be considered in critically ill patients. Finally, we advocate for randomized placebo-controlled clinical trials used to study drugs like tocilizumab and remdesivir to include children in addition to adults with COVID-19 given our patient’s presentation and response.
Acknowledgments
Parental consent was obtained before publication of this report. We thank the patient’s family for allowing us to share this case. We also acknowledge the other clinicians and staff of Children’s Healthcare of Atlanta at Scottish Rite for their commitment to this patient’s and others’ care in the setting of this outbreak.
Glossary
- ARDS
acute respiratory distress syndrome
- COVID-19
coronavirus disease 2019
- CRP
C-reactive protein
- CRS
cytokine release syndrome
- HD
hospital day
- IL-6
interleukin-6
- iNO
inhaled nitric oxide
- ITP
immune thrombocytopenia
- IVIg
intravenous immunoglobulin
- PCR
polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Footnotes
Dr Patel conceptualized and designed the study, collected data, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Chandrakasan, Mickells, Yildirim, Kao, and Bennett were involved in analysis and interpretation of data, critically reviewed for important intellectual content, and revised the manuscript; and all authors approved of the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Dr Bennett receives research funding from Novartis and has participated in advisory boards for Novartis and Dova Pharmaceuticals; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Patel is supported by a National Institutes of Health (NIH) training grant (5T32HL139443-02). No other authors have relevant funding disclosures. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report – 93. 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200422-sitrep-93-covid-19.pdf?sfvrsn=35cf80d7_4. Accessed April 23, 2020
- 2.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China [published online ahead of print March 16, 2020]. Pediatrics. doi:10.1542/peds.2020-0702 [Google Scholar]
- 3.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team . SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-J, Ni Z-Y, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zulfiqar A-A, Lorenzo-Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with covid-19. N Engl J Med. 2020;382(18):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [published online ahead of print March 20, 2020]. Int J Antimicrob Agents. doi:10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [published online ahead of print March 9, 2020]. Clin Infect Dis. doi:10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Journal of the American Medical Association Network Learning. Coronavirus in New York - report from the front lines. March 2020. Available at: https://edhub.ama-assn.org/jn-learning/video-player/18331693. Accessed March 25, 2020
- 9.Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia [published correction appears in Blood Adv. 2020;4(2):252]. Blood Adv. 2019;3(23):3829–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang Univ (Med Sci). 2020;49(2):215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19 [published online ahead of print April 10, 2020]. N Engl J Med. doi:10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Specialty Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2016;128(11):1533]. Blood. 2014;124(2):188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published correction appears in Intensive Care Med. doi:10.1007/s00134-020-06028-z]. Intensive Care Med. 2020;46(5):846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun D, Li H, Lu X-X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study [published online ahead of print March 19, 2020]. World J Pediatr. doi:10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka T, Narazaki M, Ogata A, Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26(1):88–96 [DOI] [PubMed] [Google Scholar]
- 19.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michot J-M, Albiges L, Chaput N, et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report [published online ahead of print April 2, 2020]. Ann Oncol. doi:10.1016/j.annonc.2020.03.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience [published online ahead of print April 6, 2020]. J Med Virol. doi:10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov ; US National Library of Medicine. Tocilizumab in COVID-19 pneumonia (TOCIVID-19). Identifier: NCT04317092. 2020. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04317092. Accessed April 8, 2020
- 23.ClinicalTrials.gov ; US National Library of Medicine. A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA). Identifier: NCT04320615. 2020. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04320615. Accessed April 8, 2020
- 24.ClinicalTrials.gov ; US National Library of Medicine. Sarilumab COVID-19. Identifier: NCT04327388. 2020. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04327388. Accessed April 8, 2020
- 25.ClinicalTrials.gov ; US National Library of Medicine. Efficacy and safety of emapalumab and anakinra in reducing hyperinflammation and respiratory distress in patients with COVID-19 infection. Identifier: NCT04324021. 2020. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04324021. Accessed April 8, 2020