Abstract
Background
Hypoxia-ischemia (HI) is the most common cause of brain injury in newborns and the survivors often develop cognitive and sensorimotor disabilities that undermine the quality of life. In the current study, we examined the effectiveness of flupirtine, a potassium channel opener, shown previously in an animal model to have strong anti-neonatal-seizure efficacy, to provide neuroprotection and alleviate later-life disabilities caused by neonatal hypoxic-ischemic injury.
Methods
The rats were treated with a single dose of flupirtine for four days following HI induction in 7-day-old rats. The first dose of flupirtine was given after the induction of HI and during the reperfusion period. The effect of treatment was examined on acute and chronic brain injury, motor functions, and cognitive abilities.
Results
Flupirtine treatment significantly reduced HI-induced hippocampal and cortical tissue loss at acute time-point. Furthermore, at chronic time-point, flupirtine reduced contralateral hippocampal volume loss and partially reversed learning and memory impairments, but failed to improve motor deficits.
Conclusion
The flupirtine treatment regimen used in the current study significantly reduced brain injury at acute time-point in an animal model of neonatal hypoxic-ischemic encephalopathy. However, these neuroprotective effects were not persistent and only modest improvement in functional outcomes were observed at chronic time-points.
Introduction
Hypoxia-ischemia (HI) is the major cause of brain injury and mortality in newborns (1). The resultant hypoxic-ischemic encephalopathy (HIE) is often associated with sensorimotor dysfunctions, cognitive deficits, and epilepsy in later life (2). Children with moderate HIE show delay in school readiness when compared with age-matched control group (3) and over 80% of adolescents with moderate hypoxic-ischemic injury at birth show cognitive deficits (4). As a result, many children with neonatal HIE require lifetime care and support, which puts a tremendous emotional and economic burden on the individual, family, and society (5).
HI is the most common cause of neonatal seizures (6). Both, animal and clinical research suggest than neonatal seizures may worsen the brain injury and contribute to adverse neurologic outcomes (7, 8). Unfortunately, the drugs currently used to treat neonatal seizures are not effective in large number of patients and have undesired side-effects (9–11). We recently showed that flupirtine (ethyl-N-[2-amino-6-(4-fluorophenylmethyl-amino)pyridin-3-yl] carbamate) is more efficacious than phenobarbital, the current drug of choice, in treating neonatal seizures in an animal model of hypoxic-ischemic encephalopathy (12). Flupirtine mediates its action mainly by modulating KCNQ channels and has been used as an analgesic in Europe for more than 30 years (13). KNCQ channels are voltage gated, depolarization activated potassium channels, and flupirtine action shifts the voltage required to open these channels towards a more negative potential (14–17). In addition, flupirtine has been shown to activate G-protein-regulated inwardly rectifying K+ channels (GIRK) (18) (but also see (15)), and to shift the gating of GABAA receptors to lower GABA concentrations (15). As a result of these actions, flupirtine has been shown to provide protection after ischemia-induced brain and retinal injury, and it has also been shown to improve cognitive deficits caused by ischemia, repetitive febrile seizures, and prolong restrain stress in adult rats (19–23). Therefore, in the current study, we used a rat model of neonatal HIE to examine the effects of treating the pups after HI-induced brain injury and analyzed its effects on the size of the lesion, neuronal death, sensorimotor dysfunction, and learning and memory.
Methods
An overview of the experimental design is provided in Table 1.
Table 1.
Overview of experimental design
| Test and age at testing | Number of rats in each group | Statistical test used for comparison |
|---|---|---|
| Cresyl violet staining P10 (3d after HI-induction) |
HI+V = 5 HI+F = 6 |
Unpaired t-test |
| MRI P34–49 (27–42d after HI-induction) |
HI+V = 15 HI+F =15 CTL = 6 |
Kruskal-Wallis test followed by Dunn’s multiple comparison post-hoc test |
| Grip Strength test P47–49 (40–42d after HI-induction) |
HI+V = 12 HI+F =12 Sham+V = 9 Sham+F = 8 |
One-way ANOVA test followed by Tukey’s multiple comparison post-hoc test Spearman test to measure correlation between the ipsilateral cortical volume and contralateral forepaw grip strength |
| Morris water maze (MWM) test P118–138 (111–131d after HI-induction) |
HI+V = 18 HI+F =12 Sham+V = 9 Sham+F = 8 |
Two-way repeated measures ANOVA test followed by Tukey’s multiple comparison post-hoc test to evaluate performance during trials Factorial ANOVA to evaluate performance during probe test Pearson test to measure correlation between the ipsilateral hippocampal volume as measured by MRI and the average distance travelled to reach the platform |
Animals
Animal experiments were performed in accordance with the NIH guidelines for the care and use of laboratory animals, and according to the protocol approved by the Institutional Animal Care and use Committee (IACUC) of the University of Colorado Anschutz Medical Campus (UC-AMC). Also, all efforts were made to reduce animal suffering and the number of animals used. Timed pregnant Sprague-Dawley rats were received at gestational day 14 from Charles River Laboratories. Since males are more severely affected by HI and have worse neurologic outcomes than females with hypoxic-ischemic injury (24), in our earlier study, the results of which formed the foundation for the current study, the efficacy of flupirtine to treat HI-induced neonatal seizures was examined in male pups (12). Hence, all the experiments in the current study were performed on male pups only.
Hypoxia-ischemia protocol
HI was induced in postnatal day 7 (P7) rats by Rice-Vannucci method described in detail in our previous publication (25). Briefly, the right common carotid artery of the pups was double ligated with a 4–0 polyglycolic acid suture under isoflurane anesthesia and after 2 hours of recovery time with the dam, exposed to hypoxic environment (oxygen concentration: 8 – 8.3%, temperature: 36.50 Celsius, humidity: 60 to 70%) for 2 hours. The pups were treated with analgesic (0.1 mg/kg buprenorphine hydrochloride) once every 12 hours for 48 hours and housed with the dam until weaning following HI-induction. The sham group pups were treated exactly like HI pups except that their carotid artery was identified, but not ligated, and were not exposed to hypoxia.
Drug treatment
All drugs were administered to the pups via intraperitoneal injection. The pups were selected randomly for the vehicle (DMSO) or the experimental drug (Flupirtine maleate) treatment on the day of the experiment, and whenever possible, pups from each litter were divided equally among the treatment groups. Additionally, because the study was conducted in multiple batches, each treatment group consisted of pups from multiple litters. The pups were administered a single dose of 25 mg/kg flupirtine maleate (Sigma, St. Louis, MO) or an equivalent volume of vehicle every 24 hours for 4 days after HI induction (total 4 doses). To replicate treatment protocol in the clinical practice, the first dose was given five minutes after the pups were reintroduced to room air following 2 hours of hypoxia, i.e., 5 minutes in the reperfusion period (26). The drug was freshly prepared on the day of use (12 mg flupirtine maleate dissolved in 1 ml of DMSO). This dose of flupirtine and the treatment regimen was selected as it was observed to significantly reduce the seizure burden during the acute phase of the hypoxic-ischemic injury (12).
Histology
To determine the effect of flupirtine treatment on HI-induced brain injury at an acute time-point, the brain sections were stained with cresyl violet. Two to three hours after the 4th dose of flupirtine was administered, the pups were anesthetized and perfused transcardially with cold phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde. Brains were removed and postfixed overnight at 4o C in 4% paraformaldehyde. Brains were then cut into 15 μm thick coronal sections with cryostat and three brain sections at the dorsal hippocampus level that were 150 μm apart were stained with cresyl violet using a protocol that is routinely used in our laboratories (27). Briefly, brain sections were first incubated in increasing concentration of ethanol to remove lipids. The sections were then rehydrated and incubated in a 0.1% solution of cresyl violet for 10 min. Following the incubation, the excess stain was removed using a clearing agent and sections were then air-dried and coverslipped using Permount (Fisher Scientific, Pittsburg, PA). Brain sections of the vehicle and the drug treated rats were stained in parallel, and the investigator performing the count was blinded to the experimental conditions. To determine the tissue loss, the cresyl violet stained whole brain section image was captured using the Aperio ImageScope microscope system (Leica Biosystems, Buffalo Grove, IL) at 20X magnification. To determine the tissue loss, the area of the remaining ipsilateral (to ligated carotid artery) and contralateral hippocampus and cortex in the brain sections were measured using the ImageJ program. The tissue loss was calculated using the following equation: [100 – ratio (ipsilateral/contralateral) x 100].
MRI
To examine HI-induced brain injury, 27–42 days after HI induction, animals were anesthetized with 2% isoflurane, and imaged once with a 4.7-T MRI scanner (PharmaScan, Bruker BioSpin, Billerica, MA). T2-weighted images were acquired using the following parameters: field of view (FOV) 3.2 cm; slice thickness 1.2 mm with no gap between slices; 356X256 matrix size with spatial resolution of 180 μm/pixel; repetition time/echo time (TR/TE) = 4000/80ms; 8 repetitions. Using ImageJ program volume of right (side ipsilateral to ligated carotid artery) and left (contralateral side) cortex and the hippocampus that included subiculum (it was not possible to accurately differentiate the boundary between subiculum and the hippocampus at the current resolution) was calculated by an investigator who was blinded to the treatment and experimental conditions. Analysis was performed by applying region of interests (ROI) on each anatomical slice, summing all ROI areas (in mm2) and multiplying by slice thickness.
Behavioral testing
Forelimb grip strength test
The forelimb grip strength test was used to evaluate motor function and deficit following neonatal HI. Grip strength of left forelimb (contralateral side of the ligated carotid artery) was measured using a Grip Strength meter (Ugo Basile, Italy) 40–42 days after induction of HI at P7. The rats were gently restrained by the scruff of the dorsal neck with one hand, and another hand holding the base of its tail. The animal’s forepaw was placed on a wire grid and once the animal gripped the wire its tail was gently pulled backwards and the maximum strength of the grip prior to grip release was recorded. The animal was tested 6 times and the results were averaged.
Morris water maze test
The rats were subjected to a 6-day Morris Water Maze (MWM) test 111–131 days after the induction of HI at P7 to identify possible HI-induced cognitive deficits. Briefly, the rats were introduced to a black water tank, where they had to find a submerged platform using cues placed around the room’s walls. The rats underwent four trials per day for 5 days, and the latency and the mean distance traveled to reach the platform was measured. Twenty-four hours after the last trial, a 2 minute probe test was conducted with the platform removed and the time and distance each rat swam in the former platform quadrant were recorded and analyzed.
Statistical analysis
GraphPad Prism statistical software (GraphPad Software Inc., San Diego, CA) was used for statistical analysis. Differences were considered significant at p < 0.05. The results are presented as the mean ± standard error of means (SEM).
Results
Effect of flupirtine treatment on HI-induced acute brain injury
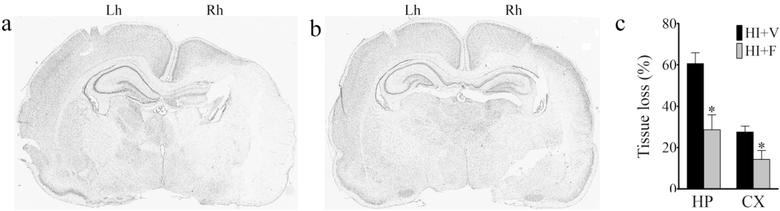
Flupirtine treatment significantly reduced HI-induced hippocampal and cortical tissue loss (Fig. 1). Compared to a 60.66% hippocampal tissue loss in the vehicle treated HI rats, flupirtine treated rats only lost 28.71% hippocampus tissue area (P = 0.007, unpaired t- test). Similarly, compared to 27.54% cortical tissue loss in the vehicle treated HI rats, flupirtine treated rats only lost 14.4% cortical tissue area (P = 0.037, unpaired t- test).
Fig. 1. Effect of flupirtine treatment on neonatal HI-induced acute hippocampal and cortical injury.
Representative cresyl violet stained brain sections of HI rat treated with (a) vehicle, and (b) flupirtine sacrificed 3 days after HI-induction at P7. The ipsilateral side of the ligated carotid artery (Rh = right hemisphere) shows severe damage to hippocampus and cortex when compared to the contralateral side (Lh = left hemisphere). Flupirtine treatment significantly reduced the tissue loss as depicted by the preservation of tissue architecture. (c) Tissue loss represented as % ratio of ipsilateral and contralateral hippocampal and cortical area. N = 5 for HI+V and 6 for HI+F group; *p < 0.05, unpaired t- test.
Effect of flupirtine treatment on HI-induced chronic brain injury
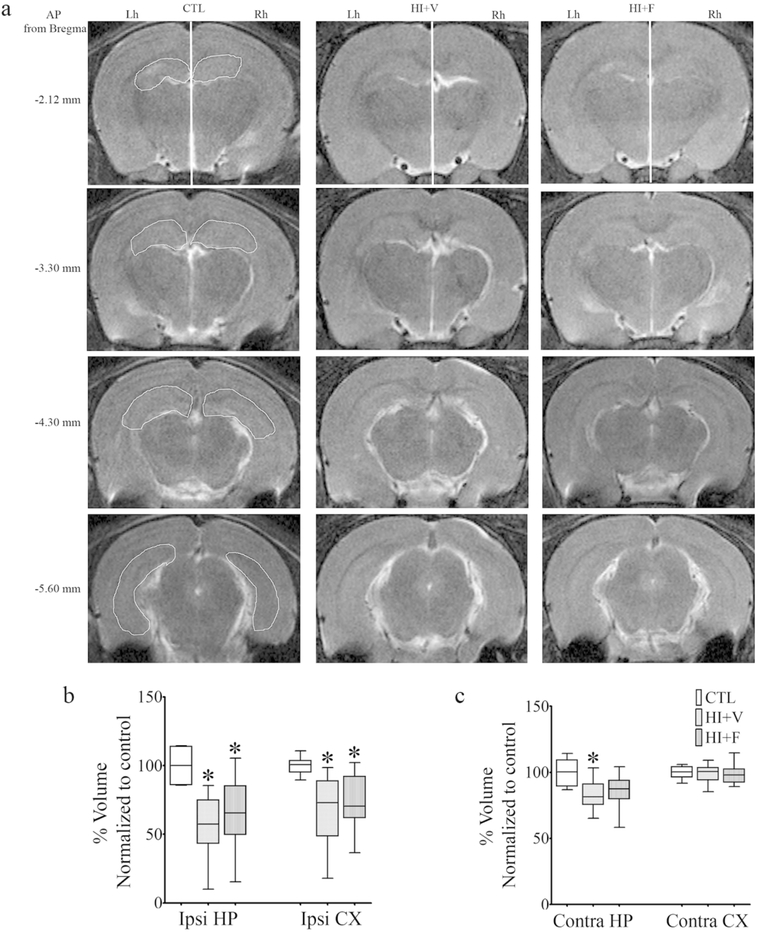
MRI was conducted approximately one month after HI and revealed a visibly smaller right hemisphere (ipsilateral side to ligated carotid artery) in HI rats (Fig. 2a) in addition to a reduction in ipsilateral and contralateral hippocampal volume (Fig. 2b, c) and ipsilateral cortical volume (Fig. 2b), suggesting that the acute injury evolves into a chronic injury. A significant reduction in ipsilateral hippocampal and cortical volume was observed in both vehicle and flupirtine treated HI rats when compared to naive adult control rats (Fig. 2b; Kruskal-Wallis test, p < 0.005, followed by Dunn’s multiple comparison post-hoc test, p < 0.05). Further, a significant reduction in contralateral hippocampal volume was also observed in HI rats treated with vehicle (Kruskal-Wallis test, p < 0.02, followed by Dunn’s multiple comparison post-hoc test, p < 0.05), however, it was no longer significant in rats treated with flupirtine when compared to control rats (Fig. 2c). No observable reduction in the contralateral cortex was found in either vehicle or flupirtine treated HI rats.
Fig. 2. Effect of flupirtine treatment on chronic brain injury caused by neonatal HI.
(a) Representative MRI scans from naive control (CTL), HI rats treated with the vehicle (HI+V), and HI rats treated with flupirtine (HI+F) at comparable anterior to posterior (AP) coordinates from bregma from Paxinos and Watson rat brain atlas (4th Edition) shown on the left of the scans. The white line in the top three scans divides the brain in the left hemisphere (Lh, contralateral side of the ligated carotid artery) and the right hemisphere (Rh, ipsilateral side). A visually noticeable smaller ipsilateral hemisphere compared to the contralateral hemisphere of HI rats and Rh and Lh of control rat can be seen in scans of HI rats. A representative manually drawn outline used to determine the volume of the hippocampus is also shown in the scans of control rats. Volume of (b) ipsilateral (ipsi) and (c) contralateral (contra) hippocampus (HP) and cortex (CX) in CTL (n = 6), HI+V (n = 15), and HI+F (n = 15) rats. *Statistically different from the CTL group; *p < 0.05, Kruskal-Wallis test followed by Dunn’s multiple comparison post-hoc test.
Effect of flupirtine treatment on HI-induced motor function deficits
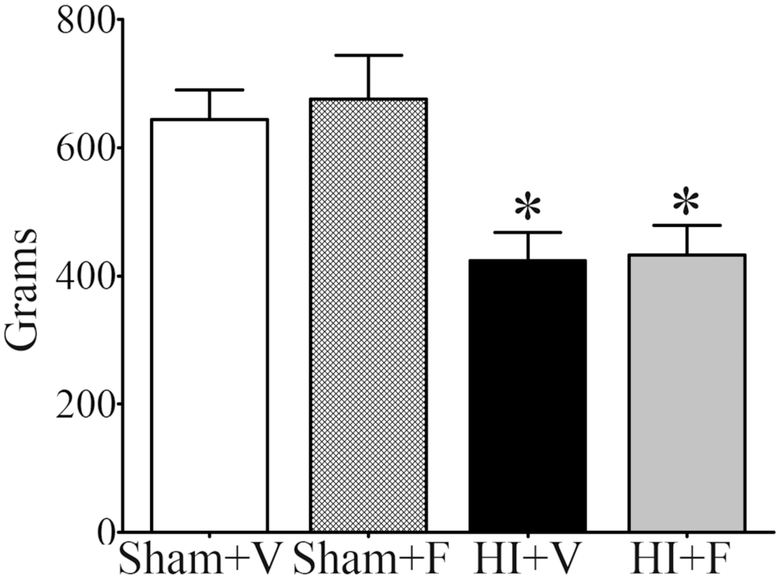
A significant reduction was detected in the test for contralateral forepaw grip strength in adult rats exposed to HI at P7 and treated with vehicle when compared to sham rats treated with either vehicle or flupirtine, and repeated flupirtine treatment failed to improve strength (Fig. 3; One-way ANOVA, F3, 37 = 6.87, p = 0.0009, followed by Tukey’s multiple comparison post-hoc test, p < 0.05). Sham rats treated with flupirtine had similar contralateral forepaw strength as the sham rats treated with the vehicle, which suggests that flupirtine itself does not negatively impact motor development (Fig. 3). A significant positive correlation was observed between the loss of ipsilateral cortical volume as measured by MRI and reduction in grip strength of the contralateral forepaw in HI rats treated with the vehicle (Spearman r = 0.57, p = 0.03, n=12).
Fig. 3. Effect of neonatal HI and flupirtine treatment on motor functions in adulthood.
Histograms show measurements of grip strength of the contralateral forepaw in adult rats exposed to either HI at P7 and treated either with the vehicle (HI+V, n = 12) or flupirtine (HI+F, n = 12), or sham treatment and treated either with the vehicle (Sham+V, n = 9) or flupirtine (Sham+F, n = 8). *Statistically different from Sham+V and Sham+F group; *p < 0.05, One-way ANOVA test followed by Tukey’s multiple comparison post-hoc test.
Effect of flupirtine treatment on HI-induced cognitive deficits
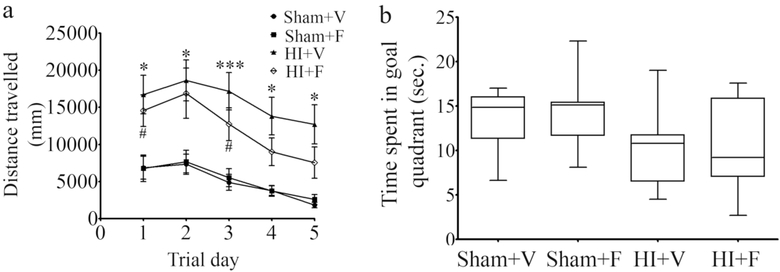
Our MRI results (Fig. 2), and studies from various labs suggest that neonatal HI injures hippocampus (28, 29). The MWM test was selected to determine the effect of neonatal HI on cognition in later life because it is considered a robust and reliable test to identify cognitive deficits associated with hippocampal dysfunction (30). Testing of adult rats subjected to HI at P7 on the MWM revealed spatial learning deficits in these rats (Fig. 4a). Specifically, vehicle treated HI rats significantly travelled longer distance and took more time to find the platform on all 5 days of trial (p < 0.05, Tukey’s multiple comparison post-hoc test) compared to sham rats treated with either vehicle or flupirtine, whereas, the performance of flupirtine treated HI rats was not significantly different from sham rats treated with flupirtine on all 5 days and sham rats treated with vehicle on day 2, 4, and 5 of trials (p < 0.05, Tukey’s multiple comparison post-hoc test; Fig. 4a). This suggests that flupirtine, at least partially, reverses some of the learning impairments caused by HI (Fig. 4a; Two-way Repeated Measures ANOVA, Time effect: F3, 133 = 10.1, p < 0.0001, Treatment effect: F3, 43 = 7.3, p = 0.0005, Time x Treatment interaction: F12, 172 = 0.3, p = 0.98). The performance of sham rats treated with flupirtine on all trial day was very similar to sham rats treated with vehicle, suggesting that flupirtine itself has no negative effect on learning and memory of rats (Fig. 4a). For the probe test, conducted 24 hours after the last trial and which measures robustness of reference memory, factorial ANOVA revealed a significant effect of the injury (F1, 43 = 8.37, p = 0.006) on the amount of time spent in the goal quadrant during the first 30 seconds of the probe test suggesting that the injury significantly reduces the strength of memory (Fig. 4b). On an average, 33% of flupirtine treated HI rats spent equal or more time than the average time spent by sham rats in the goal quadrant. In contrast, only 16% of vehicle treated HI rats spent time in the goal quadrant that was equal or more than the average time spent by sham rats. However, statistically, a significant interaction between the treatment and injury was not observed. Similarly, a significant effect of injury was observed on the distance taken by the rats to reach the platform location (path efficiency) in the probe test (Factorial ANOVA, F1, 44 = 8.86, p = 0.005; Fig. 5a). On an average flupirtine treated HI rats traveled shorter distance (348 cm) than vehicle treated HI rats (563 cm), but the difference did not reach statistical significance. A significant negative correlation was observed between the ipsilateral hippocampal volume as measured by MRI and the average distance travelled to reach the platform on the first four days of trial in HI rats treated with the vehicle (Pearson r = −0.64, p = 0.02; n=12). Similarly, a significant negative correlation was also observed between the ipsilateral hippocampal volume of HI rats and the path efficiency in the probe test (Pearson r = −0.60, p = 0.04; n=12). Both treatment HI groups of rats contained some rats that performed very badly, but, interestingly, we observed that among the well performing HI rats, flupirtine treated HI rats took a more direct route to reach the platform location than the vehicle treated HI rats during the probe test (Fig. 5b).
Fig. 4. Effect of neonatal HI and flupirtine treatment on learning and memory in adulthood.
(a) The line diagram shows the mean distance travelled by adult rats subjected to neonatal HI and treated with either vehicle (HI+V, n = 18, the data from treated (n = 12) and non-treated (n = 6) HI rats was combined as the values were statistically not significantly different) or flupirtine (HI+F, n = 12) and sham treatment and treated with either vehicle (Sham+V, n= 9) or flupirtine (Sham+F, n = 8) rats to find the hidden platform using spatial cues on trial days. * represents a statistically significant difference for HI+V group when compared to Sham+V and Sham+F group and # represents a statistically significant difference for HI+F when compared to Sham+V group. ***p < 0.0005, **p < 0.005, *, # p < 0.05, Two-way ANOVA with Repeated Measures test followed by Tukey’s multiple comparison post-hoc test. (b) The box and whiskers plot show amount of time spent by rats in the goal quadrant during the probe test. A significant overall effect of injury on time spent in the goal quadrant was observed between the groups (Factorial ANOVA, p = 0.006). For the probe test n for HI+V = 18, HI+F = 12, Sham+V = 9, and Sham+F, n = 8.
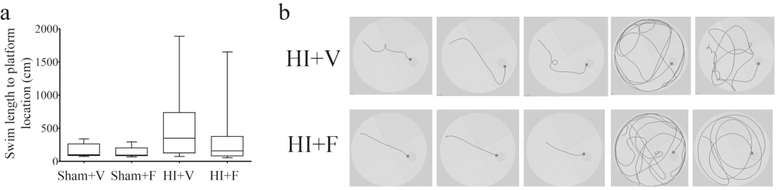
Fig. 5. Effect of neonatal HI and flupirtine treatment on memory in adulthood.
(a) The box and whiskers plot show the distance travelled by rats to reach the platform location during the probe test. A significant overall effect of injury on path efficiency was observed between the groups (Factorial ANOVA, p = 0.005). (b) The representative picture shows the route taken by 3 best performing rats and 2 worst performing rats to reach the platform location (black square). The best performing flupirtine treated HI rats took direct and shortest route to reach the platform location. N for HI+V = 18, HI+F = 12, Sham+F = 8, and Sham+V = 9.
Discussions
The key findings of the current study are that the administration of flupirtine at a clinically relevant time point reduces acute hippocampal and cortical injury caused by a hypoxic-ischemic event in neonatal rats. Moreover, flupirtine given during this critical period of development did not negatively impact motor and cognitive functions in rats. Furthermore, the finding reported here suggests that flupirtine could be used to promote an improvement in the chronic brain injury and cognitive deficits.
The treatment was initiated after a prolonged hypoxic-ischemic episode and during the reperfusion period in an animal model that replicates the etiology of a human disorder and the acute clinical symptoms, including seizures, and long-term neurologic outcome such as motor and cognitive deficits. Thus, the current study was designed to closely replicate clinical settings. The flupirtine dose and treatment duration was selected based on our earlier study in which it significantly reduced acute seizure burden (12). Studies in animal models and clinical research findings suggest that neonatal seizures contribute to brain injury and adverse neurologic outcome (7, 8). A reduction in hippocampal and cortical tissue loss at an acute time point, and partial and modest improvement in chronic brain injury and cognitive deficits in HI rats treated with flupirtine supports these earlier observations. However, based on the current experimental design, it is not possible to determine whether these protective effects of flupirtine are because of reduced seizure burden or independent of its effect on seizures.
The morphology studies suggest that the brain regions that show injury at the acute time point have an evolution of the injury at the chronic time point. Flupirtine given after HI-induction significantly reduced hippocampal and cortical tissue loss at an acute time-point. However, this neuroprotective effect of flupirtine did not sustain for longer time. Flupirtine was ineffective in significantly reducing the size of ipsilateral hippocampal and cortical injury and caused only small reduction in contralateral hippocampal injury at the chronic time-point. It is possible that the dosing regimen used in the current study prolongs the life of injured cell but is unable to prevent its loss.
The result of the MWM test suggests that flupirtine treatment partially improved the ability to learn the task and remember the learned task. Further, a direct correlation between the size of hippocampal injury and robustness of learning and memory was observed. Both, vehicle and flupirtine treated group of rats contained some rats with profound injury that performed badly, however, within those rats that performed well and took the shorter route to the platform location in probe test, the flupirtine treated HI rats appear to take more direct and shorter route (Fig. 5b). This suggests that may be at the current treatment regimen flupirtine improved cognitive abilities of those rats that relatively had moderate and less severe injury and not of those rats with profound injury. The partial and smaller improvements in long-term outcomes could also be related to the dosing frequency and the duration of treatment. According to our recent study, the half-life of flupirtine, determined using a highly sensitive LC-MS method, is 4.9 hours in cortex and hippocampus and 3.6 hours in serum of neonatal rats (31). Based on the half-life of flupirtine in the brain and the efficacy of flupirtine to reduce HI-induced seizures (12), flupirtine treated rats have approximately 75% less seizures over four days of treatment. It is possible that 75% reduction in seizure burden is not sufficient to significantly improve long-term neurologic outcome, specifically in rats with severe injury. Further, since in the majority of the animals in our HI model, the seizures are observed until 72 hours after HI, we treated the rats only for four days (12, 25). It is possible that the longer treatment may have provided more larger and complete effect. In a rat model of repetitive febrile seizures, a single dose of flupirtine (similar to the one we used in this study) given every day for 8 days reduced seizure burden, decreased brain injury, and improved cognitive deficits (22). In an another study, 25 mg/kg flupirtine given before restrain stress for 21 days prevented spatial learning impairment in the MWM test and reduced hippocampal neuronal death induced by repetitive stress in adult mice (23).
The majority of currently available antiepileptic drugs have been shown to cause neuronal death and behavioral impairments in animals when given during the critical periods of brain development (10, 32). Phenobarbital, the current first-choice drug for the treatment of neonatal seizures, has been associated with lower IQ in children (32). Repeated doses of phenobarbital, phenytoin, and lamotrigine given during early developmental period caused spatial learning deficits during the adulthood (33). Retigabine, an analogue of flupirtine, cause blue discoloration and vision impairment in humans, however, retigabine treatment of normal neonatal rats did not impair learning and memory in the later life (34). Similarly, treatment of rats with flupirtine during the critical developmental period did not adversely affect the development of motor and cognitive functions. Flupirtine has previously been used in children (13, 35). The most common side-effects of flupirtine are dizziness, dry mouth, nausea, fatigue, and heartburn (35). In a case study of an adult patient with lower back pain, ingestion of very high dose of flupirtine (10 times the therapeutic dose for lower back treatment) was associated with the appearance of sharp waves and sharp slow-wave complexes in the EEG (36). In recent years, however, long-term use of flupirtine has been shown to cause hepatotoxicity. Seven deaths have been reported related to flupirtinecaused liver injury following more than 17 million average daily prescriptions (37, 38). Further, on an average, the liver injury was observed after 4 months of treatment (37). Our study in an animal model and clinical studies suggest that seizures resolve within 4–5 days of onset following neonatal hypoxia-ischemia (25, 39), therefore, is less likely that flupirtine will be used as antiseizure medication in neonates for a prolonged period of time. However, efforts are underway to develop more potent analogues of flupirtine that are devoid of such side-effects (40).
Taken together, the current study suggests that flupirtine can reduce brain injury. However, better drug regimen and improved drug delivery such as slow release may more effectively alleviate the long-term neurologic deficits.
Acknowledgement
We thank the University of Colorado Anschutz Medical Campus Behavior and In Vivo Neurophysiology Core (BINC), Animal Imaging Core, and the Biorepository Core for providing facilities and support to perform behavior tests, MRI, and brain imaging.
Financial support
This work was supported by the NIH/NICHD HD065534 grant and Citizens United for Research in Epilepsy (CURE) Prevention of Acquired Epilepsy award to Yogendra H. Raol and NIH/NINDS NS089698 grant to Marco I. Gonzalez. Michael J. Diaz was supported by the University of Colorado GEMS program (which is supported by the NIH grant 5R25HL103286-10). The funders had no role in study design, data collection and analysis, in the writing of the manuscript, and in the decision to submit the article for publication.
Statement of financial support
This work was supported by the NIH/NICHD HD065534 grant and Citizens United for Research in Epilepsy (CURE) Prevention of Acquired Epilepsy award to Yogendra H. Raol and NIH/NINDS NS089698 grant to Marco I. Gonzalez. Michael J. Diaz was supported by University of Colorado GEMS program (which is supported by the NIH grant 5R25HL103286-10). The funders had no role in study design, data collection and analysis, in the writing of the manuscript, and in the decision to submit the article for publication.
Footnotes
Disclosure of conflicts of interest
None of the authors have any conflict of interest to disclose.
Category of study
Basic science
References
- 1.Ferriero DM. Neonatal brain injury. N Engl J Med 2004;351:1985–95. [DOI] [PubMed] [Google Scholar]
- 2.Mwaniki MK, Atieno M, Lawn JE, et al. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 2012;379:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson CM, Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr Child Health 2006;11:278–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Lindstrom K, Hallberg B, Blennow M, et al. Moderate neonatal encephalopathy: pre- and perinatal risk factors and long-term outcome. Acta Obstet Gynecol Scand 2008;87:503–09. [DOI] [PubMed] [Google Scholar]
- 5.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res 2013;74:50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronen GM, Buckley D, Penney S, et al. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology 2007;69:1816–22. [DOI] [PubMed] [Google Scholar]
- 7.Wirrell EC, Armstrong EA, Osman LD, et al. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr Res 2001;50:445–54. [DOI] [PubMed] [Google Scholar]
- 8.Glass HC, Glidden D, Jeremy RJ, et al. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr 2009;155:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster M, Lewis A. The treatment of neonatal seizures: a critical review of the evidence. Neonatal Paediatr Child Health Nurs 2007;10:11–19. [Google Scholar]
- 10.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A 2002;99:15089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raol YH, Zhang G, Budreck EC, et al. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience 2005;132:399–407. [DOI] [PubMed] [Google Scholar]
- 12.Sampath D, Valdez R, White AM, et al. Anticonvulsant effect of flupirtine in an animal model of neonatal hypoxic-ischemic encephalopathy. Neuropharmacology 2017;123:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harish S, Bhuvana K, Bengalorkar GM, et al. Flupirtine: Clinical pharmacology. J Anaesthesiol Clin Pharmacol 2012;28:172–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol 2009;156:1185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klinger F, Geier P, Dorostkar MM, et al. Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine. Br J Pharmacol 2012;166:1631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martire M, Castaldo P, D’Amico M, et al. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci 2004;24:592–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol 2006;575:175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornhuber J, Bleich S, Wiltfang J, et al. Flupirtine shows functional NMDA receptor antagonism by enhancing Mg2+ block via activation of voltage independent potassium channels. Rapid communication. J Neural Transm 1999;106:857–67. [DOI] [PubMed] [Google Scholar]
- 19.Rupalla K, Cao W, Krieglstein J. Flupirtine protects neurons against excitotoxic or ischemic damage and inhibits the increase in cytosolic Ca2+ concentration. Eur J Pharmacol 1995;294:469–73. [DOI] [PubMed] [Google Scholar]
- 20.Block F, Pergande G, Schwarz M. Flupirtine reduces functional deficits and neuronal damage after global ischemia in rats. Brain Res 1997;754:279–84. [DOI] [PubMed] [Google Scholar]
- 21.Osborne NN, Schwarz M, Pergande G. Protection of rabbit retina from ischemic injury by flupirtine. Invest Ophthalmol Vis Sci 1996;37:274–80. [PubMed] [Google Scholar]
- 22.Yu F, Liu Y, Wang Y, et al. Protective effect of the KCNQ activator flupirtine on a model of repetitive febrile seizures. Epilepsy Res 2011;97:64–72. [DOI] [PubMed] [Google Scholar]
- 23.Huang P, Li C, Fu T, et al. Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav Brain Res 2015;288:1–10. [DOI] [PubMed] [Google Scholar]
- 24.Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol Res Int 2012;2012:867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampath D, White AM, Raol YH. Characterization of neonatal seizures in an animal model of hypoxic-ischemic encephalopathy. Epilepsia 2014;55:985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao M, Latta P, Foniok T, et al. Cerebral blood flow response to a hypoxic-ischemic insult differs in neonatal and juvenile rats. MAGMA 2004;17:117–24. [DOI] [PubMed] [Google Scholar]
- 27.Lam PM, Gonzalez MI. Calpain activation and neuronal death during early epileptogenesis. Neurobiol Dis 2019;124:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira LO, Strapasson AC, Nabinger PM, et al. Early enriched housing results in partial recovery of memory deficits in female, but not in male, rats after neonatal hypoxia-ischemia. Brain Res 2008;1218:257–66. [DOI] [PubMed] [Google Scholar]
- 29.Rojas JJ, Deniz BF, Miguel PM, et al. Effects of daily environmental enrichment on behavior and dendritic spine density in hippocampus following neonatal hypoxia-ischemia in the rat. Exp Neurol 2013;241:25–33. [DOI] [PubMed] [Google Scholar]
- 30.Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol 1999;9:195–202. [DOI] [PubMed] [Google Scholar]
- 31.Patil MA, Matter BA, Raol YH, et al. Brain distribution and metabolism of flupirtine, a nonopioid analgesic drug with antiseizure effects, in neonatal rats. Pharmaceutics 2018;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farwell JR, Lee YJ, Hirtz DG, et al. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med 1990;322:364–69. [DOI] [PubMed] [Google Scholar]
- 33.Forcelli PA, Kozlowski R, Snyder C, et al. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther 2012;340:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankel S, Medvedeva N, Gutherz S, et al. Comparison of the long-term behavioral effects of neonatal exposure to retigabine or phenobarbital in rats. Epilepsy Behav 2016;57:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster G, Schwarz M, Block F, et al. Flupirtine: A review of its neuroprotective and behavioral properties. CNS drug reviews 1998;4:149–64. [Google Scholar]
- 36.Hoffmann O, Gommert LR, Egert M. Paradoxical cerebral cortical hyperexcitability following flupirtine overdose. J Toxicol Clin Toxicol 2004;42:913–16. [DOI] [PubMed] [Google Scholar]
- 37.Puls F, Agne C, Klein F, et al. Pathology of flupirtine-induced liver injury: a histological and clinical study of six cases. Virchows Arch 2011;458:709–16. [DOI] [PubMed] [Google Scholar]
- 38.Michel MC, Radziszewski P, Falconer C, et al. Unexpected frequent hepatotoxicity of a prescription drug, flupirtine, marketed for about 30 years. Br J Clin Pharmacol 2012;73:821–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure 2015;33:60–65. [DOI] [PubMed] [Google Scholar]
- 40.Bock C, Surur AS, Beirow K, et al. Sulfide Analogues of Flupirtine and Retigabine with Nanomolar KV 7.2/KV 7.3 Channel Opening Activity. ChemMedChem 2019;14:952–64. [DOI] [PubMed] [Google Scholar]