Abstract
Context
Many female survivors of adolescent and young adult cancers (AYA survivors) have shortened reproductive lifespans. However, the timing and duration of ovarian function after cancer treatment are largely unknown.
Objective
To model the trajectory of ovarian function over two decades following cancer treatment and evaluate how trajectories vary by treatment gonadotoxicity and age.
Design
In a prospective cohort, AYA survivors aged 18-39 at variable times since cancer treatment completion provided dried blood spots (DBS) every 6 months for up to 18 months. Anti-Müllerian hormone (AMH) levels were measured using the Ansh DBS AMH enzyme-linked immunosorbent assay. The mean AMH trajectory was modeled for the entire cohort and separately by treatment gonadotoxicity and age using functional principal components analysis.
Results
763 participants, mean (standard deviation) enrollment age 33.3 (4.7) and age at cancer diagnosis 25.9 (5.7) years, contributed 1905 DBS samples. The most common cancers were breast (26.9%), lymphoma (24.8%), and thyroid (18.0%). AMH trajectories differed among survivors by treatment gonadotoxicity (low, moderate, or high) (P < 0.001). Following low or moderately gonadotoxic treatments, AMH levels increased over 2-3 years and plateaued over 10-15 years before declining. In contrast, following highly gonadotoxic treatment, AMH levels were lower overall and declined shortly after peak at 2-3 years. Younger age at treatment was associated with higher trajectories, but a protective effect of younger age was not observed in survivors exposed to highly gonadotoxic treatments (Pinteraction < 0.001).
Conclusions
In this large AYA survivor cohort, timing and duration of ovarian function strongly depended on treatment gonadotoxicity and age at treatment. The findings provide novel, more precise information to guide reproductive decision-making.
Keywords: AMH, ovarian reserve, reproductive lifespan, adolescent and young adult cancer, functional principal components analysis
Nearly 70 000 adolescents and young adults (AYA) aged 15 to 35 are diagnosed with cancer each year in the United States, a 6-fold higher incidence than in children (1). The most common AYA cancer types include breast, leukemia, lymphoma, gynecologic, gastrointestinal, sarcoma, skin, and thyroid (2). AYA cancer survivors exhibit higher long-term survival compared with older adult cancer survivors, and survival rates in female AYA survivors are higher than in males (3). Hence, there are almost 400 000 female AYA survivors younger than aged 40, many of whom experience shortened reproductive lifespans, infertility, and/or primary ovarian insufficiency due to gonadotoxic cancer treatments (1). The window of ovarian function remaining after cancer treatment is largely unknown but clinically important for fertility and contraception decision-making, as well as to meet the informational needs voiced by AYA survivors (4-7).
Anti-Müllerian hormone (AMH) is a well-characterized biomarker of ovarian aging in women without cancer (8-13). Levels of AMH, a glycoprotein expressed by granulosa cells of small growing ovarian follicles, peak at puberty, plateau from puberty to age 25 and decline thereafter to undetectable levels in women in their 40s to 50s (14,15). In childhood and AYA cancer survivors, small cross-sectional and prospective cohort studies show time- and cancer treatment-dependent changes in AMH. Levels initially fall with ovarian injury from gonadotoxic treatments and subsequently rise with ovarian function recovery for some patients in the first 5 years after treatment (16-18). AMH levels are also lower with increasing gonadotoxicity of cancer treatments (19-21). Studies of ovarian function in cancer survivors have been limited by small sample size or short duration of follow-up. As a result, the long-term trajectory of ovarian function over reproductive years, and how it may differ by cancer treatments, is largely unknown (22,23).
An efficient study design is needed to capture the span of reproductive years after cancer treatment, because prospective cohort studies following a large cohort of AYA survivors from diagnosis between aged 15-35 until the later reproductive years is cost prohibitive, has limited feasibility, and has considerable time lag before yielding results. The cross-sequential study design is an innovative approach to estimate the window of ovarian function after cancer treatment (24-26). The design is a hybrid between a prospective cohort study and a cross-sectional study. This approach pairs (i) prospectively collecting serial AMH levels from each AYA survivor to generate individual trajectories and account for intra-individual variation with (ii) analyzing the pool of trajectories at variable times since cancer treatment to generate an overall trajectory of ovarian function over 2 decades following cancer treatment.
The objective of this study was to estimate the long-term trajectory of AMH following cancer treatments in female AYA cancer survivors. We hypothesized that the overall trajectory for the sample of AYA survivors would rise following the end of primary cancer treatment, plateau, and then fall over time. We further hypothesized that the trajectory would vary by gonadotoxicity of cancer treatments and age at cancer treatment.
Methods
The Reproductive Window Study is a cross-sequential study of the ovarian function of female AYA survivors. Eligibility criteria included females with cancer diagnoses between ages 15-35, aged 18-40 at study enrollment, completion of primary cancer treatment, presence of at least one ovary, and no uncontrolled endocrinopathies (eg, thyroid and adrenal disease). AYA survivors could enter the study from 1 day to 25 years posttreatment. The included cancer types were selected by highest incidence in this population: breast, leukemia, lymphoma, gynecologic (cervix, uterus, ovary), gastrointestinal (intestines, gall bladder, pancreas), sarcomas, skin, and thyroid. AYA survivors were recruited from the California and Texas Cancer Registries (36.0%), University of California, San Diego Health System (29.6%), cancer advocacy organizations (10.8%), physician referrals (3.9%), and other sources (19.7%). The State of California Committee for the Protection of Human Subjects and the Institutional Review Boards at the University of California, San Diego, and the Texas Department of State Health Services approved this study.
Individuals in the cancer registries or UC San Diego Health System who met age and cancer diagnosis criteria were mailed recruitment letters with directions to the online study portal. All other potential participants were also directed to the online study portal via telephone calls or emails. On the secure, Web-based study portal, potential participants registered, answered screening questions, reviewed study requirements, and completed informed consent documents in order to enroll. Followed for up to 18 months, enrolled participants were asked to complete an online questionnaire through the study portal and self-collect dried blood spots (DBS) every 6 months. Study questionnaires collected self-reported information on cancer, reproductive (fertility, contraception, menstrual pattern), medical, demographic, and lifestyle characteristics using questions derived from large cancer and reproductive cohort studies (9,27).
Participants provided consent for HIPAA and medical record release for study staff to obtain primary cancer treatment records. Cancer diagnosis and treatment data were abstracted from primary medical records by 2 board-certified pediatric oncologists and 1 board-certified reproductive endocrinologist using the Childhood Cancer Survivor Study methods and case report forms with high agreement on re-review of 25% of abstracted data (28).
DBS collections were timed to the early follicular phase (cycle days 3-7) for menstruating individuals, on day 7 of the hormone-free interval in individuals on combined hormonal contraception, and on a random day for amenorrheic individuals or those on menopausal hormone therapy. Participants were instructed to puncture their finger pad and apply up to 5 drops of whole blood to the blood spot filter paper, following written and picture instructions. Telephone or video calls with study staff were deployed during each participant’s first DBS collection for quality control and repeated with subsequent collections as needed. Participants were then instructed to allow samples to dry at room temperature for at least 4 h prior to placement in a gas impermeable plastic bag with desiccant and shipment back to UC San Diego via 2-day mail. Once received, DBS samples were inspected for quality and frozen at −80C.
AMH analysis
DBS were assayed for AMH levels (limit of detection [LOD] 0.03 ng/mL, LOQCV<20% 0.06 ng/mL, inter-assay and intra-assay coefficient of variation [CV] < 10%) using an enzyme-linked immunosorbent assay designed specifically for the measurement of AMH in human DBS specimens (Product AL0.129, AnshLabs, Webster, TX, US). DBS AMH concentrations are traceable to the manufacturer’s recombinant human AMH standard and are corrected for the DBS dilution factor so that values assigned are relative to the subject’s serum levels. The dilution recovery of DBS specimens containing 0.319 to 11.967 ng/mL was 92% to 113%. Using 29 matched serum and DBS samples ranging from 0.745 to 16.326 ng/mL AMH in serum, DBS AMH levels measured in these samples have been compared to serum levels measured with the Ansh picoAMH enzyme-linked immunosorbent assay (Product #AL0.124). Passing Bablok analysis of the results yielded the following Regression: DBS AMH ng/mL = −4.404 + 0.065 Serum AMH ng/mL (r = 0.96; P < 0.001).
Statistical analysis
AMH samples were rightly skewed and thus were natural log transformed to meet assumptions for statistical modeling. The assay lower LOD for AMH was 0.03 ng/mL. Values below the LOD were imputed using randomly generated values from a uniform distribution ranging from half the LOD to the LOD (0.015-0.03). AMH levels of >12.32 ng/mL were above the linear portion of the standard curve and replaced with the value 12.32 ng/mL. In total 7.1% were below the LOD and 0.6% were >12.32 ng/mL. For the primary analysis, all AMH samples were used. In sensitivity analysis, AMH samples were restricted to participants who were not on hormonal contraception, GnRH agonist, or tamoxifen (29). A second model estimated AMH trajectories after removing participants who contributed only one sample. A third model excluded participants aged 18 to 25 at DBS sample in order to estimate AMH trajectories without the impact of age-related increases in AMH between aged 15 to 25 (14).
Participants were classified by exposure to high, moderate, and low gonadotoxicity treatments based on existing literature (30-33). High gonadotoxicity treatments included any exposure to pelvic radiation, stem cell or bone marrow transplants (autologous or allogeneic), or cyclophosphamide equivalent dose (CED) of ≥ 7 grams/m2. Low gonadotoxicity treatments included surgery only (excluding hysterectomy and/or oophorectomy), endocrine therapy only, radioiodine treatment, cervical trachelectomy. All remaining treatment exposures (e.g., CED < 7 grams/m2), any alkylating chemotherapy exposure, other chemotherapy, and targeted therapy, hysterectomy, and unilateral oophorectomy were grouped as moderate gonadotoxicity.
Functional principal components analysis (FPCA) was used to model the mean trajectory of AMH following cancer treatment. FPCA is an appropriate statistical approach to estimate curvilinear functions with sparse, irregularly spaced observations. Each participant contributed up to 4 repeated measures of AMH over 18 months that generated small individual (usually nonlinear) trajectories over a short time interval. Across participants, the study captured AMH data over 0 to 25 years since cancer treatment, but data at any one time may be sparse. The FPCA method we adopted is ideally suited to combine this information across larger windows of time. This approach estimates functional principal components (FPC), which serve as predictors for modeling trajectories (34,35). We denote Xi(t) as the AMH trajectory for the ith participant where i = 1,2, . . . N, and t as years since cancer treatment, Xi(t) is expressed as
where μ(t) is the mean AMH trajectory, ϕ k(t) is the kth FPC, and ξ ik is the associated ith participant’s kth FPC score. We implemented Peng and Paul’s method to estimate model components μ(t),ϕ k(t),and ξ ik and to select an optimal number of FPCs (K) based on approximate leave-one-curve-out cross-validation scores (36). The FPC scores are projections of AMH trajectories onto the lower-dimensional space spanned by the FPCs. These scores can then be used to compare AMH trajectories across clinical or demographic subgroups using standard statistical methods. Graphical representations of mean curves were truncated when fewer than 10 remaining participants contributed to the data for a given group.
Differences in trajectories between gonadotoxicity and/or age at cancer treatment groups were tested by fitting analysis of variance models using estimated FPC scores for each FPC. The fpca package from the statistical programming language R (ver. 3.6.1) was used for our FPCA analysis (37,38). A 2-sided significance level of 0.05 was used for testing hypotheses.
Results
Between March, 2015 and September, 2018, 1150 eligible participants were consented, of which 829 (72%) provided consent for HIPAA and medical record release. Among these 829 participants, there was at least one usable DBS sample in 763 (92%). Baseline characteristics of this cohort of 763 participants are shown in Table 1. Mean attained age (standard deviation) was 33.3 (4.7) years, mean age at cancer diagnosis was 25.9 (5.7) years; 73.8% were white, 20.2% were Hispanic, and the most common cancer types were breast (26.9%), lymphoma (24.8%), and thyroid (18.0%). The cohort contributed 1905 AMH samples (239 four time points, 161 three time points, 103 two time points, 260 one time point) at a median 7.3 (interquartile ratio 4.8-9.9) years posttreatment (606 samples from 0 < 5 years, 859 from 5 < 10 years, 252 from 10 < 15 years, 134 from 15<20 years, and 54 from 20-26 years).
Table 1.
Participant baseline characteristics by gonadotoxicity group
| Characteristic | Overall N (%) (N = 763) | Low N (%) (N = 209) | Moderate N (%) (N = 442) | High N (%) (N = 95) | P-Value |
|---|---|---|---|---|---|
| Mean age (SD) | 33.3 (4.7) | 33.8 (4.8) | 33.4 (4.6) | 31.9 (4.7) | 0.003 |
| Age at enrollment (year) 18-24 25-30 31-35 ≥ 36 | 45 (5.9) 179 (23.5) 287 (37.6) 251 (32.9) | 11 (5.3) 49 (23.4) 67 (32.1) 82 (39.2) | 24 (5.4) 95 (21.5) 180 (40.7) 142 (32.1) | 8 (8.4) 30 (31.6) 36 (37.9) 21 (22.1) | 0.04 |
| Race White Black Asian/Pacific Islander Other | 563 (73.8) 24 (3.1) 56 (7.3) 120 (15.7) | 149 (71.3) 2 (1.0) 18 (8.6) 40 (19.1) | 338 (76.5) 19 (4.3) 24 (5.4) 61 (13.8) | 65 (68.4) 3 (3.2) 13 (13.7) 14 (14.7) | 0.01 |
| Hispanic ethnicity | 154 (20.2) | 45 (21.5) | 88 (19.9) | 17 (17.9) | 0.73 |
| Current smoking | 33 (4.3) | 11 (5.3) | 18 (4.1) | 3 (3.2) | 0.92 |
| Body mass index (kg/m2) <18.5 18.5-24.9 25-29.9 ≥30 | 23 (3.0) 349 (45.7) 175 (22.9) 196 (25.7) | 8 (3.8) 95 (45.5) 41 (19.6) 59 (28.2) | 10 (2.3) 201 (45.5) 115 (26.0) 106 (24.0) | 4 (4.2) 48 (50.5) 17 (17.9) 22 (23.2) | 0.27 |
| Partnered relationship status | 525 (68.8) | 139 (66.5) | 322 (72.9) | 54 (56.8) | 0.006 |
| Education Did not complete college College graduate | 170 (22.3) 593 (77.7) | 38 (18.2) 171 (81.8) | 101 (22.9) 341 (77.1) | 21 (22.1) 74 (77.9) | 0.39 |
| Income < $51 000 ≥ $51 000 | 183 (24.0) 580 (76.0) | 46 (22.0) 163 (78.0) | 101 (22.9) 341 (77.1) | 31 (32.6) 64 (67.4) | 0.1 |
| Cancer characteristics | |||||
| Mean age at dx (SD) | 25.9 (5.7) | 26.1 (5.5) | 26.4 (5.7) | 23.9 (5.4) | <0.001 |
| Age at cancer dx (years) 15-24 25-30 ≥ 31 | 325 (42.6) 259 (33.9) 177 (23.2) | 91 (43.5) 68 (32.5) 50 (23.9) | 165 (37.3) 162 (36.7) 114 (25.8) | 58 (61.1) 26 (27.4) 11 (11.6) | < 0.001 |
| Mean years since dx (SD) | 7.4 (4.8) | 7.7 (4.2) | 7.0 (4.9) | 7.9 (5.2) | 0.1 |
| Years since diagnosis 0-2 3-4 5-8 ≥ 9 | 122 (16.0) 145 (19.0) 293 (38.4) 203 (26.6) | 26 (12.4) 24 (11.5) 98 (46.9) 61 (29.2) | 80 (18.1) 105 (23.8) 150 (33.9) 107 (24.2) | 15 (15.8) 15 (15.8) 37 (38.9) 28 (29.5) | <0.001 |
| Cancer type Blood/leukemia Bone/sarcoma Breast Gynecologic Gastrointestinal Lymphoma Skin Thyroid | 63 (8.3) 50 (6.6) 205 (26.9) 72 (9.4) 23 (3.0) 189 (24.8) 24 (3.1) 137 (18.0) | 0 (0.0) 12 (5.7) 26 (12.4) 10 (4.8) 7 (3.3) 0 (0.0) 22 (10.5) 132 (63.2) | 35 (7.9) 18 (4.1) 178 (40.3) 45 (10.2) 9 (2.0) 151 (34.2) 2 (0.5) 4 (0.9) | 26 (27.4) 20 (21.1) 0 (0.0) 10 (10.5) 6 (6.3) 32 (33.7) 0 (0.0) 1 (1.1) | <0.001 |
| Surgery | 519 (68.0) | 202 (96.7) | 276 (62.4) | 30 (31.6) | < 0.001 |
| Radiation | 339 (44.4) | 92 (44.0) | 190 (43.0) | 52 (54.7) | 0.11 |
| Chemotherapy | 514 (67.4) | 3 (1.4) | 408 (92.3) | 92 (96.8) | < 0.001 |
| Biologic therapy | 33 (4.3) | 2 (1.0) | 28 (6.3) | 3 (3.2) | 0.003 |
| Bone marrow or stem cell transplant | 31 (4.1) | 1 (0.5) | 1 (0.2) | 28 (29.5) | < 0.001 |
| Endocrine therapy (tamoxifen, GnRH agonist) | 130 (17.0) | 15 (7.2) | 113 (25.6) | 1 (1.1) | < 0.001 |
| Cancer Recurrence | 58 (7.6) | 20 (9.6) | 8 (1.8) | 28 (29.5) | < 0.001 |
| Reproductive characteristics | |||||
| Hysterectomy | 17 (2.2) | 4 (1.9) | 10 (2.3) | 2 (2.1) | 1.0 |
| Unilateral oophorectomy | 60 (7.9) | 5 (2.4) | 49 (11.1) | 3 (3.2) | < 0.001 |
| Ever been pregnant | 356 (46.7) | 95 (45.5) | 224 (50.7) | 28 (29.5) | < 0.001 |
| Prior live birth | 294 (38.5) | 85 (40.7) | 180 (40.7) | 23 (24.2) | 0.003 |
| Menses past yeara 0 1-3 4-9 10-12 | 63 (12.7) 34 (6.9) 64 (12.9) 334 (67.5) | 8 (5.5) 9 (6.2) 6 (4.1) 122 (84.1) | 29 (10.2) 19 (6.7) 49 (17.3) 187 (65.8) | 24 (45.3) 4 (7.5) 6 (11.3) 19 (35.8) | < 0.001 |
| Menses occurring more than 60 days apartb | 80 (18.5) | 16 (11.7) | 46 (18.0) | 14 (48.3) | < 0.001 |
aExcludes participants on hormonal contraception, GnRH agonist, tamoxifen, menopausal hormone therapy (n = 495)
bExcludes participants on hormonal contraception, GnRH agonist, tamoxifen, menopausal hormone therapy and amenorrheic in prior year (n = 432).
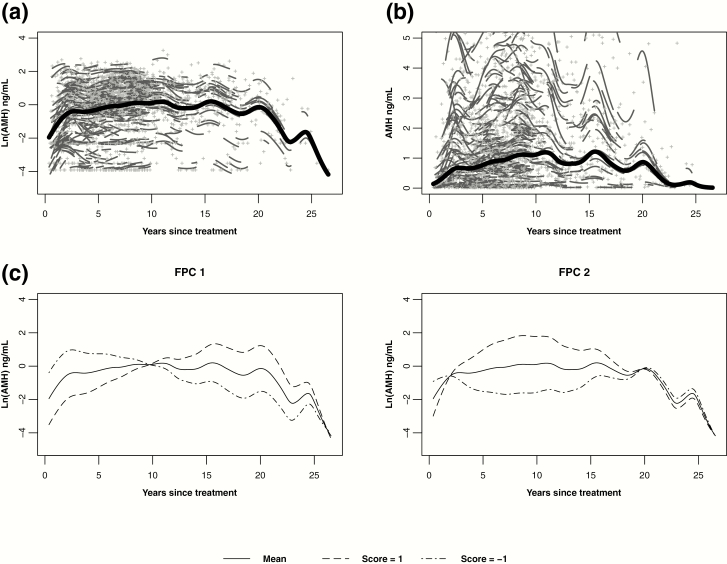
For the entire cohort, the predicted mean AMH trajectory after cancer diagnosis began at a low AMH level (geometric mean 0.14 ng/mL, 95% confidence interval 0.07-0.28). This was followed by an initial rise over 2 to 3 years, a prolonged plateau until approximately 15 years posttreatment, and a final decline (Fig. 1a and b). This trajectory was optimally described by two FPCs, with FPC 1 accounting for the majority of the total variation in AMH levels (62%) over time. High FPC 1 scores corresponded to lower AMH levels in the first years posttreatment and then slowly rising over time (Fig. 1c). FPC 2 accounted for 38% of the variation in AMH levels, and high scores corresponded to a steeper rise in AMH over the first 10 years posttreatment, followed by a decline (Fig. 1c). Combinations of FPC 1 and FPC 2 scores specific to each participant translated to the predicted individual AMH trajectories in Fig. 1.
Figure 1.
Predicted mean log-transformed (A) and untransformed (B) AMH trajectories over years since cancer treatment (bold line), individual log-transformed AMH levels (+), and individual trajectories (short lines related to +). (C) Predicted mean log-transformed AMH trajectory and hypothetical trajectories with FPC scores of 1 and −1. Participants with these positive and negative scores, respectively, have differing additive effects on the mean. FPC 1 contributed nearly 62% of the total variation in AMH levels over time, while FPC 2 accounted for approximately 38% of the variation in AMH.
Seven hundred forty-six participants (98%) had available primary cancer treatment records that allowed for gonadotoxicity group assignment. By cancer treatment gonadotoxicity groups, 209 (28%), 442 (59%), and 95 (13%) of participants were exposed to low, moderate, and high gonadotoxicity treatments, respectively (Table 1). Accordingly, participants’ cancer type and treatments were significantly different by group. Participants in the high gonadotoxicity group tended to be younger at diagnosis and had longer time since diagnosis, compared to those in the low or moderate groups. On demographic characteristics, study enrollment, age, and partner status were significantly different; participants in the high gonadotoxicity treatment group tended to be younger and unpartnered. On reproductive characteristics, prior pregnancy, prior live birth, amenorrhea and oligomenorrhea, were also different between groups; participants in the high gonadotoxicity group tended not to have pregnancies or live births and were more likely to have oligomenorrhea or amenorrhea.
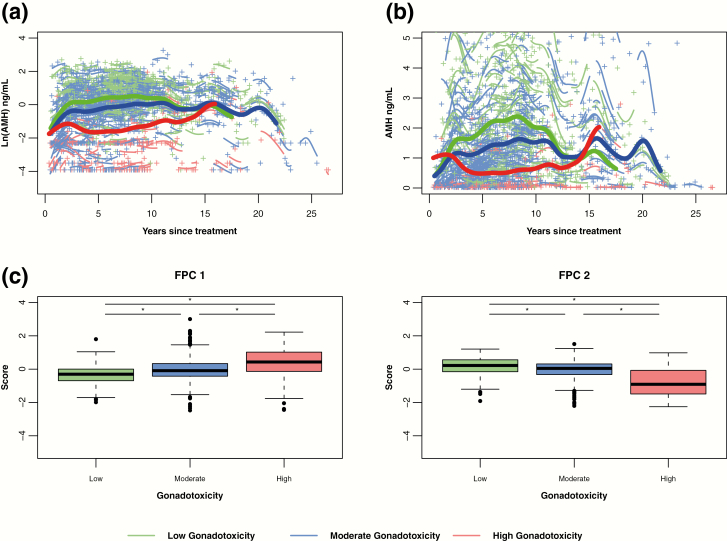
Mean AMH trajectories were significantly different by gonadotoxicity group, based on tests of FPC 1 and FPC 2 (P < 0.001 overall and pairwise) (Fig. 2a and b). The trajectories of all 3 groups rose initially. The trajectory for high gonadotoxicity (red curve) had a noticeably steeper decline after its initial rise in the first 2 to 3 years since cancer treatment, compared to the trajectories of the moderate (blue curve) and low (green curve) gonadotoxicity groups. In the first 10 to 15 years following cancer treatment, increasing toxicity was associated with consistently lower AMH levels. Reconvergence of the mean AMH curves occurred around 15 years, with relative paucity of high gonadotoxicity group data after 10 to 15 years. Sensitivity analysis using enrollment age as the time variable, instead of years since treatment, yielded similar findings (data not shown).
Figure 2.
By gonadotoxicity group, predicted mean log-transformed (A) and untransformed (B) AMH trajectories over years since cancer treatment (bold lines with green for low, blue for moderate, red for high). Mean curves are truncated when the number of individual participants remaining in the group is fewer than 10. Individual log-transformed AMH levels (+) and predicted trajectories (short lines related to +) also are depicted by the color of the gonadotoxicity group (green for low, blue for moderate, red for high). (C) Boxplots of FPC 1 and FPC 2 scores by gonadotoxicity groups. Both FPC scores differed significantly by gonadotoxicity group (P < 0.001). Unadjusted pairwise comparisons denoted by horizontal lines (*P < 0.001): low vs moderate (FPC 1: P < 0.001, FPC 2: P < 0.001), low vs high (FPC 1: P < 0.001, FPC 2: P < 0.001), moderate vs high (FPC 1: P < 0.001, FPC 2: P < 0.001).
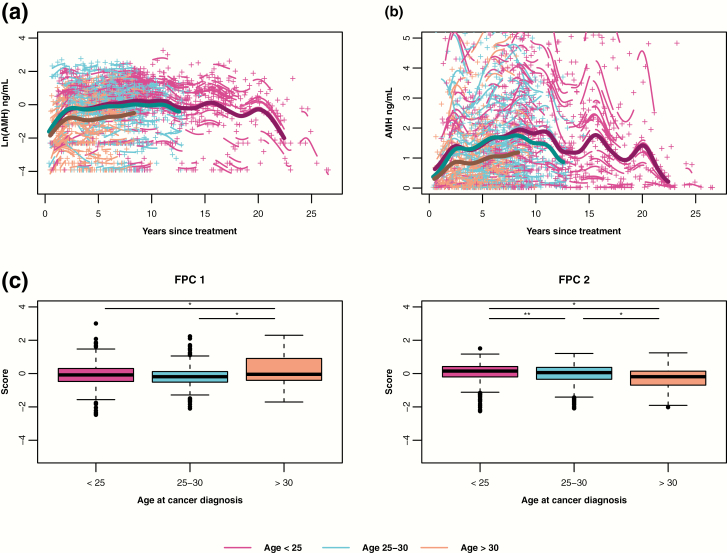
Mean AMH trajectories were also significantly different by age at cancer treatment (P < 0.001 overall) (Fig. 3a and b). At treatment, 325 participants (43%) were aged 15 to 24, 259 (34%) were aged 25 to 30, and 177 (23%) were aged 31 to 35. The trajectory of the aged > 30 group (orange curve) was marked by consistently lower AMH than those of the aged < 25 (pink curve) and aged 25 to 30 (blue curve) groups. The aged < 25 compared to aged 25 to 30 did not differ on FPC 1 scores, but did differ on FPC 2 scores, as evidenced by the separation of trajectories between the aged < 25 and aged 25 to 30 groups after 5 years.
Figure 3.
By age at cancer treatment groups, predicted mean log-transformed (A) and untransformed (B) AMH trajectories over years since cancer treatment (bold lines with maroon for age < 25, blue for aged 25-30, and orange for aged >30). Mean curves are truncated when the number of individual participants remaining in the group is fewer than 10. Individual log-transformed AMH levels (+) and predicted trajectories (short lines related to +) also are depicted by the color of the age group (maroon for aged <25, blue for age 25-30, and orange for aged >30). (C) Boxplots of FPC 1 and FPC 2 scores by age group. Both FPC scores differed significantly by age group (P < 0.001). Unadjusted pairwise comparisons denoted by horizontal lines (*P < 0.001, **P = 0.01): <25 vs 25 to 30 (FPC 1: P = 0.32, FPC 2: P = 0.01), <25 vs > 30 (FPC 1: P < 0.001, FPC 2: P < 0.001), 25 to 30 vs >30 (FPC 1: P < 0.001, FPC 2: P < 0.001).
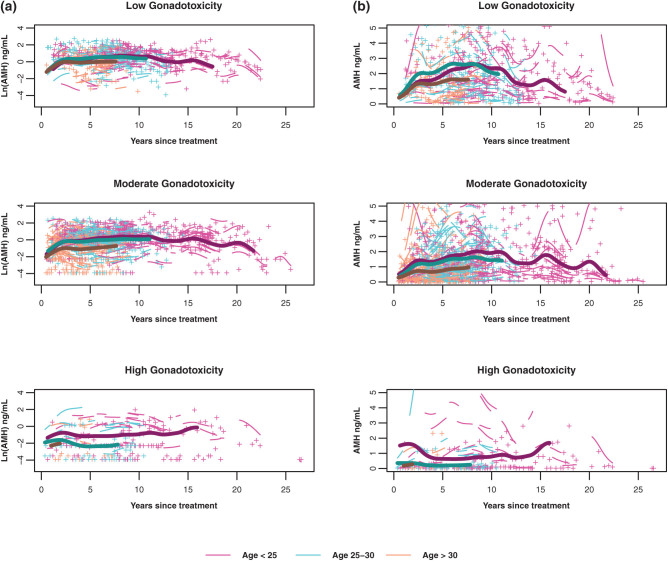
As mean AMH trajectories differed by both gonadotoxicity of cancer treatment and age at diagnosis, we tested for an interaction to assess whether the relationship between AMH trajectory and gonadotoxicity varied by age at cancer diagnosis (Fig. 4a and b). The overall interaction between age and gonadotoxicity was significant (FPC 1: P < 0.001; FPC 2: P = 0.09). For the low gonadotoxicity group (top panel), AMH trajectories were similar across age groups with some separation of aged > 30 group (orange curve) from the younger groups over time. For the moderate gonadotoxicity group (middle panel), there was greater separation of aged > 30 from aged < 25 (pink curve) and aged 25 to 30 (blue curve). In contrast, among participants in the high gonadotoxicity group, the AMH trajectories were similar for the aged 25 to 30 and aged > 30 groups but were different from that for the youngest age group.
Figure 4.
By age at cancer treatment groups, predicted mean log-transformed (A) and untransformed (B) AMH trajectories over years since cancer treatment (bold lines with maroon for aged <25, blue for aged 25-30, and orange for aged >30) among participants exposed to low gonadotoxicity (left panel), moderate gonadotoxicity (middle panel) and high gonadotoxicity (right panel) treatments. Mean curves are truncated when the number of individual participants remaining in the group is fewer than 10. Individual log-transformed AMH levels (+) and predicted trajectories (short lines related to +) also are depicted by the color of the age group (maroon for aged <25, blue for aged 25-30, and orange for aged >30).
For sensitivity analysis, AMH trajectories were modeled in the subset of 513 participants who were not on hormonal contraception, GnRH agonist, or tamoxifen (39). Second, AMH trajectories were modeled without the 260 participants who contributed only one sample (39). Third, because AMH levels increase in adolescence and plateau between aged 15 to 25, we excluded the 45 participants in this age group (39). Modeling trajectories by age at AMH measurement showed similar curves to the primary analysis by years since treatment, because of the co-linearity of these 2 time measures (39). For all 4 analyses, the shape of the overall curves as well as those stratified by treatment gonadotoxicity and age were similar to the findings in the overall cohort. Finally, when follicle-stimulating hormone data from participants who were not on hormonal birth control, GnRH agonists, tamoxifen, and menopausal hormone therapy were modeled (n = 495), results were consistent, that is, inverted from AMH curves (39).
Discussion
The window of ovarian function is clinically important to AYA survivors in making fertility, contraception, menopausal hormone therapy, and cancer treatment decisions. By using a hybrid cross-sectional and longitudinal design (24-26), we estimated trajectories of ovarian function as measured by AMH over the first 20 years after cancer treatment. We observed an extended duration of time during which AMH levels appear to be stable after initial recovery following cancer treatment and prior to decline. The shape of trajectories was strongly related to treatment gonadotoxicity. In contrast, age at diagnosis impacted the magnitude of recovery (ie, overall AMH levels), while the shape of the trajectories was similar. These findings significantly extend 2 prior observations. Previous cross-sectional work suggested that higher gonadotoxicity is associated with lower AMH levels (19-21,40). Additionally, small prospective cohort studies observed high pretreatment AMH levels, followed by a fall during cancer treatment and initial rise, but largely lacked longitudinal data beyond the first 5 years (16-18,41). Prior to the current study, there were sparse data to estimate long-term trajectories (22,23).
The overall predicted AMH trajectory behaved as hypothesized. The earliest section of the trajectory reflects the time period immediately following cancer treatment. The mean AMH level in the immediate posttreatment period was low, because of destruction of growing ovarian follicles by cancer treatments aimed to stop proliferation. From prior studies, AMH levels fall and reach a nadir during receipt of gonadotoxic treatments and the immediately ensuing time period (16,17,42). When there are residual primordial ovarian follicles after cancer treatment, then growth of those follicles into the primary, secondary, preantral, and antral follicle stages will result in proliferation of granulosa cells that express AMH (43). Accordingly, prior cohort studies show a rise in AMH levels in the first 12-24 months posttreatment (17,18,44,45). In our data, AMH trajectories showed recovery that occurred by approximately 2 to 3 years following treatment.
Over the long span of reproductive years after cancer treatment, increasing treatment gonadotoxicity was related not only to lower AMH levels, but different patterns of AMH over time. Following similar peaks in recovery by 2 to 3 years, divergence occurred. For the low and moderate gonadotoxicity groups, a long duration of plateau in AMH levels was observed, suggesting a wide window of ovarian function after recovery and before the decline that would be anticipated with advanced reproductive age. This is consistent with normative data in the general population, in which there is also a plateau or a modest decline from mid-teens to early 30s (14,46,47). Interestingly, although mean AMH levels in our samples were still relatively low at the peak of recovery, the wide plateau following peak suggests that, overall, there is not a more rapid decline in ovarian reserve. Our observation contrasts with what is observed in the general population, where AMH levels decline steeply during the late reproductive stage when levels are low (14,46,48). Importantly, in the absence of an international standard for AMH, our AMH results cannot be directly compared to the data reported in these studies of women without cancer (49).
The AMH trajectory for the high gonadotoxicity group dropped soon after peaking and stayed low thereafter. Reconvergence of the mean gonadotoxicity curves occurred around 15 years, which we postulate is a result of the relative lack of high toxicity samples at 10 to 15 years. These longitudinal data significantly extend prior observations of low AMH levels after high-dose alkylating chemotherapy, preparative regimens for stem cell therapy, and pelvic radiation (20,50). While AMH levels in the general population do not appear to be related to time to pregnancy (51), AMH is associated with yield in ovarian stimulation and time to menopause (48,52-56). The identification of a possible, although narrow, window of ovarian function in women exposed to highly toxic therapies raises opportunities for posttreatment fertility preservation or, conversely, the need to prevent unintended pregnancy.
AMH trajectories were lower with older age at treatment, but profiles were notably parallel over the duration of follow-up. Among survivors within the same gonadotoxicity group, chronologically older survivors, expected to have lower pretreatment ovarian reserve (8-13), exhibited less recovery. Our data are consistent with a smaller prospective cohort study in which higher pretreatment AMH levels were associated with a higher recovery rate of AMH levels posttreatment (57).
Aided by sample size and distribution of treatment gonadotoxicity, we observed that survivors who were aged 25 to 30 at treatment and were exposed to either low or moderate gonadotoxicity treatments exhibited an AMH pattern similar to survivors who were younger than 25 at treatment. However, with exposure to high gonadotoxicity treatments, the AMH trajectory of this 25- to 30-year-old age group now tracked with that of the treatment aged > 30 group—with a small recovery followed by rapid decline. These findings suggest that the protective effects of younger age, presumably by higher ovarian reserve, become diminished in the latter 20s when exposed to high-gonadotoxicity treatments.
To date, longitudinal data on the long-term trajectory of function after cancer treatment are scarce. Small prospective cohort studies have measured AMH levels starting at cancer diagnosis but have yet to characterize the ovarian function trajectory beyond 2 to 5 years (16-18,41). A recent cohort study of 170 childhood and AYA survivors showed that the change in AMH between ages 15 and 40 were similar between survivors and similarly aged controls (22). An additional study assessed 192 adult survivors of childhood cancers for AMH levels at 16 years following cancer treatment (range 5-43) and approximately 3 years later (23). Results also suggested that the slope of change may be similar to women without cancer. These data are complementary to our observation of a wide window of ovarian function in many survivors.
Recently, a number of studies have reported on using highly sensitive AMH assays to predict with more precision the final menstrual period (FMP) in the general population (52,55,56). Combinations of age and very low levels of AMH (10 pg/mL) predicted FMP within 12 months (Positive Predictive Value 51%-79% for aged < 48-51) (56). Conversely, levels > 100 pg/mL predicted no FMP within 36 months (Negative Predictive Value 90%-97%). These types of prediction would be of utility in AYA survivors, but would need to take into account time since cancer treatment as AMH levels exhibit the initial recovery, in contrast to time-related changes in women who have not had cancer treatment.
The FPCA approach is novel to estimating patterns of change in ovarian reserve markers with reproductive aging. Many studies that aim to capture the span of reproductive years are cross-sectional. The few existing longitudinal studies are hampered by small sample sizes and follow-up/duration. Our hybrid cross-sequential design addresses these limitations but requires novel analytic approaches, such as FPCA, which accounts for complexity in curve estimation and investigates for dominant modes of variation even when data are sparse. Furthermore, FPCA has been well-studied in the sparse functional data setting (58). Examples include studying patterns of activity in Alzheimer’s disease, weight growth trajectories, and variations of glomerular filtration curves (59-61). Two principal components best described our data, and these 2 FPC were significantly different by gonadotoxicity and age, known predictors of advanced reproductive age, supporting validity.
There was considerable overlap across these groups when considering individual trajectories. This suggests that exposure categories for reproductive risk, such as CED and pelvic radiation, are inadequate and contribute to misclassification. Our future work with this cohort’s treatment data is to use latent class modeling to explore hidden patterns and combinations of individual drugs, chemotherapy, or biologic treatment regimens and participant characteristics that are related to AMH profiles to improve precision of estimates on individual survivors’ long-term pattern of ovarian function.
Several limitations warrant discussion. While AMH does describe ovarian function, levels do not appear clinically useful in prediction of time to pregnancy in healthy women or in case reports of cancer survivors (18,51). Thus, regardless of AMH level, young cancer survivors interested in fertility ought to attempt pregnancy. However, AMH is related to time to menopause and number of oocytes retrieved (48,52-56). Hence, for young survivors who are not yet ready to conceive but are known to have a narrowed window of residual ovarian function, AMH may be useful to determining whether and when to attempt fertility preservation post cancer treatment. There may also be clinical implications for breast cancer survivors whose endocrine therapy approach depends on whether she is premenopausal or postmenopausal (62). Second, our data are too few to estimate with precision the change points when peak recovery or start of decline occur, but our data do indicate a more narrow time period for exploration. Perhaps pooling longitudinal data across studies may aid in generating more precise estimated. On the approach, we caution that the FPCA method is not robust in detecting outliers, because outliers in the functional data setting have multiple dimensions that cannot easily be accounted. Because one-third of our cohort contributed only one DBS time point, we observed greater variability without serial measures. Lack of pretreatment AMH levels precludes using levels to predict posttreatment trajectories. Finally, the data compare only AYA survivors without an additional comparison with healthy controls.
In summary, the Window cohort provides novel data to characterize the span of ovarian function following cancer treatment in AYA survivors, an understudied population compared to childhood cancer survivors. We demonstrate that ovarian function recovers over the first 2 to 3 years after the end of cancer treatment and then, depending on the gonadotoxicity of treatment and age at treatment, plateaus for variable amounts of time prior to decline. These results advance knowledge that informs clinical care regarding the residual window of ovarian function and research efforts to generate more precise reproductive risks.
Acknowledgments
We wish to thank Window Study participants, Stupid Cancer!, Fertile Action, and the California and Texas Cancer registries for their partnership in this project. We also wish to acknowledge Ajay Kumar, PhD, and Bahnu Kalra, PhD, for development of the DBS AMH assay.
Financial Support: NIH HD080952, NSF DGE-1650112. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health, National Institute of Child Health and Human Development.
Additional Information
Disclosure Summary: PMS works for AnshLabs, and ACD works for Bluebird Bio, Inc. These companies did not sponsor, support, or have oversight of this research.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. American Cancer Society. Cancer treatment and survivorship: facts and figures 2019-2021. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 2. Coccia PF, Altman J, Bhatia S, et al. Adolescent and young adult oncology. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;10(9):1112-1150. [DOI] [PubMed] [Google Scholar]
- 3. Liu L, Moke DJ, Tsai KY, et al. A reappraisal of sex-specific cancer survival trends among adolescents and young adults in the United States. J Natl Cancer Inst. 2019;111(5):509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorman JR, Bailey S, Pierce JP, Su HI. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv. 2012;6(2):200-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorman JR, Usita PM, Madlensky L, Pierce JP. Young breast cancer survivors: their perspectives on treatment decisions and fertility concerns. Cancer Nurs. 2011;34(1):32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammond CT, Beckjord EB, Arora NK, Bellizzi KM, Jeffery DD, Aziz NM. Non-Hodgkin’s lymphoma survivors’ fertility and sexual function-related information needs. Fertil Steril. 2008;90(4):1256-1258. [DOI] [PubMed] [Google Scholar]
- 7. Thewes B, Meiser B, Taylor A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23(22):5155-5165. [DOI] [PubMed] [Google Scholar]
- 8. Broer SL, Eijkemans MJ, Scheffer GJ, et al. Anti-Müllerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96(8):2532-2539. [DOI] [PubMed] [Google Scholar]
- 9. Freeman EW, Sammel MD, Gracia CR, et al. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83(2):383-392. [DOI] [PubMed] [Google Scholar]
- 10. Randolph JF Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90(11):6106-6112. [DOI] [PubMed] [Google Scholar]
- 11. Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimüllerian hormone concentration. Menopause. 2011;18(7):766-770. [DOI] [PubMed] [Google Scholar]
- 12. Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-Müllerian hormone. J Clin Endocrinol Metab. 2013;98(2):729-735. [DOI] [PubMed] [Google Scholar]
- 13. Tepper PG, Randolph JF Jr, McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hagen CP, Aksglaede L, Sørensen K, et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95(11):5003-5010. [DOI] [PubMed] [Google Scholar]
- 15. de Kat AC, van der Schouw YT, Eijkemans MJ, et al. Back to the basics of ovarian aging: a population-based study on longitudinal anti-Müllerian hormone decline. BMC Med. 2016;14(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21(10):2583-2592. [DOI] [PubMed] [Google Scholar]
- 17. Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97(6):2059-2067. [DOI] [PubMed] [Google Scholar]
- 18. Dezellus A, Barriere P, Campone M, et al. Prospective evaluation of serum anti-Müllerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer. 2017;79:72-80. [DOI] [PubMed] [Google Scholar]
- 19. van Beek RD, van den Heuvel-Eibrink MM, Laven JS, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin’s lymphoma during childhood. J Clin Endocrinol Metab. 2007;92(10):3869-3874. [DOI] [PubMed] [Google Scholar]
- 20. van den Berg MH, Overbeek A, Lambalk CB, et al. ; DCOG LATER-VEVO Study Group Long-term effects of childhood cancer treatment on hormonal and ultrasound markers of ovarian reserve. Hum Reprod. 2018;33(8):1474-1488. [DOI] [PubMed] [Google Scholar]
- 21. Larsen EC, Müller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307-5314. [DOI] [PubMed] [Google Scholar]
- 22. Cameron K, Sammel MD, Prewitt M, Gracia C. Differential rates of change in measures of ovarian reserve in young cancer survivors across the reproductive lifespan. J Clin Endocrinol Metab. 2019;104(5):1813-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Kooi AL, van den Heuvel-Eibrink MM, van Noortwijk A, et al. Longitudinal follow-up in female childhood cancer survivors: no signs of accelerated ovarian function loss. Hum Reprod. 2017;32(1):193-200. [DOI] [PubMed] [Google Scholar]
- 24. Kujala S, Miron-Shtaz T, Jokinen J. The cross-sequential approach: a short-term method for studying long-term user experience. J. Usability Stud. 2019;14(2):105-116. [Google Scholar]
- 25. Donaldson G, Horn JL. Age, cohort, and time development muddles: easy in practice, hard in theory. Exp Aging Res. 1992;18(3-4):213-222. [DOI] [PubMed] [Google Scholar]
- 26. Dorn LD, Beal SJ, Kalkwarf HJ, Pabst S, Noll JG, Susman EJ. Longitudinal impact of substance use and depressive symptoms on bone accrual among girls aged 11-19 years. J Adolesc Health. 2013;52(4):393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Groves RM, Mosher W, Lepkowski J, Kirgis NG;. National Center for Health Statistics. Planning and development of the continuous National Survey of Family Growth.. Vital Health Stat. 2009;1(48):1-64. [PubMed] [Google Scholar]
- 28. Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shandley LM, Spencer JB, Fothergill A, et al. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril. 2017;107(1):243-252.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chemaitilly W, Li Z, Krasin MJ, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude lifetime cohort. J Clin Endocrinol Metab. 2017;102(7):2242-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738-744. [DOI] [PubMed] [Google Scholar]
- 32. Levine JM, Whitton JA, Ginsberg JP, et al. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2018;124(5):1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poorvu PD, Frazier AL, Feraco AM, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr. 2019;3(1):pkz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramsay JO, Silverman BW.. Principal components analysis for functional data. In Functional data analysis. New York, NY: Springer; 2005:147-172. [Google Scholar]
- 35.Ramsay JO, Silverman BW.Regularized principal components analysis. In Functional data analysis. New York, NY: Springer New York; 2005:173-185. [Google Scholar]
- 36. Peng J, Paul D. A geometric approach to maximum likelihood estimation of the functional principal components from sparse longitudinal data. J Comput Graph Stat. 2009;18(4):995-1015. [Google Scholar]
- 37. Peng J, Paul D. fpca: restricted MLE for functional principal components analysis. R package version 0.2-1. https://cran.r-project.org/web/packages/fpca/fpca.pdf. Published 2011. [Google Scholar]
- 38. Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 39. Su HI, Kwan B, Whitcomb BW, et al. Data from: modeling variation in the reproductive lifespan of female adolescent and young adult cancer survivors using AMH. Figshare NIH Repository. Deposited March 21, 2020. 10.35092/yhjc.12016353. [DOI] [PMC free article] [PubMed]
- 40. Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31(2):231-239. [DOI] [PubMed] [Google Scholar]
- 41. Anderson RA, Cameron DA. Pretreatment serum anti-Müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96(5):1336-1343. [DOI] [PubMed] [Google Scholar]
- 42. Evranos B, Faki S, Polat SB, Bestepe N, Ersoy R, Cakir B. Effects of radioactive iodine therapy on ovarian reserve: A prospective pilot study. Thyroid. 2018;28(12):1702-1707. [DOI] [PubMed] [Google Scholar]
- 43. Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77-83. [DOI] [PubMed] [Google Scholar]
- 44. Mörse H, Elfving M, Lindgren A, Wölner-Hanssen P, Andersen CY, Øra I. Acute onset of ovarian dysfunction in young females after start of cancer treatment. Pediatr Blood Cancer. 2013;60(4):676-681. [DOI] [PubMed] [Google Scholar]
- 45. Henry NL, Xia R, Schott AF, McConnell D, Banerjee M, Hayes DF. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist. 2014;19(1):68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-Müllerian hormone from conception to menopause. Plos One. 2011;6(7):e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lie Fong S, Visser JA, Welt CK, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nair S, Slaughter JC, Terry JG, et al. Anti-Müllerian hormone (AMH) is associated with natural menopause in a population-based sample: the CARDIA Women’s Study. Maturitas. 2015;81(4):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Su HI, Sammel MD, Homer MV, Bui K, Haunschild C, Stanczyk FZ. Comparability of antimüllerian hormone levels among commercially available immunoassays. Fertil Steril. 2014;101(6):1766-72.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116(3):592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. Jama. 2017;318(14):1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Depmann M, Eijkemans MJC, Broer SL,et al. Does AMH relate to timing of menopause? Results of an individual patient data meta- analysis. J Clin Endocrinol Metab. 2018. [DOI] [PubMed] [Google Scholar]
- 53. Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91(3):705-714. [DOI] [PubMed] [Google Scholar]
- 54. Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17(1):46-54. [DOI] [PubMed] [Google Scholar]
- 55. Finkelstein JS, Lee H, Karlamangla A, et al. Anti-Mullerian hormone and impending menopause in late reproductive age: the study of women’s health across the nation. J Clin Endocrinol Metab. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Santoro N, Finkelstein JS, Lee H, et al. Anti-Mullerian hormone for predicting the final menstrual period: the Study of Women’s Health Across the Nation (SWAN). J Clin Endocrinol Metab. 2020;105(4):e1862–e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dillon KE, Sammel MD, Prewitt M, et al. Pretreatment antimüllerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99(2):477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. James G, Hastie T, Sugar C. Principal component models for sparse functional data. Biometrika. 2000;87(3):587-602. [Google Scholar]
- 59. Che M, Kong L, Bell RC, Yuan Y. Trajectory modeling of gestational weight: a functional principal component analysis approach. Plos One. 2017;12(10):e0186761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dong JJ, Wang L, Gill J, Cao J. Functional principal component analysis of glomerular filtration rate curves after kidney transplant. Stat Methods Med Res. 2018;27(12):3785-3796. [DOI] [PubMed] [Google Scholar]
- 61. Zeitzer JM, David R, Friedman L, et al. Phenotyping apathy in individuals with Alzheimer disease using functional principal component analysis. Am J Geriatr Psychiatry. 2013;21(4):391-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol. 2006;24(16):2444-2447. [DOI] [PubMed] [Google Scholar]