Using a population-based approach, this study shows improvement in survival without major morbidity among VLBW infants in California.
Abstract
Video Abstract
OBJECTIVES:
To examine trends in survival without major morbidity and its individual components among very low birth weight infants across California and assess remaining gaps that may be opportune targets for improvement efforts.
METHODS:
The study population included infants born between 2008 and 2017 with birth weights of 401 to 1500 g or a gestational age of 22 to 29 weeks. Risk-adjusted trends of survival without major morbidity and its individual components were analyzed. Survival without major morbidity was defined as the absence of death during birth hospitalization, chronic lung disease, severe peri-intraventricular hemorrhage, nosocomial infection, necrotizing enterocolitis, severe retinopathy of prematurity or related surgery, and cystic periventricular leukomalacia. Variations in adjusted rates and/or interquartile ranges were examined. To assess opportunities for additional improvement, all hospitals were reassigned to perform as if in the top quartile, and recalculation of predicted numbers were used to estimate potential benefit.
RESULTS:
In this cohort of 49 333 infants across 142 hospitals, survival without major morbidity consistently increased from 62.2% to 66.9% from 2008 to 2017. Network variation decreased, with interquartile ranges decreasing from 21.1% to 19.2%. The largest improvements were seen for necrotizing enterocolitis and nosocomial infection. Bronchopulmonary dysplasia rates did not change significantly. Over the final 3 years, if all hospitals performed as well as the top quartile, an additional 621 infants per year would have survived without major morbidity, accounting for an additional 6.6% annual improvement.
CONCLUSIONS:
Although trends are promising, bronchopulmonary dysplasia remains a common and persistent major morbidity, remaining a target for continued quality-improvement efforts.
What’s Known on This Subject:
Quality improvement in NICUs has focused on reducing mortality and morbidity for very low birth weight infants. California hospitals have employed a model of collaborative quality improvement to share data and evidence-based practices.
What This Study Adds:
Over the past decade, survival without major morbidity improved from 62% to 67%. Infants who survived did so with less overall morbidity. Population-based data for quality improvement have driven change and provide further opportunities to optimize care and outcomes.
Very low birth weight (VLBW) infants in the NICU can experience adverse outcomes due to immature respiratory and immune systems, their need for advanced nutrition support, and the frequent need for invasive devices or procedures.1–3 VLBW infants frequently suffer morbidities such as chronic lung disease, nosocomial infections (NIs), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), and even death.4 Such comorbidities can lead to adverse developmental outcomes, prolonged length of stay, increased economic burden, and psychological stress for families.
In addition to biomedical advancements, a focus on collaborative quality improvement has led to population-based improvements in neonatal outcomes.5,6 Participation in collaborative organizations can spur individual institutions to aspire to benchmarks and provide an infrastructure for implementing quality-improvement initiatives.7,8
The California Perinatal Quality Care Collaborative (CPQCC) is a group of NICUs belonging to the Vermont Oxford Network and represents >90% of NICUs in the state. Established in 1997, the CPQCC partners with multiple stakeholders with a common goal of improving maternal and infant outcomes in the state of California.6 Focused projects in the CPQCC have demonstrated improvements in NI, chronic lung disease, and NEC.3,9,10
Although previous studies have examined improvements in individual outcomes over a limited number of years, there has not yet been a broad overview of long-term trends in overall neonatal outcomes in the CPQCC. Our objective was to examine the incidence of survival without major morbidity and its individual components among VLBW infants during 2008 through 2017 with the goal of characterizing long-term trends, quantifying improvement, and providing current estimates of the rates of various outcomes. We also aimed to assess remaining gaps to foster the development of future targets for quality improvement.
Methods
Study Cohort
We analyzed clinical and organizational data from 142 NICUs in the CPQCC (>90% of all California NICUs).6 We included all hospitals that were members of the CPQCC at any time point during the study period because this represented a population-based cohort in which there was a gradual increase in the number of NICUs during this study period with some NICUs closing or changing ownership but still caring for similar populations. All VLBW infants who were cared for in the CPQCC and born between January 1, 2008, and December 31, 2017, were included. Specifically, we included infants who were between 401 and 1500 g or between 22 and 29 weeks’ gestational age at birth (n = 60 272 admissions). We excluded infants who died in the delivery room (n = 3234) and infants with severe congenital anomalies (n = 1262; using mortality-based criteria for severity) for an analytic cohort of 55 776 admissions of 49 333 unique infants. Analyses were performed on the unique infant cohort. This study was approved by the Stanford University Institutional Review Board.
Main Outcomes
The primary outcome of interest was survival without major morbidity, which was defined as the absence of the following: infant death during birth hospitalization (counting transfers among NICUs) or within 1 year of birth after continuous hospitalization (any infants who died after being discharged from the hospital or were readmitted are not included); chronic lung disease, which was defined as receiving any supplemental oxygen at 36 weeks of age; severe IVH, which was defined as a grade 3 or 4 peri-intraventricular hemorrhage on or before day 28; NI, which was defined as a late bacterial, coagulase-negative Staphylococcus, or fungal infection after day 3; NEC as diagnosed at surgery, postmortem examination, or clinically (bilious gastric aspirate or emesis, abdominal distension, or blood in stool) and radiographically (pneumatosis intestinalis, hepatobiliary gas, or pneumoperitoneum); severe ROP or ROP surgery, which was defined as infants receiving an eye examination and whose worst stage or ROP was 3, 4, or 5 or had related surgery; and cystic PVL, which was defined as evidence of PVL on cranial ultrasound, computed tomography, or MRI. The presence or absence of each of these outcomes was evaluated across all admissions for each infant. Secondary outcomes of interest included the incidence of each individual component of survival without major morbidity.
Included Covariates
To examine trends in survival without major morbidity, we used an established risk-adjustment model from the CPQCC. We did not include level of care as categorized by the American Academy of Pediatrics11 in risk adjustment because our goal was to perform a population-based analysis of trends but present the distribution of patients in hospitals by level of care. The risk-adjustment model included infant-level factors such as sex, gestational age, 5-minute Apgar score, being small for gestational age, congenital abnormalities, multiple birth status, race and/or ethnicity, inborn or outborn status, and receipt of prenatal care. These covariates were also included in individual models for each component of survival without major morbidity.
If an infant was ever transferred to another CPQCC NICU during his or her hospital stay, this infant was defined as “outborn” because he or she was considered outborn in at least one NICU. Approximately 80% of these were categorized as “acute” transfers (ie, to receive a higher level of care). Otherwise, if an infant was initially inborn in a CPQCC NICU and never transferred, this infant was defined as “inborn.” The CPQCC race classification scheme for race and/or ethnicity includes non-Hispanic white, non-Hispanic African American, Hispanic (of any race), Asian American and/or Pacific Islander (API), and American Indian or Alaskan native and, for this analysis, combines all other groups into an “other” category. We labeled these groups as white, African American, Hispanic, API, American Indian and/or Alaskan native, and other.
Statistical Analysis
Continuous covariates were examined by using measures of central tendency such as medians and interquartile ranges (IQRs) and were categorized into equal intervals for analyses (Table 1). To calculate adjusted rates of survival without major morbidity and each of its individual component outcomes, we fitted multivariable logistic regression models, adjusting for the covariates included in the established models, as noted above. From these models, we calculated the expected number of cases per year for each outcome. For each year, we then calculated a standardized incidence ratio, which is the ratio of observed to expected cases. Multiplying the standardized incidence ratio by the population mean rate resulted in the adjusted rate. Confidence intervals for the adjusted rate were calculated by using the Poisson exact method. To examine trends, we calculated the absolute and percent changes in adjusted rates from 2008 to 2017. The slope for change in each outcome per year was calculated by regressing the adjusted rates on birth year. We also examined variation in adjusted rates across all hospitals over time by bootstrapping adjusted rates and calculating the IQR for each year then calculating the difference in IQR from 2008 to 2017 with 10 000 iterations. We tested if the mean change in IQR was statistically different from 0 using t tests.
TABLE 1.
Infant Characteristics (2008–2017; n = 49 333)
| Overall | |
|---|---|
| Patient characteristics | |
| Sex, n (%) | |
| Female | 23 713 (48.1) |
| Male | 25 609 (51.9) |
| Missing | 11 (0.02) |
| Gestational age, wk | |
| Median (IQR) | 28 (26–30) |
| <27, n (%) | 14 013 (28.4) |
| 27–29, n (%) | 18 569 (37.6) |
| ≥30, n (%) | 16 747 (33.9) |
| Missing, n (%) | 4 (0.01) |
| Small for gestational age, n (%) | |
| No | 35 349 (71.7) |
| Yes | 13 980 (28.3) |
| Missing | 4 (0.01) |
| Birth wt, g | |
| Median (IQR) | 1119 (840–1340) |
| <401, n (%) | 148 (0.3) |
| 401–750, n (%) | 8516 (17.3) |
| 751–1000, n (%) | 10 714 (21.7) |
| 1001–1250, n (%) | 12 364 (25.1) |
| 1251–1500, n (%) | 16 277 (33.0) |
| >1500, n (%) | 1314 (2.7) |
| Birth location, n (%) | |
| Inborn in a CPQCC center and never transferred | 38 564 (78.2) |
| Outborn (≥1 transfer) | 10 769 (21.8) |
| Race and/or ethnicity, n (%) | |
| Non-Hispanic African American | 6466 (13.1) |
| Hispanic | 22 229 (45.1) |
| Non-Hispanic white | 13 031 (26.4) |
| API | 6045 (12.3) |
| American Indian and/or Alaskan native | 154 (0.3) |
| Other | 1159 (2.4) |
| Missing | 249 (0.5) |
| 5-min Apgar score, median (IQR) | 8 (7–9) |
| Hospital level characteristics, n (%) | |
| Level of care | |
| 2 | 26 (18.1) |
| 3 | 89 (62.2) |
| 4 | 25 (17.5) |
| None of the above | 2 (1.4) |
| Urban or rural | |
| Rural | 3 (2.1) |
| Urban | 139 (97.2) |
| Teaching hospital | |
| No | 121 (84.6) |
| Yes | 21 (14.7) |
We examined trends in the numbers of comorbidities over time among infants who survived with at least 1 major morbidity. The slope for change in comorbidities per year was calculated by regressing the number of comorbidities (per 100 infants) on birth year. Lastly, to assess the potential for additional quality improvement, we reestimated the model for each outcome during the most recent 3 years (2015–2017) but included a dummy variable indicating whether each hospital was in the top quartile of performance for each outcome. We then reassigned every hospital to be in the top quartile and recalculated the predicted number of each outcome for every hospital. The predicted cases before reassignment equals the observed number of cases of each outcome, and the difference between the observed cases and predicted cases after reassignment equals the number of cases of survival without major morbidity that would be increased or, for negative individual outcomes, the number of cases that would be prevented if all hospitals performed similar to hospitals in the top quartile. Statistical and graphical analyses were conducted with SAS (version 9.4; SAS Institute, Inc, Cary, NC) and SigmaPlot 11.0 (Systat Software, Inc, San Jose, CA).
Results
Cohort and Hospital Characteristics
Characteristics of 49 333 VLBW infants who were cared for in the CPQCC network between 2008 and 2017 are shown in Table 1. The median gestational age was 28 (IQR 26–30) weeks; 28.3% were small for gestational age, ∼39% were <1000 g at birth, 17.5% were cared for in level 4 NICUs, and 14.7% were cared for in teaching hospitals during their first admission. The cohort included the following distribution: 26.4% non-Hispanic white, 13.1% African American, 45.1% Hispanic, 12.3% API, and 2.7% other. The percentage of the most immature infants, born at 22, 23, and 24 weeks’ gestation, remained stable at an average of 0.6%, 3.5%, and 7.1%, respectively, from 2008 to 2017.
Long-term Trends in Incidence of Survival Without Major Morbidity and Its Components
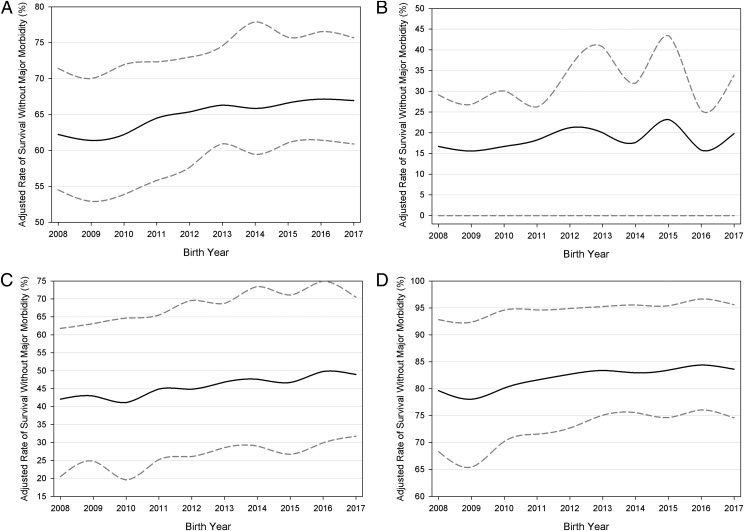
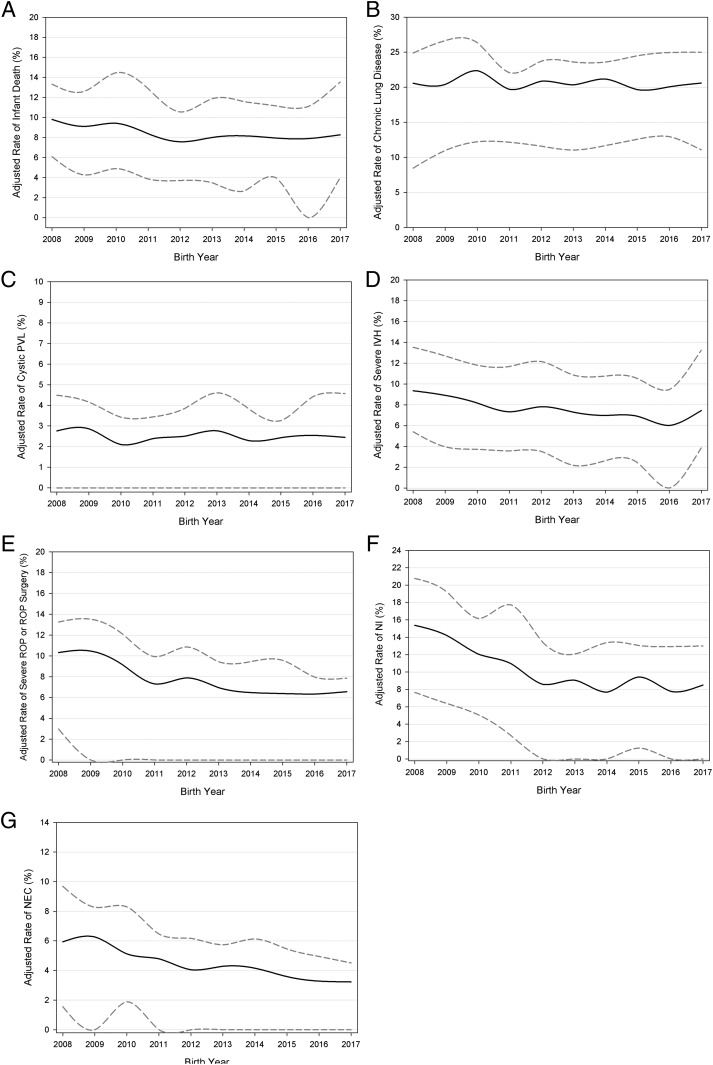
From 2008 to 2017, survival without major morbidity improved significantly among VLBW infants in the CPQCC network (P < .001), increasing from 62.2% to 66.9% for an overall 7.6% change (Fig 1, Table 2). When stratified by gestational age, infants <27 weeks of age contributed most significantly to the overall improvement; we observed the largest change among infants at <25 (18.7%) and 25 to 27 (16.5%) weeks’ gestation, whereas infants >27 weeks of age showed a 5.2% increase (Fig 1). Infant death decreased significantly from 2008 to 2017. Among the individual outcomes that comprise survival without major morbidity, the largest improvements were seen in NEC (45.6% reduction in incidence) and NI (44.7% reduction in incidence). Significant decreases in severe IVH, severe ROP, or ROP surgery were also observed (Fig 2, Table 2). However, rates of chronic lung disease (P = .44) and PVL (P = .46) remained stagnant (Fig 2, Table 2).
FIGURE 1.
Long-term trends in adjusted rates. The lower and upper bands indicate IQR among NICUs. The y-axis scale differs for each figure panel. A, Survival without major morbidity among all infants. B, Survival among infants <25 weeks of age. C, Survival among infants 25 to 27 weeks of age. D, Survival among infants >27 weeks of age.
TABLE 2.
Annual Proportional and Absolute Changes in Adjusted Rates of Survival Without Major Morbidity and Its Components (2008–2017)
| Outcome | Starting Rate (2008)a | Current Rate (2017)a | Change in IQR (2008–2017)b | Absolute Change, % | Percent Change, % | Average Change per y, %c | Change per y, P |
|---|---|---|---|---|---|---|---|
| Survival without major morbidity | 62.2 | 66.9 | −1.84d | 4.7 | 7.6 | 0.7 | <.001 |
| Infant death | 9.8 | 8.3 | −2.03d | −1.5 | −15.7 | −0.2 | .01 |
| Chronic lung disease | 20.6 | 20.6 | 3.04d | 0.02 | 0.1 | −0.07 | .44 |
| Cystic PVL | 2.8 | 2.5 | −2.31d | −0.3 | −11.3 | −0.02 | .46 |
| Severe peri-intraventricular hemorrhage | 9.4 | 7.5 | −2.07d | −1.9 | −20.4 | −0.3 | .002 |
| Severe ROP or ROP surgery | 10.3 | 6.6 | −6.14d | −3.8 | −36.4 | −0.5 | <.001 |
| NI | 15.4 | 8.5 | −8.54d | −6.9 | −44.7 | −0.8 | <.001 |
| NEC | 5.9 | 3.2 | −5.64d | −2.7 | −45.6 | −0.3 | <.001 |
Per 100 infants.
Change in IQR across all hospitals.
Slope for regressing each outcome rate on birth year.
Denotes statistical significance.
FIGURE 2.
Long-term trends in adjusted rates. The lower and upper bands indicate IQR among NICUs. The y-axis scale differs for each figure panel. A, Infant death. B, Chronic lung disease. C, Cystic PVL. D, Severe peri-intraventricular hemorrhage. E, Severe ROP or ROP surgery. F, NI. G, NEC.
We also observed a significant decrease in variation across the network for adjusted hospital rates of survival without major morbidity. The IQR decreased from 21.1% in 2008 to 19.2% by 2017 (P < .001). Variation decreased significantly for adjusted hospital rates of infant death, severe IVH, NI, NEC, severe ROP or ROP surgery, and cystic PVL (P < .001). For chronic lung disease, however, variation in hospital rates increased during the last decade (Table 2).
Long-term Trends in Morbidities
For infants who survived with major morbidity, the number of morbidities decreased over time (Table 3). The percentage of infants who survived with ≥4 morbidities decreased from 1.22% in 2008 to 0.73% in 2017, a 40.2% decrease over the 10-year period (P = .004). Similarly, the percentage of infants who survived with 2 or 3 morbidities decreased significantly over time, with a 18.7% and 40.0% decrease in infants with 2 and 3 morbidities, respectively.
TABLE 3.
Trends in Infant Morbidities Over Time (2008–2017)
| Morbidities | Birth y | Percent Change, %a | Average Change per y, %b | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||||
| 0 | 62.4 | 60.6 | 61.8 | 64.8 | 65.7 | 65.8 | 65.7 | 67.1 | 67.3 | 67.3 | 7.8 | 0.7 | <.001 |
| 1 | 20.7 | 22.2 | 22.5 | 22.1 | 21.7 | 21.0 | 21.9 | 20.9 | 21.1 | 20.1 | −2.9 | −0.1 | .13 |
| 2 | 11.4 | 11.3 | 11.3 | 9.4 | 9.4 | 9.4 | 9.0 | 9.0 | 8.7 | 9.3 | −18.4 | −0.3 | .002 |
| 3 | 4.3 | 4.4 | 3.5 | 2.8 | 2.6 | 3.1 | 2.7 | 2.5 | 2.3 | 2.6 | −40.0 | −0.2 | .002 |
| ≥4 | 1.2 | 1.5 | 1.0 | 0.9 | 0.7 | 0.8 | 0.7 | 0.5 | 0.6 | 0.7 | −41.7 | −0.1 | .004 |
Absolute change from 2008 to 2017.
Slope for average change per y as calculated by linear regression of rates of morbidities on birth year.
Potential for Future Improvement in Outcomes
We assessed the potential for additional improvement if all hospitals had performed as well as the top quartile over most recent years of 2015 through 2017 (Table 4). If all hospitals performed as well as the hospitals in the top quartile, an additional 621 infants would survive without major morbidity, an additional 6.6% improvement per year, on average. Among the individual components, we could expect further substantial reductions in infant death (15.6%), chronic lung disease (24.9%), severe IVH (18.3%), severe ROP or ROP surgery (27.8%), NI (22.1%), and NEC (33.3%) per year.
TABLE 4.
Potential Number of Cases Improved or Prevented if all Hospitals Performed as Well as the Top Quartile of Hospitals (2015–2017)
| Outcomea | No. Observed Cases | Current Rate, per 100 Infants | Potential Expected No. Casesb | Potential Expected Rate, per 100 Infants | Average Cases Improved or Prevented per y | Average Potential Improvement or Decrease in Outcomes per y, % |
|---|---|---|---|---|---|---|
| Survival without major morbidityc | 9449 | 66.9 | 11 311 | 80.5 | 621 | 6.6 |
| Infant death | 1133 | 8.3 | 603 | 4.3 | −177 | −15.6 |
| Chronic lung disease | 2712 | 20.6 | 739 | 5.7 | −727 | −24.9 |
| Severe peri-intraventricular hemorrhage | 886 | 7.5 | 399 | 3.0 | −162 | −18.3 |
| Severe ROP or ROP surgery | 729 | 6.6 | 120 | 1.1 | −203 | −27.8 |
| NI | 1108 | 8.5 | 372 | 2.8 | −245 | −22.1 |
| NEC | 470 | 3.2 | 0 | 0.0 | −157 | −33.3 |
PVL was not included because of the small number of cases.
Expected rates calculated through multivariate logistic regression.
Survival without major morbidity is a positive outcome.
Discussion
We present a population-based analysis of trends in outcomes among VLBW infants in California NICUs over the last decade. A steady, significant improvement in survival without major morbidity was observed along with a significant decrease in all major morbidities except for chronic lung disease and PVL. The most significant improvement was seen in the lowest gestational age groups.
It is promising to note that there has been steady progress in mortality across populations for very preterm birth.12–15 A study of trends and variation in the Vermont Oxford Network, which includes NICUs in the CPQCC, reported a steady trend of improvement for VLBW infants for mortality and across morbidities from 2005 to 2014, with mortality decreasing from 14.0% to 10.9%.16 Although that the methods in that previous study and the current study differ somewhat, we note that the mortality trends are similar between studies, with slightly lower mortality rates being found in California over the overlapping times of study, starting at 10% in 2008 and then ranging generally from 7.5% to 8.5% in recent years despite stable proportions of infants being born at the earliest gestational ages. A study of 7336 infants in the European network called Effective Perinatal Intensive Care in Europe reported a mortality rate of 9.2% during 2011 to 2012.17 The predominantly academic NICUs of the Neonatal Research Network in the United States have shown steady progress for infants born at each gestational age, including those born at 23 weeks.18
As survival improves for those born at the earliest gestational ages, there may be concern about survivors having a higher likelihood of morbidities even as care and attitudes surrounding periviable gestations evolve.19 Nevertheless, similar to other populations, we found significant improvements in survival without major morbidity over this 10-year period.20,21 The composite measure of survival without major morbidity does not take into account whether a neonate has 1, 2, or more morbidities. In California, neonates born more recently were less likely to have any morbidity. Of those having at least 1 morbidity, the likelihood of having 3 or more morbidities decreased significantly (Table 3).
Having a higher number of morbidities has been associated with higher risk of adverse neurodevelopmental outcomes at toddler age and early school age even after risk adjustment.22,23 Although it is important to reduce individual morbidities for the purpose of reducing burden on the health care system and families, perhaps the most important reason to reduce morbidities is to improve long-term cognitive and neurodevelopmental outcomes.
When we examined individual morbidities, we found significant reductions in rates of outcomes such as infection and NEC. The largest declines occurred in these 2 outcomes for which quality-improvement activities have been well studied and implemented successfully, including across the CPQCC.3,10,24,25 The toolkit Nutritional Support of the VLBW Infant is the most downloaded document on the CPQCC Web site and covers best practices surrounding prevention of NEC, such as breast milk provision and feeding protocols.26 Prevention of hospital-acquired infection has been a key quality-improvement goal across hospitals, and the CPQCC’s toolkit has been frequently used alongside statewide dissemination and collaborative activities.3,27–29
Other morbidities, such as cystic PVL, did not improve significantly over this time period. Areas such as NEC and hospital-acquired infection have been more amenable to quality-improvement work, with clear linkages of practices and processes to outcomes observed. This is less possible for other morbidities related to preterm birth. These considerations have informed what may be most relevant for quality assessment. For a regional quality-improvement network such as the CPQCC, the areas that are most amenable to improvement are high-value targets. In previous work, through a Delphi process, experts chose the following 9 measures to comprise a composite measurement of quality: antenatal steroid administration, moderate hypothermia on admission, pneumothorax, health care–associated infection, chronic lung disease, timely eye examination for ROP screening, discharge on human milk, mortality, and growth velocity.30,31 These measures may be considered most amenable to quality improvement.
Rates of chronic lung disease did not decrease, and adjusted rates increased slightly. Other groups have reported a similar lack of improvement for this outcome.14,15,21 There are some indications that quality improvement that focuses on respiratory management, including during the first moments after birth, could have an impact on chronic lung disease.32 Variation across centers also indicates that management strategies in the NICU are likely to influence rates.33 Ultimately, chronic lung disease remains a significant challenge, with implications for long-term financial and health considerations for families and society seen.34 Advances in both translational research and quality improvement are likely needed to move the needle for this condition.
In considering what types of practice changes and actions may have led to improvements in outcomes, we propose that the partnership of biomedical science and quality improvement has been a key contributor to the advancement in neonatology. Although the benefits of antenatal steroids were discovered in the 1970s, collaborative quality-improvement activities in California have furthered dissemination steadily over the past 20 years.35–37 We believe that the observed improvement in survival without major morbidity is a result of multifactorial contributions, including continuous advancements in care, real-time data measurement, and active collaborative quality-improvement efforts. The growth of CPQCC, the Vermont Oxford Network, and other state perinatal organizations has also allowed for increased learning and sharing of best practices.5,38 In this context, we recommend ongoing emphases on both biomedical research and quality improvement for neuroprotection and chronic lung disease.
In considering the variation of outcomes across hospitals, we calculated the potential for additional improvement if all hospitals performed as well as the hospitals in the top quartile, recognizing that opportunities for further improvement exist (Table 4). Although quality improvement has traditionally been a voluntary activity for clinicians and hospitals, future efforts could particularly target these lower-performing hospitals with resources and guidance to close these gaps. In the CPQCC, the implementation of Baby-MONITOR (a composite measure of quality) will facilitate real-time measures of each hospital’s quality of care, directing attention to the areas that are most in need of attention.31 Furthermore, our recently implemented disparity dashboard can reveal opportunities to improve equity in care within hospitals where disparities in processes and outcomes exist.
This study should be viewed in the context of its design. We were not able to investigate factors such as detailed care policies of individual hospitals, safety culture, provider burnout, or the availability of family-centered care, all of which can play an important role in influencing quality of care in the NICU and therefore affect infant outcomes. It is our goal to advance in the assessment of such factors, which may explain some of the variation in processes and outcomes that still remains across what may be considered similar NICUs.
Conclusions
Outcomes for VLBW infants have improved dramatically, even in the past decade. However, challenges remain for morbidities such as chronic lung disease. In addition, despite significant overall improvement and decreasing variation in the CPQCC network overall, significant variation in rates of survival without major morbidity continues across hospitals. Strategies using data to shine a light on opportunities for improvement will facilitate further gains in neonatal care.
Glossary
- API
Asian American and/or Pacific Islander
- CPQCC
California Perinatal Quality Care Collaborative
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NI
nosocomial infection
- PVL
periventricular leukomalacia
- ROP
retinopathy of prematurity
- VLBW
very low birth weight
Footnotes
Dr Lee had full access to all of the data in this study, takes responsibility for the integrity of the data and the accuracy of the analyses, acquired funding for this study, conceptualized and designed the study, selected data for inclusion in analyses, analyzed the data, interpreted the results, and helped draft initial manuscript; Dr Liu selected data for inclusion in analyses, executed the analysis, and helped interpret the results and draft the initial manuscript; Drs Profit, Hintz, and Gould helped interpret the results; and all authors revised the manuscript and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Sponsored in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R01 HD087425). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2020-0436.
References
- 1.Payne NR, Finkelstein MJ, Liu M, Kaempf JW, Sharek PJ, Olsen S. NICU practices and outcomes associated with 9 years of quality improvement collaboratives. Pediatrics. 2010;125(3):437–446 [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27(4):293–301 [DOI] [PubMed] [Google Scholar]
- 3.Wirtschafter DD, Powers RJ, Pettit JS, et al. Nosocomial infection reduction in VLBW infants with a statewide quality-improvement model. Pediatrics. 2011;127(3):419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019–1026 [DOI] [PubMed] [Google Scholar]
- 5.Horbar JD, Rogowski J, Plsek PE, et al. ; NIC/Q Project Investigators of the Vermont Oxford Network . Collaborative quality improvement for neonatal intensive care. Pediatrics. 2001;107(1):14–22 [DOI] [PubMed] [Google Scholar]
- 6.Gould JB. The role of regional collaboratives: the California Perinatal Quality Care Collaborative model. Clin Perinatol. 2010;37(1):71–86 [DOI] [PubMed] [Google Scholar]
- 7.Shah V, Warre R, Lee SK. Quality improvement initiatives in neonatal intensive care unit networks: achievements and challenges. Acad Pediatr. 2013;13(suppl 6):S75–S83 [DOI] [PubMed] [Google Scholar]
- 8.Profit J, Soll RF. Neonatal networks: clinical research and quality improvement. Semin Fetal Neonatal Med. 2015;20(6):410–415 [DOI] [PubMed] [Google Scholar]
- 9.Lapcharoensap W, Bennett MV, Powers RJ, et al. Effects of delivery room quality improvement on premature infant outcomes. J Perinatol. 2017;37(4):349–354 [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Kurtin PS, Wight NE, et al. A quality improvement project to increase breast milk use in very low birth weight infants. Pediatrics. 2012;130(6). Available at: www.pediatrics.org/cgi/content/full/130/6/e1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Committee on Fetus and Newborn Levels of neonatal care. Pediatrics. 2012;130(3):587–597 [DOI] [PubMed] [Google Scholar]
- 12.Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004–2007 and 2014–2016. JAMA. 2019;321(12):1188–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancel PY, Goffinet F, Kuhn P, et al. ; EPIPAGE-2 Writing Group . Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study [published correction appears in JAMA. 2015;169(4):323]. JAMA Pediatr. 2015;169(3):230–238 [DOI] [PubMed] [Google Scholar]
- 14.Shah PS, Sankaran K, Aziz K, et al. ; Canadian Neonatal Network . Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol. 2012;32(2):132–138 [DOI] [PubMed] [Google Scholar]
- 15.Kusuda S, Fujimura M, Uchiyama A, Totsu S, Matsunami K; Neonatal Research Network, Japan . Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr Res. 2012;72(5):531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horbar JD, Edwards EM, Greenberg LT, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 2017;171(3):e164396. [DOI] [PubMed] [Google Scholar]
- 17.Zeitlin J, Manktelow BN, Piedvache A, et al. ; EPICE Research Group . Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ. 2016;354:i2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314(10):1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raju TN, Mercer BM, Burchfield DJ, Joseph GF Jr.. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American college of obstetricians and gynecologists. Obstet Gynecol. 2014;123(5):1083–1096 [DOI] [PubMed] [Google Scholar]
- 20.Rüegger C, Hegglin M, Adams M, Bucher HU; Swiss Neonatal Network . Population based trends in mortality, morbidity and treatment for very preterm- and very low birth weight infants over 12 years. BMC Pediatr. 2012;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grisaru-Granovsky S, Reichman B, Lerner-Geva L, et al. ; Israel Neonatal Network . Population-based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum Dev. 2014;90(12):821–827 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF; Trial of Indomethacin Prophylaxis in Preterms (TIPP) Investigators . Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289(9):1124–1129 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt B, Roberts RS, Davis PG, et al. ; Caffeine for Apnea of Prematurity (CAP) Trial Investigators; Caffeine for Apnea of Prematurity CAP Trial Investigators . Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167(5):982–986.e2 [DOI] [PubMed] [Google Scholar]
- 24.Greenberg RG, Cochran KM, Smith PB, et al. Effect of catheter dwell time on risk of central line-associated bloodstream infection in infants. Pediatrics. 2015;136(6):1080–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantorowska A, Wei JC, Cohen RS, Lawrence RA, Gould JB, Lee HC. Impact of donor milk availability on breast milk use and necrotizing enterocolitis rates. Pediatrics. 2016;137(3):e20153123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wight N, Kim J, Rhine W, et al. Nutritional support of the VLBW infant. 2018. Available at: https://www.cpqcc.org/resources/nutritional-support-vlbw-infant. Accessed April 21, 2020
- 27.Bowles S, Pettit J, Mickas N, Nisbet C, Proctor T, Wirtschafter D. Neonatal hospital-acquired infection prevention. 2007. Available at: https://www.cpqcc.org/content/neonatal-hospital-acquired-infection-prevention. Accessed April 21, 2020
- 28.Wirtschafter DD, Pettit J, Kurtin P, et al. A statewide quality improvement collaborative to reduce neonatal central line-associated blood stream infections. J Perinatol. 2010;30(3):170–181 [DOI] [PubMed] [Google Scholar]
- 29.Powers RJ, Wirtschafter DW. Decreasing central line associated bloodstream infection in neonatal intensive care. Clin Perinatol. 2010;37(1):247–272 [DOI] [PubMed] [Google Scholar]
- 30.Profit J, Gould JB, Zupancic JA, et al. Formal selection of measures for a composite index of NICU quality of care: Baby-MONITOR. J Perinatol. 2011;31(11):702–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Profit J, Kowalkowski MA, Zupancic JA, et al. Baby-MONITOR: a composite indicator of NICU quality. Pediatrics. 2014;134(1):74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HC, Powers RJ, Bennett MV, et al. Implementation methods for delivery room management: a quality improvement comparison study. Pediatrics. 2014;134(5). Available at: www.pediatrics.org/cgi/content/full/134/5/e1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapcharoensap W, Gage SC, Kan P, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015;169(2):e143676. [DOI] [PubMed] [Google Scholar]
- 34.Lapcharoensap W, Lee HC, Nyberg A, Dukhovny D. Health care and societal costs of bronchopulmonary dysplasia. NeoReviews. 2018;19(4):e211–e223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirtschafter DD, Danielsen BH, Main EK, et al. ; California Perinatal Quality Care Collaborative . Promoting antenatal steroid use for fetal maturation: results from the California Perinatal Quality Care Collaborative. J Pediatr. 2006;148(5):606–612 [DOI] [PubMed] [Google Scholar]
- 36.Profit J, Goldstein BA, Tamaresis J, Kan P, Lee HC. Regional variation in antenatal corticosteroid use: a network-level quality improvement study. Pediatrics. 2015;135(2). Available at: www.pediatrics.org/cgi/content/full/135/2/e397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HC, Lyndon A, Blumenfeld YJ, Dudley RA, Gould JB. Antenatal steroid administration for premature neonates in California. Obstet Gynecol. 2011;117(3):603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pai VV, Lee HC, Profit J; Improving Uptake of Key Perinatal Interventions Using Statewide Quality Collaboratives . Improving uptake of key perinatal interventions using statewide quality collaboratives. Clin Perinatol. 2018;45(2):165–180 [DOI] [PubMed] [Google Scholar]