Abstract
Introduction:
Annual lung cancer screening with low-dose computed tomography is recommended for adults aged 55 to 80 years with a greater than or equal to 30 pack-year smoking history who currently smoke or quit within the past 15 years. The 50% who are current smokers should be offered cessation interventions, but information about the impact of adding cessation to screening is limited.
Methods:
We used an established lung cancer simulation model to compare the effects on mortality of a hypothetical one-time cessation intervention and annual screening versus annual screening only among screen-eligible individuals born in 1950 or 1960. Model inputs were derived from national data and included smoking history, probability of quitting with and without intervention, lung cancer risk and treatment effectiveness, and competing tobacco-related mortality. We tested the sensitivity of results under different assumptions about screening use and cessation efficacy.
Results:
Smoking cessation reduces lung cancer mortality and delays overall deaths versus screening only across all assumptions. For example, if screening was used by 30% of screen-eligible individuals born in 1950, adding an intervention with a 10% quit probability reduces lung cancer deaths by 14% and increases life years gained by 81% compared with screening alone. The magnitude of cessation benefits varied under screening uptake rates, cessation effectiveness, and birth cohort.
Conclusions:
Smoking cessation interventions have the potential to greatly enhance the impact of lung cancer screening programs. Evaluation of specific interventions, including costs and feasibility of implementation and dissemination, is needed to determine the best possible strategies and realize the full promise of lung cancer screening.
Keywords: Lung cancer screening, Smoking cessation interventions, Lung and tobacco-related mortality, Cancer Intervention and Surveillance Modeling Network (CISNET)
Introduction
Despite recent progress, lung cancer is the second most common cancer in women and men and the leading cause of cancer death in the United States.1,2 At least 80% of lung cancers could be averted by avoiding smoking initiation.3 Among those who already have a long history of cigarette use, lung cancer screening can reduce lung cancer mortality.4–6 On the basis of this evidence, lung cancer screening using low-dose computed tomography (LDCT) scans is now recommended by the U.S. Preventive Services Task Force for adults aged 55 to 80 years with greater than or equal to 30 pack-year smoking history who currently smoke or quit within the past 15 years.7
In addition to directly increasing life expectancy through early stage detection and initiation of treatment, lung cancer screening has been hypothesized to be a teachable moment, in which current smokers might be motivated to quit smoking.8–11 Cessation counseling and referral to cessation interventions have been recommended as a key component of lung screening programs7,12 because smoking cessation decreases lung cancer and other tobacco-related morbidity and mortality risks. Nevertheless, little is known about the efficacy of cessation interventions in the screening setting. There are clinical trials underway to fill this gap,11,13–16 but results are not expected for several years.17 Furthermore, little is known about the potential synergistic effects of joint screening and smoking cessation programs on population lung cancer and tobacco-related morbidity and mortality rates.
In this study, we used an established Cancer Intervention and Surveillance Modeling Network (CISNET) model to estimate reductions in lung cancer, premature deaths, and gains in life years with cessation interventions delivered at the point of lung cancer screening versus screening only. We tested the magnitude of cessation benefits under varying assumptions of lung screening use and cessation efficacy. The results of this study are intended to provide guidance on the conduct of future research evaluating specific interventions, including costs and feasibility of their implementation and dissemination.
Materials and Methods
We used an established CISNET lung cancer microsimulation model to portray the processes of smoking initiation and cessation and the impact of cessation and screening on lung cancer incidence and mortality and allcause mortality. The model has been described in detail elsewhere.18 Briefly, the model uses individual U.S. smoking histories as input to simulate random lung cancer outcomes, including the onset of preclinical and clinical lung cancer, and survival or death in the lack of screening. The model then simulates the impact of interventions such as smoking cessation or screening on individual smoking and lung cancer outcomes.
Population and the CISNET Smoking History Generator
The smoking history generator (SHG) is a microsimulation model that produces U.S. cohort-specific smoking histories by age and sex for cohorts born from 1864 to present.19–21 The SHG has been reported to reproduce the actual history of smoking in the United States.19 The SHG simulates individual smoking histories (number of daily cigarettes smoked per age) and age at death from competing causes of death using U.S. estimates of smoking initiation, cessation, intensity (cigarettes per day), and mortality rates by smoking status. To accomplish this, the model used data from the Integrated Public Use Microdata Series Health Surveys, a harmonized version of the National Health Interview Survey from 1965 to 2015,22 the Human Mortality Database,23 and data on lung cancer mortality from the Cancer Prevention Study-I24 and the Cancer Preventigon Study-II.25
The modeling of age-specific smoking status and intensity is used to simulate individual cumulative smoking exposure (pack-years) time since quit for former smokers to determine screening eligibility.
We utilized the SHG to generate smoking histories of 1 million men and 1 million women for the 1950 and 1960 U.S. birth cohorts to represent smoking patterns of the population. We picked the sample size on the basis of earlier work that suggests that 1 million individuals are sufficient to obtain stable estimates. The 1950 and 1960 birth cohorts were chosen because they are now in the middle of their screening eligibility according to current guidelines (70 y/o for 1950 and 60 y/o for 1960 in the year 2020) and they are representative of different periods of the tobacco epidemic (higher smoking prevalence for 1950 versus decreasing prevalence for 1960).
University of Michigan Lung Cancer Screening and Smoking CISNET Model
University of Michigan Lung Cancer Screening and Smoking (MichiganLung) is a discrete state microsimulation model that simulates the natural history of lung cancer (onset, histologic type, stage progression, clinical detection, and mortality or survival) and the outcomes of LDCT screening and any resulting follow-up and treatment intervention.18,26,27 The model consists of three main components: natural history, screening, and cost-utility analysis. The model simulates individual preclinical and clinical lung cancer histories and outcomes per computed tomography (CT) screen and has been found to reproduce the relatively short-term outcomes of a few rounds of CT screening in randomized control trials by arm, lung cancer histologic diagnosis, and stage.18,28 The model uses estimates of the sensitivity and specificity per CT screen by lung cancer histologic diagnosis and stage29 to simulate the lifetime impact of annual CT screening for individuals in the U.S. population.26,27
In this study, the MichiganLung model was used to simulate lung cancer and screening outcomes among screen-eligible individuals from the 1950 and 1960 birth cohorts under different scenarios of screening uptake and under different cessation intervention rates.
Modeling Cessation
A hypothetical smoking cessation intervention at the point of the first screen was incorporated into the MichiganLung model (see diagram in the appendix). Every current smoker undergoing LDCT screening for the first time also undergoes a one-time hypothetical smoking cessation intervention. An individual going through the hypothetical cessation intervention was assumed to have a probability of quitting owing to the intervention.
If an individual quits, the age- and sex-specific other-cause mortality on the basis of the updated smoking history is generated using the SHG mortality rates by smoking status. In addition, the individual’s lung cancer natural history after a cessation intervention is resi-mulated with the following considerations: (1) Individuals who did not have lung cancer in the no-screening scenario but gained extra life years owing to quitting smoking earlier might get lung cancer during the additional years of life. (2) Individuals who were diagnosed with lung cancer in the no-screening scenario and quit smoking more than 2 years before the onset of their lung cancer may have their lung cancer incidence age delayed or completely eliminated on the basis of their reduced lung cancer risk. If the age of lung cancer incidence is delayed, histologic type and stage progression are updated accordingly. (3) Individuals who were diagnosed with lung cancer in the no-screening scenario but quit smoking less than 2 years before their lung cancer diagnosis keep their original lung cancer age at diagnosis and lung cancer natural history (histologic type and stage progression) but have improved other-cause mortality.
Outcomes and Scenarios
We considered the following three main outcomes: lung cancer deaths averted, premature all-cause deaths averted (deaths delayed), and life years gained (LYG). We compared these and the number of people screened and the number of individuals going through the cessation intervention for different scenarios of screening uptake (percentage of eligible individuals screened) and of the probability of quitting owing to the intervention. In particular, we evaluated the impact of varying screening uptake from 5% to 100% in 5% increments and varying the probability of quitting owing to the intervention from 0% to 30% in 2.5% increments informed by the estimated efficacy of current trials30–33 and a recent meta-analysis that focused on studies of smokers likely to be eligible for screening on the basis of age and smoking history.17
We provide results per 100,000 individuals at age 45. Because the proportion and characteristics of screen-eligible individuals vary by birth cohort and scenario, our results are calculated “per population” (including both screened and unscreened individuals) rather than “per screened population” so that they are comparable across cohorts.
Results
As individuals age, the proportion that is both a current smoker and eligible for screening decreases (Fig. 1). Approximately 10% and 14.9% of the population are screening-eligible current smokers at age 55, decreasing to about 2.7% and 3.5% at age 80 in the 1960 and 1950 birth cohorts, respectively.
Figure 1.
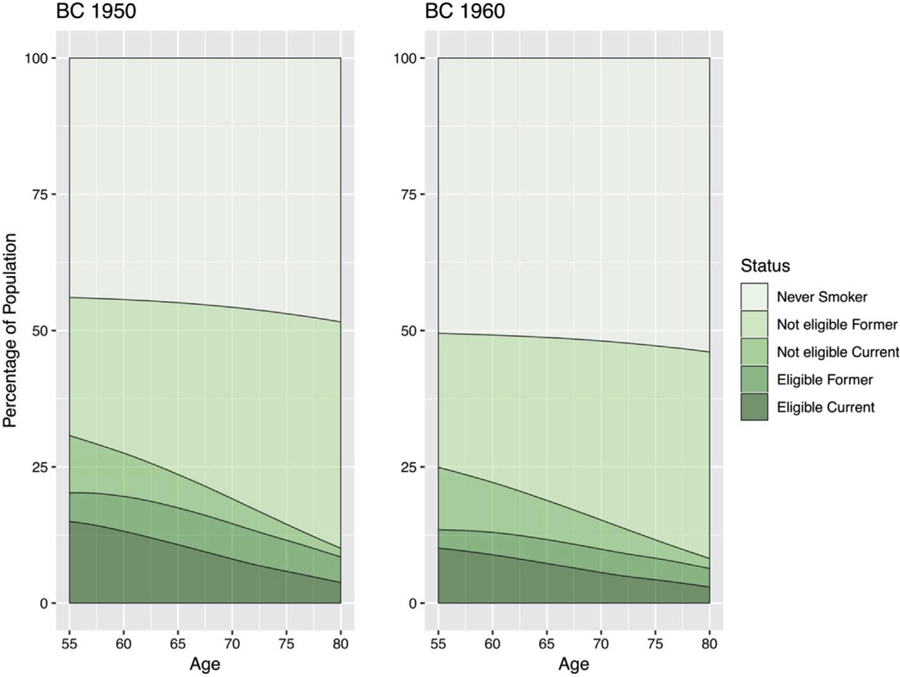
Screening eligibility by age and smoking status for birth cohorts 1950 (left) and 1960 (right) under the current U.S. Preventive Services Task Force guideline.
Overall, the magnitude of projected gains from smoking cessation interventions depends on screening uptake and the probability of quitting as a result of the intervention. For screening uptake rates ranging from 10% to 100%, the percent reduction in lung cancer deaths with cessation versus screening alone ranged from 3% to 52% with cessation probabilities from 5% to 25% (Table 1), whereas the percent increase in life years varied from 40% to over 200% (Table 2).
Table 1.
Lung Cancer Deaths Averted Per 100,000 Population at Age of 45 Years (by Selected Cessation Probabilities and Uptake) for 1950 and 1960 Birth Cohorts
| Screening Uptake | Probability of Cessation Owing to Intervention | |||||
|---|---|---|---|---|---|---|
| Birth cohort 1950 | ||||||
| 0% | 5% | 10% | 15% | 20% | 25% | |
| 100% | 807 | 881 | 955 | 1029 | 1090 | 1173 |
| 70% | 549 | 623 | 665 | 712 | 758 | 815 |
| 50% | 402 | 436 | 479 | 516 | 548 | 591 |
| 30% | 244 | 257 | 278 | 303 | 337 | 350 |
| 20% | 158 | 177 | 189 | 195 | 212 | 231 |
| 10% | 80 | 93 | 94 | 107 | 108 | 118 |
| Birth cohort 1960 | ||||||
| 0% | 5% | 10% | 15% | 20% | 25% | |
| 100% | 425 | 453 | 496 | 538 | 576 | 612 |
| 70% | 298 | 323 | 346 | 372 | 408 | 435 |
| 50% | 211 | 227 | 251 | 271 | 286 | 306 |
| 30% | 131 | 135 | 156 | 162 | 172 | 184 |
| 20% | 81 | 95 | 102 | 110 | 117 | 123 |
| 10% | 42 | 46 | 49 | 54 | 59 | 61 |
Table 2.
Life Years Gained Per 100,000 Population at Age of 45 Years (by Selected Cessation Probabilities and Uptake) for 1950 and 1960 Birth Cohorts
| Screening Uptake | Probability of Cessation Owing to Intervention | |||||
|---|---|---|---|---|---|---|
| Birth cohort 1950 | ||||||
| 0% | 5% | 10% | 15% | 20% | 25% | |
| 100% | 12,083 | 17,415 | 22,500 | 27,456 | 32,573 | 37,895 |
| 70% | 8243 | 12,137 | 15,639 | 19,352 | 22,781 | 26,419 |
| 50% | 6010 | 8584 | 11,263 | 13,702 | 16,418 | 19,003 |
| 30% | 3645 | 5116 | 6580 | 8286 | 9913 | 11,400 |
| 20% | 2355 | 3438 | 4398 | 5432 | 6437 | 7413 |
| 10% | 1203 | 1746 | 2311 | 2849 | 3275 | 3767 |
| Birth cohort 1960 | ||||||
| 0% | 5% | 10% | 15% | 20% | 25% | |
| 100% | 6460 | 9540 | 13,105 | 16,377 | 19,436 | 22,986 |
| 70% | 4480 | 6785 | 9068 | 11,484 | 13,766 | 16,229 |
| 50% | 3228 | 4833 | 6551 | 8218 | 9887 | 11,515 |
| 30% | 1998 | 2901 | 4034 | 4912 | 5913 | 6830 |
| 20% | 1256 | 1926 | 2641 | 3362 | 3854 | 4614 |
| 10% | 677 | 986 | 1313 | 1619 | 1984 | 2262 |
For example, for the 1950 birth cohort, assuming a 30% screening uptake, the number of lung cancer deaths averted per 100,000 population would increase from 244 in the no-cessation intervention scenario to 278 (14% higher) for an intervention with a 10% probability of quitting. The number of deaths averted would increase to 350 (43% higher versus no-cessation) for an intervention with a 25% probability of quitting. Similarly, under a cessation intervention with 5% quit probability, the number of lung cancer deaths averted would increase from 93 per 100,000 population under a 10% uptake scenario to 881 per 100,000 (843% higher) under a 100% uptake scenario. In general, the number of lung cancer deaths averted for the 1950 birth cohort is about twice that for the 1960 birth cohort in all scenarios.
The number of LYG also increases with both screening uptake and the probability of quitting. For instance, for the 1950 birth cohort, assuming a 30% screening uptake, the number of LYG per 100,000 population would increase from 3645 in the no-cessation intervention scenario to 6580 (80% higher) with an intervention with 10% quit probability. Similarly, under a cessation intervention with 5% quit probability, the number of LYG would increase from 1746 per 100,000 population screened under a 10% uptake scenario to 17,415 per 100,000 (897% higher) under a 100% uptake scenario. In general, the amount of LYG is about 65% to 80% higher for the 1950 birth cohort.
Figure 2 reveals the cumulative LYG (left), lung cancer deaths averted (middle), cumulative all-cause deaths delayed by age (top right), and age-specific all-cause deaths delayed (bottom right) for different quit probabilities (0%, 5%, 10%, 15%, 20%, 25%) under a 30% uptake scenario. The top panels correspond to the 1950 birth cohort, and the bottom panels to the 1960 birth cohort. Cumulative LYG and lung cancer deaths averted increase as a function of age, with more gains with higher cessation probability. In contrast, cumulative deaths averted from all causes increase but then peak around age of 80 years and then decrease because everyone in the simulation eventually dies. The figure of the age-specific all-cause death reveals how premature deaths are delayed by screening and cessation programs by about two decades. Figure 3 reveals the heat maps of lifetime cumulative LYG (left), lifetime cumulative lung cancer deaths averted (middle), and deaths from all causes delayed by age of 80 years (right) for screening uptake ranging from 5% to 100% and quit probabilities ranging from 0% to 30%. The top panel shows the heat maps for the 1950 birth cohort, and the bottom panels for the 1960 birth cohort. We can see that for medium to high levels of screening uptake, the quitting probabilities have a considerable impact on the resulting LYG and delayed deaths from all causes by age of 80 years (nonlinear pattern), but that they have a lesser effect on lung cancer deaths averted (horizontal linear pattern).
Figure 2.
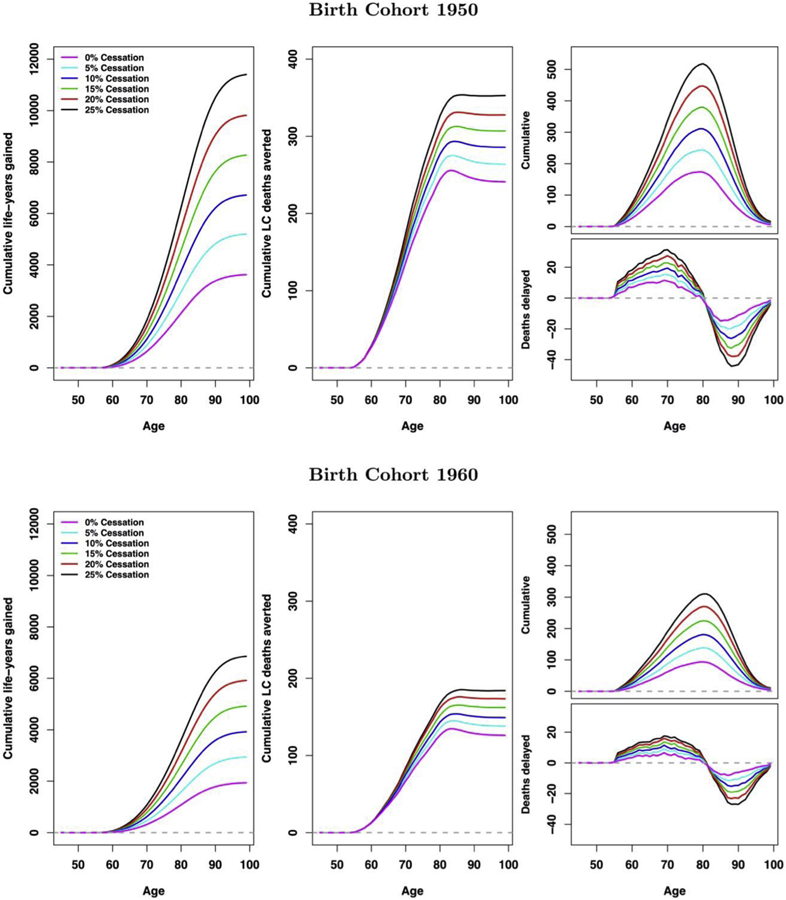
Cumulative life years gained (left), cumulative lung cancer deaths averted (middle), cumulative deaths averted (top right), and deaths delayed (bottom right) with the probability of quitting being 0%, 5%, 10%, 15%, 20%, and 25% under screening uptake of 30% for 1950 (top panel) and 1960 (bottom panel) birth cohorts. All results are presented per 100,000.
Figure 3.
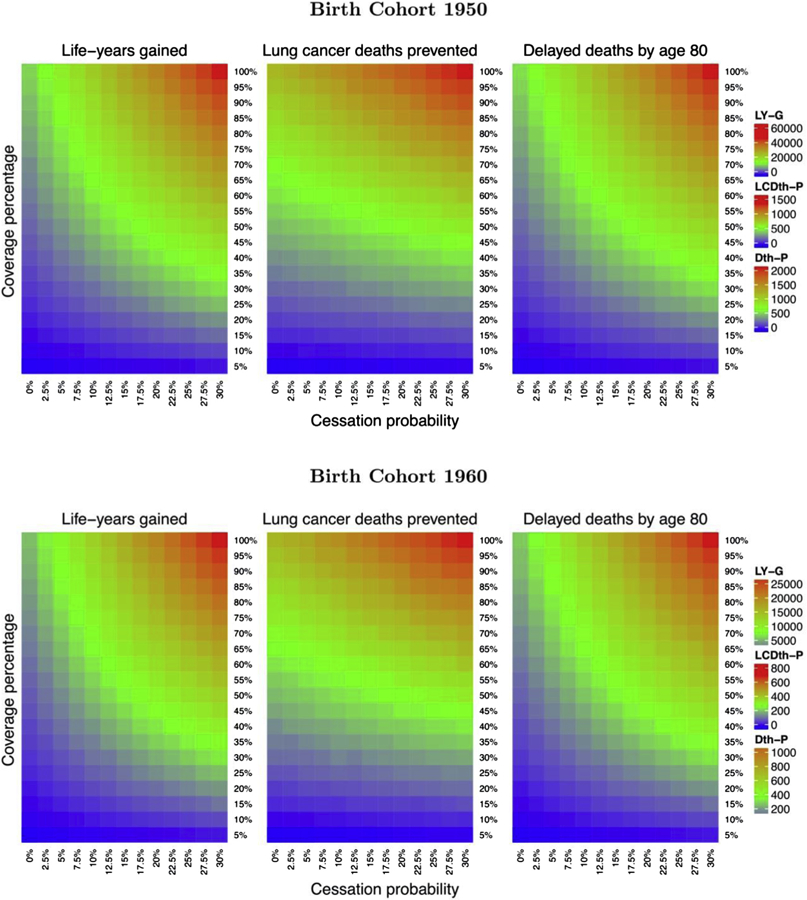
Heatmaps of life years gained (left), lung cancer death averted (middle), and deaths delayed by age of 80 years (right) under different assumptions of screening uptake (5%–100% with 5% increment) and the probability of quitting (0%–30% with 2.5% increment) for birth cohorts 1950 (top panel) and 1960 (bottom panel).
Discussion
This model-based analysis provides clinical and policy-relevant data on the potential mortality impact of mandated delivery of smoking cessation interventions to U.S. smokers at the time of lung cancer screening. Providing a one-time smoking cessation intervention to current cohorts of adults eligible for lung cancer screening is projected to save considerable additional lives beyond those expected with annual LDCT screening alone. We found that even a modest quit rate results not only in fewer lung cancer deaths but also in a large increase in LYG and delays in overall mortality. These incremental benefits occur largely because smoking cessation not only reduces the rates of developing lung cancer but also substantially extends life owing to a reduction of other tobacco-related conditions such as cardiovascular disease, chronic obstructive pulmonary disease, and other smoking-related cancers.3,34 This conclusion was robust across a wide variety of assumptions, but the absolute magnitude of the overall population benefits of providing cessation at the time of screening is expected to vary considerably on the basis of the dynamic effects of the joint combinations of cohort- and age-specific smoking rates, screening uptake, and the probability of quitting after cessation interventions.
From the clinical perspective, these results under-score the importance of provider discussions of smoking cessation in visits for decision making about lung cancer screening. One provider barrier to universal offering of smoking cessation to current smokers eligible for screening is the lack of evidence regarding effective interventions in the context of lung screening.11,17 There are few trials to date in the lung cancer screening setting, but early studies suggest intervention cessation probabilities from 1.4% to 20%.30–33 The National Cancer Institute’s Smoking Cessation at Lung Examination initiative includes eight trials designed to provide a robust evidence base of feasible, scalable approaches to providing smoking cessation, including combinations of pharmacotherapy, in-person counseling, and telephone counseling.11,13–16 The National Cancer Institute—Lung Population-based Research to Optimize the Screening Process initiative is now also evaluating the implementation of screening-based cessation programs across diverse health systems.35 However, the results of these trials and studies will not be available for several years.17 Until then, our results suggest that even modestly successful interventions will provide meaningful health benefits beyond those obtainable with screening alone.
There are also several policy implications of our findings. First, although the U.S. Preventive Services Task Force recommends and the Centers of Medicare and Medicaid Services mandates inclusion of smoking cessation to all current smokers undergoing lung cancer screening in the United States,7,12 formal integration of cessation programs is not yet required for certification or reimbursement of lung cancer screening services. The Centers of Medicare and Medicaid Services does require a shared decision-making visit for reimbursement of the screening examination. Smoking cessation is expected to be discussed during this visit.12 There is wide variability in the content of these shared decision-making visits across health systems and providers.36 For instance, a recent study found that provider discussion of smoking cessation was often limited by short visit durations and patients’ resistance and lack of interest, with referral rates to a local cessation clinic or quitline below 25%.36 Optimal integration of cessation programs into the screening process is a challenge, as the intervention of choice will likely vary for a given setting and population, area resources, and the screening workflow process.35 Further research regarding the effectiveness and cost-effectiveness of cessation interventions at the point of screening would help provide the basis for the development of standards and best practices for cessation programs within lung screening. Guidelines similar to those by the Joint Commission for inpatient smoking cessation might also be useful to accelerate the inclusion of effective cessation service delivery into screening programs.37
Another policy-level consideration is that the uptake of lung cancer screening among the screen-eligible population has been low in the United States, varying widely by region, with estimates ranging from 18% in Florida to 7% in Nevada.38 Our results indicate that even with a 20% screening rate, which seems achievable in the near future, lung cancer screening could result in 158 lung cancer deaths averted per 100,000 for the 1950 birth cohort compared with no screening. Different interventions are being proposed to improve screening uptake, including education programs to increase physician’s knowledge of screening recommendations, and interventions to increase patient awareness and facilitate access for hard-to-reach populations.39 Given the high costs of lung cancer care, it is possible that investments in interventions to increase screening uptake (and increase early stage diagnosis) would be cost-effective, especially given the high costs of new treatment paradigms for more advanced-stage lung cancer.40 However, even if lung cancer screening were used annually by 100% of eligible adults and saved lung cancer care costs, screening will not affect other tobacco-related morbidity and mortality. Thus, complementing screening with cessation interventions is needed to maximize its potential benefits.
Overall, our model analysis contributes to the growing clinical and policy discussions about how to maximize the potential of lung cancer screening and tobacco control in the United States.41–44 Previous modeling analyses have assessed the cost-effectiveness of lung screening with and without cessation programs under different assumptions of the efficacy of the cessation intervention, and all found that adding cessation to lung screening improved outcomes.45–47 Villanti et al.45 found that adding a cessation intervention with 1.25% to 5% quit rates could improve the cost-effectiveness of lung screening in the United States by 20% to 40%. In an analysis of the Canadian population, Goffin et al.46,47 evaluated the cost-effectiveness of annual and biennial lung screening with and without a single smoking cessation intervention. They found that adding a cessation intervention with a 22.5% quit probability would improve the cost-effectiveness of annual and biennial lung screening by 50% and result in considerable gains in quality adjusted life years. Our study extends these results by considering background cessation rates that vary by age, cohort, and sex, a wider range on the quit probabilities and screening uptake, and using a detailed model of lung cancer natural history, screening, and cessation.
Our simulation modeling is also useful to illustrate how the dynamic balance among smoking patterns, screening use, and cessation intervention effectiveness in the screening setting might affect overall population mortality rates. The success of tobacco policies and changes in lifestyle has led to a steady decline in smoking rates over the past four to five decades.21 On the basis of these trends, smoking cessation (and lung screening) had greater benefits in the 1950 birth cohort because they had higher smoking prevalence at older ages than the 1960 cohort. It will be important to reevaluate our results in future cohorts as smoking patterns evolve and possibly shift into other tobacco products.48 Modeling provides a flexible virtual laboratory platform to quickly project the impact of the changing landscape of tobacco control and targeted lung cancer therapy on the overall population lung cancer and tobacco-related disease mortality.
This modeling research used a well-established CISNET lung cancer natural history simulation model to synthetize large national studies of smoking patterns, trials screening effects, and the Surveillance, Epidemiology, and End Results population-based cancer registry data. This model has been used previously to extrapolate the benefits of lung screening from randomized controlled trials to the U.S. population, and the results of the model used in this study are similar to the other CISNET lung models.18,26,27 The model has also been used to simulate the population impact of different screening strategies for the U.S. population,18,26,27 including the relative differences of current recommendations versus risk-based screening strategies,26 the impact of patient preferences on benefits from lung cancer screening,18 and the cost-effectiveness of strategies varying the age at stopping screening.27 The model has external validity as it reproduces the short-term outcomes of a few rounds of computerized lung cancer tomography screening in randomized control trials by arm, lung cancer histology, and stage.18,28 Finally, we used a validated model of individual smoking histories in the United States that has been found to reproduce the history of smoking by birth cohort for the U.S. population.19–21
Despite use of robust CISNET models and large national datasets, there are several caveats that should be considered in evaluating our results. First, we did not evaluate cost-effectiveness of joint screening and cessation programs. This will be important in our future modeling research to evaluate the impact of specific interventions. Relatedly, we modeled only a single cessation intervention at the time of the first screen. In practice, it is likely that once cessation programs within lung screening are established, these will become available to current smokers at the time of each annual screen. Thus, our results are likely a conservative estimate of the potential impact of effective joint cessation and screening interventions. Finally, we restricted our analyses to two single birth cohorts of the U.S. population. Although these two cohorts are representative of the current eligible population, simulation of the whole U.S. population is needed to project the actual impact that these interventions will have on U.S. lung cancer and tobacco-related mortality.
In summary, our findings highlight the need for effective joint smoking cessation interventions to maximize the population benefits of lung screening. Effective cessation interventions at the point of screening could also save lives owing to decreases in other tobacco-related diseases as a consequence of smoking cessation. The future population impact of providing cessation with lung cancer screening is expected to be dynamic on the basis of changing trends in tobacco use and products, reach of screening, and ability to fully integrate cessation and screening into clinical workflow.
Supplementary Material
Acknowledgments
This research was supported by the National Cancer Institute Grants U01CA199284 (CISNET Lung) and R01CA207228 (Georgetown SCALE trial). This work was supported in part by a Cancer Prevention Research Fellowship sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation (ASPO-17–001). This work was also supported in part by the National Cancer Institute Grants U01CA12958, U01 CA183081, and R35 CA197289. The content of this article reflects the views of the authors. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. We would like to acknowledge the Smoking Cessation in Lung Examination (SCALE) Collaboration for their input and support for this work.
Footnotes
Disclosure: The authors declare no conflict of interest.
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2020.02.008.
References
- 1.Key statistics for lung cancer. https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html Accessed August 9, 2019.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Chronic Disease Prevention and Health Promotion (US). Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the national lung screening trial. N Engl J Med. 2014;371:1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA, U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. [DOI] [PubMed] [Google Scholar]
- 8.Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56:125–134. [DOI] [PubMed] [Google Scholar]
- 9.van der Aalst CM, van den Bergh KA, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch–Belgian randomised controlled lung cancer screening trial. Thorax. 2010;65:600–605. [DOI] [PubMed] [Google Scholar]
- 10.Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106:dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph AM, Rothman AJ, Almirall D, et al. Lung cancer screening and smoking cessation clinical trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) collaboration. Am J Respir Crit Care Med. 2018;197:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT). Medicare Coverage Database. 2015. CAG-00439N.
- 13.Smoking Cessation at Lung Examination: The SCALE Collaboration. https://cancercontrol.cancer.gov/brp/tcrb/scale-collaboration.html Accessed August 9, 2019.
- 14.Taylor KL, Deros DE, Fallon S, et al. Study protocol for a telephone-based smoking cessation randomized controlled trial in the lung cancer screening setting: the lung screening, tobacco, and health trial. Contemp Clin Trials. 2019;82:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu SS, Rothman AJ, Vock DM, et al. Program for lung cancer screening and tobacco cessation: study protocol of a sequential, multiple assignment, randomized trial. Contemp Clin Trials. 2017;60:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham AL, Burke MV, Jacobs MA, et al. An integrated digital/clinical approach to smoking cessation in lung cancer screening: study protocol for a randomized controlled trial. Trials. 2017;18:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadham CJ, Jayasekera JC, Advani SM, et al. Smoking cessation interventions for potential use in the lung cancer screening setting: a systematic review and meta-analysis. Lung Cancer. 2019;135:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caverly TJ, Cao P, Hayward RA, Meza R. Identifying patients for whom lung cancer screening is preference-sensitive: a microsimulation study. Ann Intern Med. 2018;169:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med. 2014;46:e31–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon J, Holford TR, Levy DT, et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann Intern Med. 2018;169:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blewett LA, Julia A, Drew R, King ML, Williams KCW. IPUMS Health Surveys: National Health Interview Survey, Version 6.4 [dataset]. Minneapolis, MN: IPUMS; 2019. [Google Scholar]
- 23.Barbieri M, Wilmoth JR, Shkolnikov VM, et al. Data resource profile: the human mortality database (HMD). Int J Epidemiol. 2015;44:1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns DM, Shanks TG, Choi W, Thun MJ, Heath CW, Garfinkel L. The American Cancer Society Cancer Prevention Study I: 12-year followup of 1 million men and women In: Burns DM, Garfinkel L, Samet JM, eds. Changes in Cigarette-Related Disease Risks and Their Implication for Prevention and Control. NCI Monograph 8. Bethesda, MD: National Institutes of Health, National Cancer Institute; 1997: 113–149. [Google Scholar]
- 25.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–2501. [DOI] [PubMed] [Google Scholar]
- 26.Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies [e-pub ahead of print]. J Natl Cancer Inst. 10.1093/jnci/djz164, accessed Septemer 30, 2019. [DOI] [PMC free article] [PubMed]
- 27.Criss SD, Cao P, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study [e-pub ahead of print]. Ann Intern Med. 10.7326/M19-0322, accessed November 5, 2019. [DOI] [PubMed]
- 28.Meza R, ten Haaf K, Kong CY, et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer. 2014;120:1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ten Haaf K, van Rosmalen J, de Koning HJ. Lung cancer detectability by test, histology, stage and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiol Biomark Prev. 2015;24:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark MM, Cox LS, Jett JR, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer. 2004;44:13–21. [DOI] [PubMed] [Google Scholar]
- 31.Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer. 2012;76:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor KL, Hagerman CJ, Luta G, et al. Preliminary evaluation of a telephone-based smoking cessation intervention in the lung cancer screening setting: a randomized clinical trial. Lung Cancer Amst. Neth. 2017;108:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremblay A, Taghizadeh N, Huang J, et al. A randomized controlled study of integrated smoking cessation in a lung cancer screening program. J Thorac Oncol. 2019;14:1528–1537. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 35.Rendle KA, Burnett-Hartman AN, Neslund-Dudas C, et al. Evaluating lung cancer screening across diverse healthcare systems: a process model from the lung PROSPR consortium. Cancer Prev Res (Phila). 2020;13:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanodra NM, Pope C, Halbert CH, Silvestri GA, Rice LJ, Tanner NT. Primary care provider and patient perspectives on lung cancer screening. A qualitative study. Ann Am Thorac Soc. 2016;13:1977–1982. [DOI] [PubMed] [Google Scholar]
- 37.Fiore MC, Goplerud E, Schroeder SA. The Joint Commission’s new tobacco-cessation measures—will hospitals do the right thing? N Engl J Med. 2012;366:1172–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zahnd WE, Eberth JM. Lung cancer screening utilization: a behavioral risk factor surveillance system analysis. Am J Prev Med. 2019;57:250–255. [DOI] [PubMed] [Google Scholar]
- 39.Wang GX, Baggett TP, Pandharipande PV, et al. Barriers to lung cancer screening engagement from the patient and provider perspective. Radiology. 2019;290:278–287. [DOI] [PubMed] [Google Scholar]
- 40.Bradley CJ, Eguchi M, Perraillon MC. Factors associated with utilization of high-cost agents for the treatment of metastatic non-small cell lung cancer [e-pub ahead of print]. J Natl Cancer Inst. 10.1093/jnci/djz223, accessed November 09, 2019. [DOI] [PMC free article] [PubMed]
- 41.Land SR, Marcus PM. Cancer screening and diagnosis: opportunities for smoking cessation intervention. J Clin Oncol. 2015;33:1631–1632. [DOI] [PubMed] [Google Scholar]
- 42.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn EE, Gould MK. Lung cancer screening and smoking cessation: never too early or too late. J Natl Cancer Inst. 2018;110:1157–1158. [DOI] [PubMed] [Google Scholar]
- 44.Gould MK. Precision screening for lung cancer: risk-based but not always preference-sensitive? Ann Intern Med. 2018;169:52–53. [DOI] [PubMed] [Google Scholar]
- 45.Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013;8, e71379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goffin JR, Flanagan WM, Miller AB, et al. Cost-effectiveness of lung cancer screening in Canada. JAMA Oncol. 2015;1:807–813. [DOI] [PubMed] [Google Scholar]
- 47.Goffin JR, Flanagan WM, Miller AB, et al. Biennial lung cancer screening in Canada with smoking cessation— outcomes and cost-effectiveness. Lung Cancer. 2016;101:98–103. [DOI] [PubMed] [Google Scholar]
- 48.Kasza KA, Borek N, Conway KP, et al. Transitions in tobacco product use by U.S. adults between 2013–2014 and 2014–2015: findings from the PATH study wave 1 and wave 2. Int J Environ Res Public Health. 2018;15:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


