Abstract
Background:
Many research studies attempting to improve locomotor function following motor incomplete spinal cord injury (iSCI) focus on providing stepping practice. However, observational studies of physical therapy strategies suggest the amount of stepping practice during clinical rehabilitation is limited; rather, many interventions focus on mitigating impairments underlying walking dysfunction.
Objective:
The purpose of this blinded-assessor randomized trial was to evaluate the effects of task-specific vs impairment-based interventions on walking outcomes in individuals with iSCI.
Methods:
Using a crossover design, ambulatory participants with iSCI > 1-year duration performed either task-specific (upright stepping) or impairment-based training for up to 20 sessions over ≤6 weeks, with interventions alternated after >4 weeks delay. Both strategies focused on achieving higher cardiovascular intensities, with training specificity manipulated by practicing only stepping practice in variable contexts or practicing impairment-based tasks targeting impairments underlying locomotor dysfunction (strengthening, balance tasks, and recumbent stepping).
Results:
Significantly greater increases in fastest overground and treadmill walking speeds were observed following task-specific vs impairment-based training, with moderate associations between differences in amount of practice and outcomes. Gains in balance confidence were also observed following task-specific vs impairment-based training, although incidence of falls was also increased with the former protocol. Limited gains were observed with impairment-based training except for peak power during recumbent stepping tests.
Conclusion:
The present study reinforces work from other patient populations that the specificity of task practice is a critical determinant of locomotor outcomes and suggest impairment-based exercises may not translate to improvements in functional tasks.
Clinical Trial Registration-URL:
https://clinicaltrials.gov/; Unique Identifier: NCT02115685
Keywords: rehabilitation, gait, high-intensity
Introduction
Over half of patients diagnosed with a spinal cord injury (SCI) are classified as motor incomplete (iSCI)1, indicating partial preservation of descending pathways and the potential for recovery of locomotor function. Indeed, restoration of locomotion function is often a main goal of patients with iSCI, their caregivers and the rehabilitation professionals who treat them2. While the extent of sensorimotor recovery early following injury can facilitate prediction of independent ambulation3,4, treatment strategies designed to enhance walking ability vary substantially and the efficacy of many interventions is uncertain.
Research to improve walking recovery following iSCI5,6 has focused primarily on task-specific (stepping) strategies. Early work in animal models of SCI suggested that provision of large amounts of stepping practice resulted in greater gains in walking function as compared to less stepping practice or practice of alternative (non-stepping) tasks7,8. Studies attempting to translate these basic research findings to the treatment of individuals with iSCI, specifically by providing large amounts of focused stepping practice on a treadmill or overground, indicate potentially greater recovery of walking performance than typically observed9-11. While the efficacy of specific strategies vary12-14, previous work in other patient populations (i.e., stroke) suggest that practice of stepping tasks at higher cardiovascular intensities and in variable contexts (overground, treadmill and stairs) may elicit significant gains in walking function. Attempts to apply these strategies to patients with iSCI15-17 resulted in participants achieving >2000 steps/session with significant gains in locomotor function, particularly as compared to lower-intensity interventions15.
Despite these findings, recent data suggest stepping practice provided to patients with iSCI during rehabilitation is limited, even in those with recovery of independent walking function18. In one study, ambulatory patients with iSCI practiced 51 steps/session during initial physical therapy sessions of inpatient rehabilitation, with non-significant increases to 115 steps/sessions closer to discharge. Interestingly, the amount of non-walking volitional leg movements (balance, transfers, leg exercises) were higher than stepping practice (143 and 218 repetitions/sessions at admission and discharge, respectively). Attention towards non-walking activities during physical therapy sessions is not limited to inpatient rehabilitation, however. Specialized activity-based therapy programs that enhance rehabilitation opportunities to patients in the later stages post-SCI also focus on both walking and non-walking tasks19-22. Specific strategies include use of postural training in development positions and standing, strength and transfer activities, in addition to locomotor training activities. Previous and more recent studies have also focused on the use of high-intensity strengthening23,24, aerobic recumbent cycling or stepping programs25-27, or combined circuit-training programs28, all of which have demonstrated some gains in walking recovery. Indeed, attention toward impairment-based activities are established strategies utilized in the treatment of iSCI29 or other neurological populations30,31.
This focus on distributed task practice of varied impairment-based tasks is, however, inconsistent with motor control literature32 and findings that emphasize the importance of the amount and specificity of practice33. Surprisingly, there are very few controlled studies that have attempted to evaluate the efficacy of non-walking, impairment-based exercises on locomotor function in patients with motor iSCI, particularly as compared to locomotor training strategies. The purpose of this study was therefore to assess changes to locomotor function and selected impairments in individuals with iSCI following task-specific (stepping) training versus impairment-based (non-stepping) interventions. Using a randomized crossover design with blinded assessments, participants > 1-year post-SCI were enrolled. Consistent with data from other patient populations (i.e., stroke)34,35, we hypothesized greater locomotor improvements would be observed with task-specific vs impairment-based practice. Such findings may provide insight into the relative value of utilizing task- vs impairment-specific exercise for improving locomotor and non-locomotor function following iSCI.
Methods
Study sample and design
Participants were recruited from outpatient clinics within a rehabilitation hospital system if they presented with motor iSCI (classified as C or D using the American Spinal Injury Association Impairment Scale) at neurological level of T10 or above of at least 1-yr duration. Additional inclusion criteria consisted of: 18-75 years old; ability to walk overground at self-selected speeds (SSS) < 1.0 m/s without physical assistance but with devices and bracing below the knee as needed; and medical clearance to participate. Exclusion criteria included: severe lower extremity contractures such that walking performance was affected (e.g., reduced dorsiflexion movement in late stance causing substantial knee hyperextension); documented history or patient-report of osteoporosis; cardiovascular or metabolic instability; existing unhealed decubiti or infection; active heterotrophic ossification; previous history of other central nervous system injury that affected their walking function; and inability to adhere to study requirements. Participants could not be enrolled in physical therapy throughout the duration of the study and provided written informed consent prior to participation. All procedures were approved by the Indiana University Institutional Review Board.
Participants were randomized to receive up to 20 sessions of either task-specific or impairment-based training over less than 6 weeks followed by the alternate training paradigm, with a delay of at least 4 weeks between interventions (Fig 1). This cross-over experimental design was employed to increase the efficiency of the data collected given the incidence of SCI as compared to other neurological disorders and the difficulties of traditional randomized trials in smaller patient populations.36 Separate data from published studies evaluating the effects of high intensity variable stepping or impairment-based interventions in individuals with iSCI were used for the initial power analyses. Specific gains of 0.14±0.10 m/s in walking speed and 30±24 m in the 6 minute walking test (6MWT) were observed following high-intensity variable stepping training.15 Conversely, gains of approximately 0.06±0.06 m/s and 14±10 m were observed in selected non-walking intervention studies24,26. Power calculations using these approximate changes revealed 16 individuals were necessary to observe significant between-group differences (82-93% power) Participants were stratified by gait speed (<0.5 or 0.5-1.0 m/s) and block-randomized (4/block) into task-specific or impairment-based training first.
Figure 1.
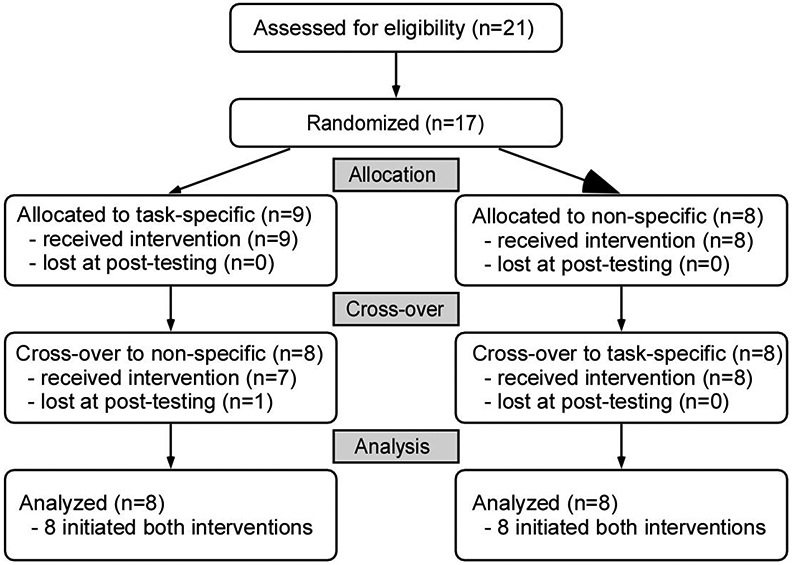
CONSORT flow diagram of randomized crossover design. One participant enrolled in task-specific training first was lost prior to initiating impairment-based training
Intervention
All participants were asked to attend all 20 sessions for either intervention within 6 weeks, with training termination after 6 weeks regardless of number of sessions attended. The number of sessions completed by an individual in the first training epoch was the targeted number of sessions for that participant during the second training epoch. Task-specific training consisted of up to 40 min of stepping practice in variable contexts within 1-hr sessions. Impairment-based training consisted of up to 40 min of non-walking interventions, including strengthening, balance tasks, aerobic conditioning and practice of transfers (sit-to-stand, supine-to-sit) to improve lower extremity and trunk strength and coordination. A primary intent of both strategies was to achieve high cardiovascular intensities, including attaining 70-80% heart rate (HR) reserve (HRR) and ratings of perceived exertion (RPE) > 1437,38. Estimates of HRs were measured continuously using either chest or arm monitors (H10 or OH1, Polar Inc, Bethpage, NY), or via pulse-oximetry systems. Targeted HR ranges were determined using age-predicted maximum HR [208-(0.7*age)]34,39 with HHR calculated using the Karvonen equation [exercise HR = % target intensity (maximum HR – resting HR) + resting HR]; this strategies was utilized instead of peak HRs achieved during baseline (BSL) exercise testing due to limitations in neuromuscular function that limit peak power (i.e., workload) achieved40. The RPE scale was used as a secondary measure in cases during which patients could not achieve the desired HR ranges secondary to autonomic dysfunction, specific medications, or individual variations15. In cases where HRs were 10-15 beats/min different from expected HR based on RPE values, therapists incrementally increased or decreased HRs with guidance from the primary investigator15. Both HRs and RPEs were documented every 3-5 minutes or more frequently with changes in exercise tasks or demands.
Primary goals of task-specific training have been detailed previously15,39 and included: 1) maximizing successful stepping practice in a specific direction (e.g., forward, backward, sideways); 2) achieving specific intensities, and 3) increasing difficulty of skilled walking tasks as tolerated. Each session was composed of 4 different stepping tasks practiced over ~10 min/session, including speed-dependent treadmill training, skill-dependent treadmill training, over ground training, and stair climbing. Speed-dependent treadmill training consisted of forward treadmill walking while maintaining targeted HRRs or RPEs. Limb swing assistance, body weight support, and nylon straps stabilizing the pelvis were provided only as needed to ensure successful stepping, characterized by positive step lengths, lack of stance-phase limb collapse, and sagittal/frontal plane stability. Skill-dependent treadmill training was performed by applying perturbations to challenge postural stability, propulsion, and limb swing during treadmill walking, and included stepping in multiple directions, over inclines and obstacles, and reducing handrail use as tolerated. Additional loads (weighted vest or leg weights) or resistance (posterior forces at the trunk or thighs) were applied as necessary to reach the targeted intensities while focusing on specific biomechanical gait components (stability, propulsion, limb swing). Perturbations were applied such that 2-5 different stepping tasks were repeated within the 10 min. Overground training focused on speed- or skill-dependent locomotor activities as described above, with use of a gait belt or overhead suspension system for safety. Additional stepping activities included walking over uneven or narrow surfaces and stepping around obstacles. Stair climbing was performed over static or rotating stairs (Stairmaster, Vancouver, WA) with attempts to use reciprocal gait patterns and progression to higher speeds and reduced handrail use as able. If the HRs/RPEs were outside the targeted range, the demands of walking tasks (i.e., speeds or loads carried) were manipulated. Stepping activity was measured using accelerometers on the ankle of the more impaired limb (StepWatch, Modus Inc, Wash DC).
Impairment-based training included rehabilitation strategies directed towards mitigating specific impairments underlying walking dysfunction, including strengthening tasks (10-15 min/session), balance activities (10 min), aerobic conditioning (10 min) and practice of transfer tasks that targeted lower extremity and trunk strength and coordination (5-10 min). Aerobic exercises included recumbent cycling or stepping (e.g., NuStep LLC, Ann Arbor, MI) and varying both the resistance and cadence to achieve desired HRs and RPEs. Specific strengthening activities included hip and knee flexion/extension and plantarflexion exercises using weight machines, and hip flexion and squatting exercises using free weights (leg weight, weighted vest). Each exercise targeted 3-4 sets of 8-20 repetitions. A specific 1-repetition maximum was not performed given previous findings that individuals with iSCI can generate ~20% greater torques during repeated vs single contractions, and intensity was assessed using HRs and RPEs as described previously41. To maintain higher HRs, minimal rest breaks were provided between sets by alternating exercises between legs or rapidly switching to other exercises (i.e., analogous to circuit training). Transfer tasks included practice of bed mobility, sit-to-stand or floor-to-sit transfers while providing verbal cues to increase speed and minimize rest breaks with use of weighted vests or leg weights to achieved desired intensities. For balance activities, participants practiced standing or sitting activities on uneven or compliant surfaces (e.g., foam, trampoline) with increasing difficulty by decreasing base of support, adding limb and torso weights, dual upper extremity/balance tasks, occluding vision and/or providing manual perturbations at the trunk to elicit reactive balance strategies. Given the difficulty of achieving higher HRs during balance tasks, challenging balance exercises were often practiced for ~1-2 min and alternated with strengthening or transfer tasks using a circuit training-type paradigm.
Outcomes
Participants were assessed prior to and following each training protocol. Primary measures included the fastest speed (FS) over short distances and peak treadmill speed. Measures of FS and peak treadmill speed have previously been shown to be sensitive to the effects of high-intensity stepping training in iSCI15,42. Assessment of FS was performed by blinded assessors, with instructions to “walk as fast as you safely can” (Zeno Walkway, ProtoKinetics LLC, Haverton, PA) with two trials averaged and preceded by a warm-up trial. Patient’s customary bracing and use of assistive devices were utilized and were identical at all assessments. Evaluation of peak treadmill speed was not blinded and evaluated during a modified graded exercise test (GXT) with simultaneous collection of 12-lead ECGs and cardiorespiratory data using indirect calorimetry (K4B2, Cosmed, Inc, Chicago, IL). During graded exercise testing, participants began walking on a motorized treadmill at 0.1 m/s for 1 minute with speed increased by 0.1 m/s every minute until the subject experienced significant gait instability, could not continue walking, requested to stop, or the investigator observed ECG abnormalities that are considered absolute criteria for exercise termination using American College of Sports Medicine guidelines43. Participants with balance deficits often needed to hold onto the handrails during testing but were not allowed to support their bodyweight (i.e., push vertically). During testing, patients wore a safety harness without body weight support and cardiorespiratory data were collected (see below). Participants’ HRs were measured continuously while blood pressures were measured immediately prior to and following testing. Peak treadmill speed was determined as the highest speed achieved for 1 minute.
Secondary blinded clinical measures included SSS on the instrumented walkway with instructions to “walk at your normal, comfortable pace”, and 6MWT with instructions to “cover as much ground as possible”. Other blinded measures include the Berg Balance Scale (BBS) and 5-times sit-to-stand (5XSTS). Subjective measures included the Activities-specific Balance Confidence (ABC) scale and the Patient-Reported Outcomes Measurement Information System (PROMIS) – Mobility score (version 1.2). Additional unblinded assessments included lower extremity motor score (LEMS), and peak O2 consumption (VO2peak) during graded exercise tests on the treadmill and peak power and VO2peak during graded recumbent stepping testing. For both tests, metabolic gases were collected on a breath-by-breath basis. During the treadmill exercise test, VO2peak was calculated as the average VO2 achieved over the last 30 sec of the peak treadmill speed. During testing on the recumbent stepper, we used a modified protocol developed for this device44 (NuStep) during which the exercise intensity (power) increased every 2 minutes while maintaining cadence between 80-90 steps/min. Peak stepping power (Powerpeak in watts) achieved during testing was determined by the highest power achieved for at least 1 min without cadence dropping below 40 steps/min, and VO2peak during the recumbent stepping test was the average VO2 during the last 30 sec of the last minute.
Incidence of serious and minor adverse events were tabulated. Serious adverse events included death, falls with injury outside of training, and cardiovascular events requiring hospitalization. Minor adverse events included musculoskeletal pain, falls without injury outside of training, dizziness, loss of consciousness, excessive shortness of breath, or episodes of hypertension, hypotension or angina that limited training.
Analysis
Training parameters of interest included total number of sessions and average steps/session, as well as minutes of stepping practice (i.e., number of minutes with steps ≥ 10), and average steps/min. With intensity measures (HR and RPE) documented every 5 minutes, we identified both peak and average HR and RPE per session. Measures of HR are reported as percentage HRR and accounted for β-blocker use by subtracting 10 beats/min from the targeted range45. Differences in training parameters were compared between interventions using paired t-tests.
For statistical analysis, data were normally distributed (Kolmogorov-Smirnov) with the exception of SSS, although parametric statistics were used for all data. Data were analyzed on-protocol from participants who participated in both interventions. Data were also analyzed using intent-to-treat (n=17) and using only those participants with completed baseline and post-testing following both interventions (n=15). Data are presented as mean ± standard deviation in the text and tables with standard errors in the figures. Outcomes assessed immediately prior to and following each training intervention were calculated. Statistical analyses of primary outcomes (FS, peak treadmill speed) were performed using a mixed model ANOVA, with primary main effects of time, including BSL and post-training (POST) measures following each training period (repeated), order (task-specific 1st or 2nd), and training condition (task-specific or impairment-based, repeated). We were specifically interested in the main effects of time (BSL vs POST) and interaction effects of time X training, and time X training X order. Bonferroni corrections were made for the primary outcomes (adjusted α=0.025), and the statistical power of the time X training effects for walking outcomes (SSS, FS, 6MWT, peak treadmill speed) are provided. Considering the potential carry-over effects of the crossover design, we also performed a separate analysis of the initial parallel-group randomization, evaluating differences in outcomes following the first training intervention only. These latter analyses also used a mixed-model ANOVA with primary main effects of time and training group. Similar analyses were performed for secondary clinical measures or metabolic measures (VO2peak during graded treadmill and recumbent stepping testing) without Bonferroni corrections. All primary and secondary outcomes were also analyzed using all participants enrolled (17 total) using both regression imputation and imputation by carrying the last number(s) forward. Statistical analyses were also performed using only the 15 participants who completed all parts of the study. Serious and minor adverse events were categorized per training intervention, with χ2 analyses to compare between-group difference in frequency.
Associations between the differences in amount of task-specific practice in each intervention and changes in locomotor outcomes were evaluated using Pearson correlation analyses. Notably, although the amount of stepping practiced was minimized during impairment-based training, the accelerometer often detected stepping during transitions between training tasks (e.g., weight machine to recumbent stepper). Further, stepping monitors may also detect “steps” when practicing impairment-based tasks were kinematically similar to stepping tasks (e.g., stepping in place, knee extension/flexion strengthening exercises). Specific differences in amount of stepping practice were calculated by subtracting the average steps/session during task-specific vs impairment-based training (i.e., Δsteps/session = average steps/sessions during task-specific training vs during impairment-based training). Further, we calculated differences in changes in outcomes following each training paradigm, (i.e., differences in the change scores for specific outcomes following task-specific vs impairment-based training)15. Correlation analyses therefore evaluated the relationship between differences in stepping activity between training interventions (per participant) vs differences in changes in outcomes (i.e., Δ6MWT or ΔSSS per participant) between interventions.
Results
Seventeen of 21 individuals who were consented fulfilled all inclusion criteria and were randomized. Fifteen participants finished all aspects of the study; one participant finished the first training intervention (task-specific) but did not wish to continue with the second intervention and their data were not included, and another subject terminated participation during the second intervention (impairment-based). Data from the latter participant are included (BSL imputed to POST). Of the 16 participants included in the analyses, 8 were randomized to task-specific training first (Fig 1). Demographic and clinical characteristics are provided in Table 1.
Table 1.
Demographics and baseline characteristics (C: cervical; T: thoracic)
| Impairment- based first (n=8) |
Task-specific first (n=8) |
|
|---|---|---|
| Clinical characteristics | ||
| Age (years) | 51±17 | 46±13 |
| gender (male/female) | 6/2 | 4/4 |
| race (white/other) | 5/3 | 7/1 |
| BMI (kg/m2) | 29±5.9 | 31±7.6 |
| duration post-iSCI (years) | 3.9±1.8 | 4.3±4.3 |
| lesion level: C1-C4 | 4 | 2 |
| C5-C8 | 2 | 2 |
| T1-T10 | 2 | 4 |
| Clinical characteristics | ||
| Lower Extremity Motor Score | 45±3.3 | 39±10 |
| ankle foot orthosis (yes/no) | 1/7 | 4/4 |
| assistive devices (yes/no) | 7/1 | 7/1 |
| baseline SSS (m/s) | 0.54±0.21 | 0.45±0.31 |
| baseline FS (m/s) | 0.71±0.25 | 0.55±0.36 |
| baseline 6MWT (m) | 194±81 | 164±134 |
| baseline treadmill speed (m/s) | 0.91±0.36 | 0.61±0.47 |
| Medication use | ||
| anti-spastics (yes/no) | 6/2 | 5/3 |
| anti-depressants (yes/no) | 1/7 | 2/6 |
| anti-hypertensives (yes/no) | 1/7 | 2/6 |
Training parameters
Table 2 details differences in training parameters for task-specific and impairment-based interventions. There were similar average number of sessions completed in each group, although differences were observed for all stepping parameters (steps/sessions, stepping minutes, and stepping rate; all p<0.01). Notably, average steps/sessions (693±437) was higher than expected during impairment-based training when walking practice was minimized. Nonetheless, sessions that focused only on task-specific (stepping) practice achieved >2200 steps/session. Despite attempts to match intensities, average and peak HRRs were different between training groups (p<0.01) favoring task-specific training (~8% greater %HRR than impairment-based interventions). Evaluation of training logs suggest greater difficulty achieving higher intensities specifically during postural training tasks. In general, 8/16 were unable to achieve >70% HRR during impairment-based training, whereas only 4/16 could not reach >70% HRR during task-specific stepping. Regardless, average and maximum RPEs were similar between groups.
Table 2.
Training parameters
| Impairment-based | Task-specific | p-values | |
|---|---|---|---|
| number of sessions | 18±3.0 | 18±1.5 | 0.84 |
| steps per sessions | 693±437 | 2206±988 | <0.001 |
| stepping minutes/session | 20±12 | 40±8.8 | <0.001 |
| stepping rate (steps/min) | 29±8.3 | 52±17 | <0.001 |
| average HRR | 57±10 | 70±11 | <0.001 |
| maximum HRR | 71±12 | 79±12 | 0.02 |
| average RPE | 18±1.3 | 17±1.2 | 0.20 |
| maximum RPE | 20±1.8 | 19±1.0 | 0.71 |
Primary and secondary outcomes
Analyses of primary outcomes (FS and peak treadmill speed) revealed significant between-group differences. Main effects of time (p<0.01) and time X training interactions were observed for FS favoring task-specific (0.14±0.18 m/s) vs impairment-based training (0.02±0.08 m/s; p=0.01; statistical power = 76%; Fig 2A and Table 3). Similar differences were observed for peak treadmill speed favoring task-specific training (0.20±0.15 vs 0.01±0.09 m/s, p<0.01; statistical power=100%; Fig 2B). No time X training X order effects were observed for either measure. Using intent-to-treat analyses or only those individuals who completed all training resulted in very similar differences in primary outcomes. Analyses of only the initial training assignment (i.e., exclusion of cross-over data) also revealed significant time X training effects for peak treadmill speed (p<0.01) but not FS (p=0.06).
Figure 2.
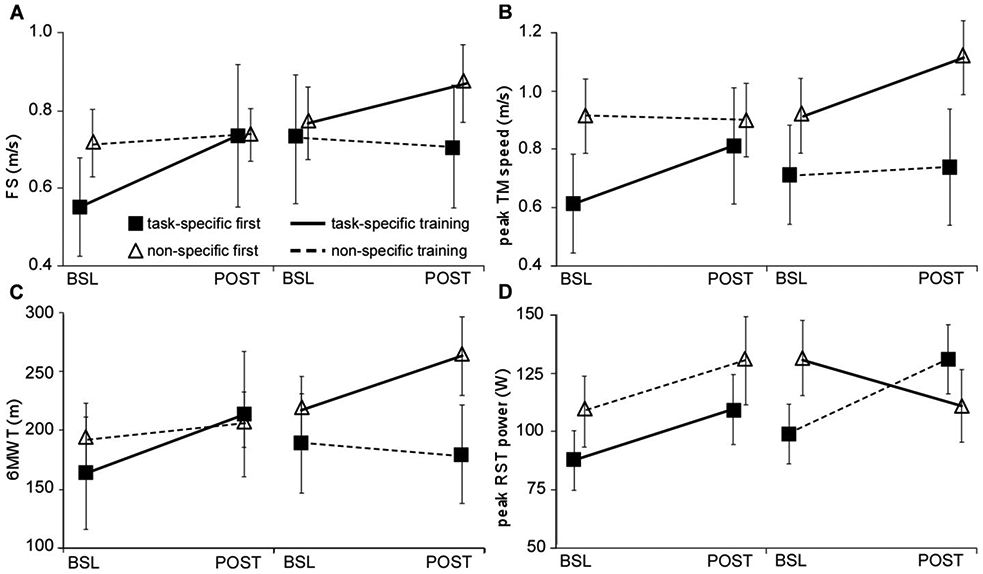
Differences in primary locomotor outcomes of A) fastest speed, B) peak treadmill speed, and secondary outcomes of C) 6MWT and D) peak RST power; BSL and POST indicated for both first and second training interventions in order received; dark lines indicate task-specific training, dashed lines indicate impairment-based training, filled squares denote task-specific first, impairment-based second; open triangles denote impairment-based first, task-specific second.
Table 3.
Pre-training (BSL) and post-training (POST) data for impairment-based vs task-specific training. Significant differences for main effect of time and time X group are provided. Recumbent stepping power is abbreviated using stepping power.
| Impairment-based | Task-specific | Crossover (p-values) |
Initial groups (p-values) |
|||||
|---|---|---|---|---|---|---|---|---|
| BSL | POST | BSL | POST | time | time X group |
time | time X group |
|
| Primary variables | ||||||||
| FS (m/s) | 0.70±0.36 | 0.71±0.33 | 0.64±0.32 | 0.78±0.41 | <0.01* | 0.01* | 0.02* | 0.06 |
| treadmill speed (m/s) | 0.81±0.41 | 0.82±0.43 | 0.76±0.43 | 0.96±0.48 | <0.01* | <0.01* | <0.01* | <0.01* |
| Secondary clinical variables | ||||||||
| SSS (m/s) | 0.53±0.28 | 0.53±0.25 | 0.51±0.26 | 0.58±0.30 | 0.10 | 0.12 | 0.10 | 0.21 |
| 6MWT (m) | 192±97 | 195±94 | 191±110 | 239±123 | <0.01* | <0.01* | <0.01* | 0.02* |
| BBS (a.u.) | 32±12 | 33±11 | 32±14 | 35±14 | 0.03* | 0.39 | 0.14 | 0.33 |
| LEMS (a.u.) | 43±6.8 | 42±10 | 42±8.0 | 41±11 | 0.53 | 0.49 | 0.49 | 0.57 |
| 5XSTS (s) | 17±5.1 | 14±4.5 | 16±8.3 | 15±4.7 | 0.29 | 0.64 | 0.91 | 0.44 |
| stepping power (W) | 103±43 | 134±48 | 110±45 | 110±43 | 0.10 | 0.04* | 0.04* | 0.99 |
| Secondary subjective variables | ||||||||
| ABC (a.u.) | 55±21 | 57±21 | 48±22 | 58±23 | 0.02* | 0.01* | 0.01* | 0.03* |
| PROMIS (a.u.) | 38±12 | 40±12 | 36±12 | 40±14 | 0.06 | 0.24 | 0.15 | 0.68 |
| Secondary metabolic variables | ||||||||
| VO2peak-treadmill (ml/kg/min) |
17±7.8 | 18±9.4 | 17±5.2 | 19±8.2 | 0.04* | 0.59 | 0.08 | 0.49 |
| VO2peak-stepping (ml/kg/min) |
15±5.2 | 16±7.3 | 14±5.0 | 14±5.1 | 0.71 | 0.58 | 0.41 | 0.87 |
For secondary clinical outcomes, significant main effects of time and time X training-group interactions were observed for 6MWT (48±31 vs 2.9±26 m, p<0.01; statistical power=100%; Fig 2C) when analyzed with the entire data set, or the initial parallel-group randomization (p<0.01, Table 3). There were no differences for SSS, BBS, 5XSTS or LEMS, with the exception of a significant time effect for BBS (e.g., statistical power for SSS=43%, p=0.12). Notably, 5 individuals could not perform the 5XSTS throughout the study and their data for this test were not included (2 with impairment-based first, and 3 with task-specific first). For subjective measures, significantly greater gains in ABC but not PROMIS were observed following task-specific vs impairment-based training (10±11 vs 1.8±11, p=0.02) using both the crossover data or analyzing only the first training intervention. A significant time X training interaction was also observed for changes in peak recumbent stepping power favoring impairment-based vs task-specific training, (27±45 vs −0.20±33 watts; p=0.04). Notably, recumbent stepping power during either intervention increased in the first training epoch, with varying effects in the second epoch. Namely, those who performed task-specific (walking) training second demonstrated decreases in peak recumbent stepping power, whereas those who performed impairment-based interventions second continued to increase recumbent stepping power. However, there were no significant time X training X order effects for recumbent stepping power (p=0.18) or any other secondary measure. Intent-to-treat analyses and inclusion of only participants who finished all training revealed similar outcomes.
For secondary metabolic measures, there was a significant main effect of time only for VO2peak during treadmill exercise tests but not for recumbent stepping tests, with no significant interactions. There were also no time X training X order effects. Similar differences were observed for intent-to-treat analyses or utilizing only those participants who completed the full protocol.
There were no serious adverse events during or outside of either training intervention. Minor adverse events included 11 falls without significant injury outside of task-specific training vs 3 outside of impairment-based training, with subsequent discomfort in 4 incidences (all during task-specific training). There were 10 incidents of soreness/AFO-related abrasions during task-specific training and 4 during impairment-based training. Other minor events during task-specific training included one incident of hypertension and one incident of anxiety related to the perception of unsteadiness such that assistance was required. Another incident of nausea was observed during impairment-based training. Between-group χ2 revealed significantly greater cumulative incidence of minor adverse events during task-specific (n=23) vs impairment-based training (n=8; p<0.01). Specific differences included greater number of falls (p=0.03) but not soreness/discomfort (p=0.10) following task-specific training.
Correlation analyses
Correlation analyses were performed to compare differences in steps/sessions between training groups (e.g., differences in steps/sessions between interventions or Δsteps/session) to differences in outcomes during each training condition (e.g., changes in FS during task-specific training vs changes during impairment-based training or ΔFS). Fig 3A-B demonstrates the significant correlation between differences in ΔFS and Δ6MWT following task-specific vs impairment-based training and Δsteps/sessions during each training paradigm (both p<0.01). Differences in ΔSSS (p< 0.01) but not Δtreadmill speed (p=0.30) were significant (not shown).
Figure 3.
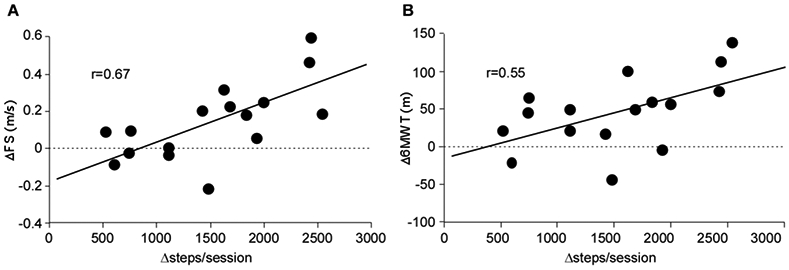
Correlations between differences in steps/sessions between training conditions (steps during task-specific minus steps during impairment-based) vs differences in changes (Δ) in selected outcome measures (i.e., ΔFS = changes in task-specific minus changes following impairment-based interventions); A) ΔFS, B) Δ6MWT (both p<0.05).
Discussion:
The present findings suggest that task-specific (stepping) training elicited greater gains in locomotor outcomes as compared to impairment-based interventions following chronic iSCI. Moderate correlations were observed between differences in changes in walking outcomes following task-specific vs impairment-based protocols and differences in steps/session. Conversely, changes in peak recumbent stepping power were greater following impairment-based training, during which recumbent stepping tasks were practiced. The combined findings suggest that the specificity of training interventions influences walking outcomes in individuals with iSCI.
The gains in locomotor measures presented here are consistent with previous studies evaluating the efficacy of high-intensity task-specific locomotor training in iSCI15-17. Specific changes with high-intensity training include mean gains in SSS (>0.05 m/s) and FS (>0.10 m/s) above small and substantial minimally clinically important differences for patients with neurological injury46. Consistent improvements in 6MWT (30-50 m) and peak treadmill speed (0.16-0.20 m/s) were also observed between these two studies. Similar gains have been observed following locomotor vs impairment-based strategies in other randomized trials recruiting patients post-stroke, although the intensities of conventional strategies were not well controlled35,47-49. Conversely, a previous multicenter trial comparing locomotor training to impairment-based exercises in patients 2-6 months post-stroke revealed no differences in functional outcomes, suggesting that large amounts of task-specific practice may not be critical50. However, neither intervention focused on achieving higher aerobic intensities and both were applied at 2 months post-stroke when the variability associated with natural recovery may mask the benefits of specific interventions. The combined data may suggest that task-specific training at higher intensities may be necessary to elicit substantial benefits, as compared to impairment-based training activities, or task-specific practice at lower intensities15. Whether these findings apply to patient early post-SCI are uncertain, given the lack of difference observed in patients post-stroke.
In the present study, the minimal gains in locomotor function following impairment-based training were surprising, particularly as compared to other selected previous studies using similar strategies19,23,24,26 and the focus of rehabilitation strategies used clinically18,20,22,51. More directly, there is substantial data indicating that impairments in balance, strength and aerobic capacity are associated with locomotor dysfunction following acute-onset neurological injury (e.g., 52,53) and therapists have hypothesized that targeting these impairments should improve walking function. However, the present findings suggest exercises directed towards these impairments result in small or inconsistent gains in gait speed or endurance. Importantly, this message in consistent with recent recommendations detailed in published clinical practice guidelines54. Discrepancies between these latter studies and reports that emphasize the potential benefits of impairment-based interventions20,22,24,26 may be due to the lack of adequate control interventions. Specifically, many impairment-based interventions are compared to strategies that elicit very small changes or no intervention. Few previous studies have evaluated the efficacy of impairment-based interventions to stepping training while attempting to control for other training parameters (time, frequency and intensity) that may influence study outcomes.
For most other non-walking assessments, the lack of differences between groups were expected given previous studies in participants with stroke or SCI suggesting other functional tasks (balance and transfers) are not compromised during stepping training15,55. However, only BBS appeared to improve over the entire course of training (main time effect) although these gains were small (1-3 pts). Differences between the present findings and previous studies in patients post-stroke demonstrating gains in balance and transfers following variable stepping practice55 may be due to the chronicity of the patient population tested here or the severity of motor impairments in iSCI (i.e., bilateral vs unilateral). The findings of differences in gains during recumbent stepping tests were, however, somewhat unexpected, given the focus on achieving higher cardiovascular intensities in both training protocols. Specifically, the gains achieved in recumbent stepping power during impairment-based training were consistent across the population, and in those who received task-specific training first. However, in participants who received task-specific training second, a decline in stepping power was observed, effectively minimizing any positive effects during impairment-based training performed initially. As such, the net gains in peak recumbent stepping power were small with walking training and significantly less than gains achieved with impairment-based strategies These observations potentially underscore the importance of specificity of neuromuscular exercise, where walking training appears to improve walking performance and recumbent stepping training may improve peak stepping power.
Importantly, the number of minor adverse events was greater during high-intensity task-specific training, including greater number of falls and a higher incidence of soreness. Increased muscle soreness and discomfort were expected in both groups during exercise activities attempting to achieve higher neuromuscular and cardiovascular demands. Greater eccentric muscle activity associated with stepping training may contribute to greater incidence of muscle soreness56, particularly in participants with low levels of daily physical activity. The increased incidence of falls was consistent with a previous study comparing stepping vs non-stepping exercise applied to patients early post-stroke50. However, these two findings are not consistent with other studies using similar training interventions. Potential reasons underlying increased fall incidence revealed here are not clear and may be related to increased fatigue or soreness post-training that interferes with performance of daily community activities. Alternatively, increased balance confidence (ABC scores) with stepping training without gains in postural stability (BBS) may result in greater attempts to perform community mobility tasks that lead to falls. Therapists must therefore consider the potential consequences of this intervention and educating patients on their potential fall-risk, regardless of perceived balance confidence. Regardless, therapists must weigh the potential consequences of adverse events compared to the outcomes achieved, and strategies are needed to ensure patients are aware of safety concerns throughout training.
Limitations of this study include the small sample size and lack of blinded assessors for the graded exercise testing, although both protocols utilized specific criteria for testing termination. The use of the cross-over design does increase the efficiency of the sample, although the potential for carry-over effects remains a concern for these designs in rehabilitation. However, our subsequent statistical analysis of only the initial training groups also revealed fairly consistent differences in selected outcomes, and the present data now allow for calculation of sample sizes for future studies that better address the comparative efficacy and generalizability of these findings. Another limitation is the lack of ability to control for training intensity as intended, particularly during practice of impairment-based balance tasks, and it is uncertain whether this limitation can be mitigated in future studies. Further the present study did not evaluate neuromuscular mechanisms underlying changes observed which has been done previously following the high- vs low-intensity in iSCI. Future studies will address potential biomechanical strategies that may account for differences in locomotor performance.
A final limitation is the accuracy of step counts during either intervention, where steps/sessions and steps/min may have overestimated actual walking practice during training. For example, while total training time during either intervention was limited to 40 min/session, average stepping time/session averaged 40 minutes, indicating stepping activity was detected during transitions between training activities (e.g., resting interval in which a participant would descend off treadmill and walk to stairwell). In addition, during impairment-based activities, the steps counted during transitions may have also contributed, although many activities that are not walking exercises (e.g., step-ups in place, knee flexion/extension exercises) may be registered as “steps” by the accelerometers. While the accuracy of the stepping monitor utilized here is the gold-standard for detecting stepping activity57, the findings raise questions regarding the accuracy of any activity monitor during non-walking tasks, which constitute a major portion of rehabilitation strategies.
Conclusions:
The present study delineates the effects of specificity of rehabilitation interventions on locomotor function in patients with motor iSCI, revealing greater walking improvements and balance confidence following stepping vs impairment-based practice. Gains in locomotor function were related to the amount of stepping practice achieved, although gains in peak power during recumbent stepping were shown following impairment-based training. However, an increase in the number of minor adverse events was observed with stepping training and therapists must educate patients on strategies to minimize these incidents. The present and previous results suggest that training specificity may be an important component of rehabilitation interventions, particularly at higher-intensities, and further work is needed to delineate potential mechanisms and generalizability of these gains.
Acknowledgements/Sources of Funding:
Funding was provided by the Indiana Brain and Spinal Cord Injury Research Foundation, the National Institute of Health R01-NS079751 and the National Institute of Disability and Rehabilitation Research- H133N110014.
Footnotes
Disclosures/Conflict of Interest: none
References
- 1.National Spinal Cord Injury Statistical Center: Facts and Figures at a Glance. Birmingham: University of Alabama at Birmingham;2018. [Google Scholar]
- 2.Ditunno PL, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal cord. 2008;46(7):500–506. [DOI] [PubMed] [Google Scholar]
- 3.van Middendorp JJ, Hosman AJ, Donders AR, et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet. 2011;377(9770):1004–1010. [DOI] [PubMed] [Google Scholar]
- 4.Hicks KE, Zhao Y, Fallah N, et al. A simplified clinical prediction rule for prognosticating independent walking after spinal cord injury: a prospective study from a Canadian multicenter spinal cord injury registry. The spine journal : official journal of the North American Spine Society. 2017;17(10):1383–1392. [DOI] [PubMed] [Google Scholar]
- 5.Huie JR, Morioka K, Haefeli J, Ferguson AR. What Is Being Trained? How Divergent Forms of Plasticity Compete To Shape Locomotor Recovery after Spinal Cord Injury. Journal of neurotrauma. 2017;34(10):1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. [DOI] [PubMed] [Google Scholar]
- 7.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. Journal of neurophysiology. 1998;79(3):1329–1340. [DOI] [PubMed] [Google Scholar]
- 8.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. Journal of neurophysiology. 1998;80(1):83–91. [DOI] [PubMed] [Google Scholar]
- 9.Barbeau H, Basso M, Behrman A, Harkema S. Treadmill training after spinal cord injury: good but not better. Neurology. 2006;67(10):1900–1901; author reply 1901-1902. [DOI] [PubMed] [Google Scholar]
- 10.Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66(4):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobkin B, Barbeau H, Deforge D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized spinal cord injury locomotor trial. Neurorehabilitation and neural repair. 2007;21(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Physical therapy. 2011;91(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexeeva N, Sames C, Jacobs PL, et al. Comparison of training methods to improve walking in persons with chronic spinal cord injury: a randomized clinical trial. The journal of spinal cord medicine. 2011;34(4):362–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JF, Musselman KE, Livingstone D, et al. Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabilitation and neural repair. 2014;28(4):314–324. [DOI] [PubMed] [Google Scholar]
- 15.Brazg G, Fahey M, Holleran CL, et al. Effects of Training Intensity on Locomotor Performance in Individuals With Chronic Spinal Cord Injury: A Randomized Crossover Study. Neurorehabilitation and neural repair. 2017;31(10-11):944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holleran CL, Hennessey PW, Leddy AL, et al. High-Intensity Variable Stepping Training in Patients With Motor Incomplete Spinal Cord Injury: A Case Series. Journal of neurologic physical therapy : JNPT. 2018;42(2):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leech KA, Kinnaird CR, Holleran CL, Kahn J, Hornby TG. Effects of Locomotor Exercise Intensity on Gait Performance in Individuals With Incomplete Spinal Cord Injury. Physical therapy. 2016;96(12):1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zbogar D, Eng JJ, Miller WC, Krassioukov AV, Verrier MC. Movement repetitions in physical and occupational therapy during spinal cord injury rehabilitation. Spinal cord. 2016. [DOI] [PMC free article] [PubMed]
- 19.Harness ET, Yozbatiran N, Cramer SC. Effects of intense exercise in chronic spinal cord injury. Spinal cord. 2008;46(11):733–737. [DOI] [PubMed] [Google Scholar]
- 20.Jones ML, Harness E, Denison P, Tefertiller C, Evans N, Larson CA. Activity-based Therapies in Spinal Cord Injury:: Clinical Focus and Empirical Evidence in Three Independent Programs. Top Spinal Cord Inj Rehabil. 2012;18(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones ML, Evans N, Tefertiller C, et al. Activity-based therapy for recovery of walking in chronic spinal cord injury: results from a secondary analysis to determine responsiveness to therapy. Archives of physical medicine and rehabilitation. 2014;95(12):2247–2252. [DOI] [PubMed] [Google Scholar]
- 22.Jones ML, Evans N, Tefertiller C, et al. Activity-based therapy for recovery of walking in individuals with chronic spinal cord injury: results from a randomized clinical trial. Archives of physical medicine and rehabilitation. 2014;95(12):2239–2246 e2232. [DOI] [PubMed] [Google Scholar]
- 23.Gregory CM, Bowden MG, Jayaraman A, et al. Resistance training and locomotor recovery after incomplete spinal cord injury: a case series. Spinal cord. 2007;45(7):522–530. [DOI] [PubMed] [Google Scholar]
- 24.Jayaraman A, Thompson CK, Rymer WZ, Hornby TG. Short-term maximal-intensity resistance training increases volitional function and strength in chronic incomplete spinal cord injury: a pilot study. Journal of neurologic physical therapy : JNPT. 2013;37(3):112–117. [DOI] [PubMed] [Google Scholar]
- 25.McLeod JC, Diana H, Hicks AL. Sprint interval training versus moderate-intensity continuous training during inpatient rehabilitation after spinal cord injury: a randomized trial. Spinal cord. 2019. [DOI] [PubMed]
- 26.DiPiro ND, Embry AE, Fritz SL, Middleton A, Krause JS, Gregory CM. Effects of aerobic exercise training on fitness and walking-related outcomes in ambulatory individuals with chronic incomplete spinal cord injury. Spinal cord. 2016;54(9):675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page SJ, Levine P, Strayer J. An electric stimulation cycling protocol for gait in incomplete spinal cord injury. Archives of physical medicine and rehabilitation. 2007;88(6):798–800. [DOI] [PubMed] [Google Scholar]
- 28.Gant KL, Nagle KG, Cowan RE, et al. Body System Effects of a Multi-Modal Training Program Targeting Chronic, Motor Complete Thoracic Spinal Cord Injury. Journal of neurotrauma. 2018;35(3):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilutti LA, Hicks AL. Rehabilitation of ambulatory limitations. Phys Med Rehabil Clin N Am. 2013;24(2):277–290. [DOI] [PubMed] [Google Scholar]
- 30.Umphred DA, Lazaro RT, Roller M and Burton G Neurological Rehabilitation. 6th ed. Maryland Heights, MO: Mosby; 2012. [Google Scholar]
- 31.O'Sullivan SB, Schmitz TJ, and Fulk G . Physical Rehabilitation. 6th ed. Philadelphia, PA: F.A. Davis; 2013. [Google Scholar]
- 32.Schmidt R, Lee T. Motor Control and Learning: A Behavioral Emphasis. Third ed. Champaign, IL: Human Kinetics Inc; 1999. [Google Scholar]
- 33.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–239. [DOI] [PubMed] [Google Scholar]
- 34.Hornby TG, Holleran CL, Hennessy PW, et al. Variable Intensive Early Walking Poststroke (VIEWS): A Randomized Controlled Trial. Neurorehabilitation and neural repair. 2016;30(5):440–450. [DOI] [PubMed] [Google Scholar]
- 35.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a "plateau" in recovery. Stroke; a journal of cerebral circulation. 2010;41(1):129–135. [DOI] [PubMed] [Google Scholar]
- 36.Frontera WR, Bean JF, Damiano D, et al. Rehabilitation Research at the National Institutes of Health. Neurorehabilitation and neural repair. 2017;31(4):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borg G Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. International journal of sports medicine. 1982;3(3):153–158. [DOI] [PubMed] [Google Scholar]
- 38.Borg GA. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 39.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilitation and neural repair. 2014;28(7):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hornby TG, Henderson CE, Plawecki A, et al. Contributions of Stepping Intensity and Variability to Mobility in Individuals Poststroke. Stroke; a journal of cerebral circulation. 2019;50(9):2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornby TG, Lewek MD, Thompson CK, Heitz R. Repeated Maximal Volitional Effort Contractions in Human Spinal Cord Injury: Initial Torque Increases and Reduced Fatigue. Neurorehabilitation and neural repair. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leech KA, Hornby TG. High-Intensity Locomotor Exercise Increases Brain-Derived Neurotrophic Factor in Individuals with Incomplete Spinal Cord Injury. Journal of neurotrauma. 2017;34(6):1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pescatello LS, ed American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 9th ed: Wolters Kluwer/Lippincott, Williams and Wilkins; 2014. [Google Scholar]
- 44.Billinger SA, VANS E, McClain M, Lentz AA, Good MB. Recumbent stepper submaximal exercise test to predict peak oxygen uptake. Medicine and science in sports and exercise. 2012;44(8):1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eynon N, Sagiv M, Amir O, Ben-Sira D, Goldhammer E, Amir R. The effect of long-term beta-adrenergic receptor blockade on the oxygen delivery and extraction relationship in patients with coronary artery disease. Journal of cardiopulmonary rehabilitation and prevention. 2008;28(3):189–194. [DOI] [PubMed] [Google Scholar]
- 46.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006;54(5):743–749. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Physical therapy. 2007;87(12):1580–1602; discussion 1603-1587. [DOI] [PubMed] [Google Scholar]
- 48.Hesse S, Bertelt C, Jahnke MT, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke; a journal of cerebral circulation. 1995;26(6):976–981. [DOI] [PubMed] [Google Scholar]
- 49.Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on 'rules of spinal locomotion' is effective in spinal cord injured persons. The European journal of neuroscience. 1995;7(4):823–829. [DOI] [PubMed] [Google Scholar]
- 50.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. The New England journal of medicine. 2011;364(21):2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Archives of physical medicine and rehabilitation. 2009;90(10):1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saraf P, Rafferty MR, Moore JL, et al. Daily stepping in individuals with motor incomplete spinal cord injury. Physical therapy. 2010;90(2):224–235. [DOI] [PubMed] [Google Scholar]
- 53.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Archives of physical medicine and rehabilitation. 2007;88(1):115–119. [DOI] [PubMed] [Google Scholar]
- 54.Hornby TG, Reisman DS, Ward IG, et al. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. Journal of neurologic physical therapy : JNPT. 2020;44(1):49–100. [DOI] [PubMed] [Google Scholar]
- 55.Straube DD, Holleran CL, Kinnaird CR, Leddy AL, Hennessy PW, Hornby TG. Effects of dynamic stepping training on nonlocomotor tasks in individuals poststroke. Physical therapy. 2014;94(7):921–933. [DOI] [PubMed] [Google Scholar]
- 56.Vila-Cha C, Hassanlouei H, Farina D, Falla D. Eccentric exercise and delayed onset muscle soreness of the quadriceps induce adjustments in agonist-antagonist activity, which are dependent on the motor task. Experimental brain research. 2012;216(3):385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Physical therapy. 2014;94(2):222–229. [DOI] [PubMed] [Google Scholar]


