Abstract
Fluorescent proteins are commonly used to label target proteins in live cells. However, the conventional approach based on covalent fusion of targeted proteins with fluorescent protein probes is limited by the slow rate of fluorophore maturation and irretrievable loss of fluorescence due to photobleaching. Here, we report a genetically encoded protein labeling system utilizing transient interactions of small, 21–28 residues-long helical protein tags (K/E coils, KEC). In this system, a protein of interest, covalently tagged with a single coil, is visualized through binding to a cytoplasmic fluorescent protein carrying a complementary coil. The reversible heterodimerization of KECs, whose affinity can be tuned in a broad concentration range from nanomolar to micromolar, allows continuous exchange and replenishment of the tag bound to a targeted protein with the entire cytosolic pool of soluble fluorescent coils. We found that, under conditions of partial illumination of living cells, the photostability of labeling with KECs exceeds that of covalently fused fluorescent probes by approximately one order of magnitude. Similarly, single-molecule localization microscopy with KECs provided higher labeling density and allowed a much longer duration of imaging than with conventional fusion to fluorescent proteins. We also demonstrated that this method is well suited for imaging newly synthesized proteins, because the labeling efficiency by KECs is not dependent on the rate of fluorescent protein maturation. In conclusion, KECs can be used to visualize various target proteins which are directly exposed to the cytosol, thereby enabling their advanced characterization in time and space.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03426-5) contains supplementary material, which is available to authorized users.
Keywords: Exchangeable labels, Live-cell localization microscopy, Coiled coil tag, Protein-PAINT
Introduction
Exchangeable fluorescent tags provide multiple advantages over traditional covalent tags, including better photostability and higher labeling density in both conventional and super-resolution microscopy of intracellular protein targets [1–3]. Moreover, using shorter and transiently interacting tags is less intrusive for maintaining the natural dynamics of protein targets than using conventional, bulky fluorescent protein tags [4]. However, only a handful of exchangeable tags are currently available for monitoring just a few specific protein targets. Most notably, these tags include LifeAct [4] (17 amino acid residues; Kd ~ 2.2 μM) and F-tractin [5] (66 amino acid residues; Kd ~ 2.7 μM) probes for specifically visualizing actin and the microtubule-associated protein MAP4 [6] (406 amino acid residues; Kd ~ 0.14 μM) for visualizing microtubules.
In this study, we sought to develop a universal labeling approach taking advantage of the unique properties of small, low-affinity tags that would be suitable for imaging a broad range of targeted proteins. Previously, peptide heterodimers, such as leucine zippers and designed coiled coils, have been tailored for imaging protein targets, although the scope of the labeling has been limited to surface-exposed parts of membrane proteins [7, 8]. Here, we chose the so-called K/E coils [9]—artificial coiled coils consisting of 3–4 heptad repeats enriched in Lys (K coils) or Glu (E coils) residues in specific positions of each heptade (Fig. 1a). These helices are small enough (21–28 amino acid residues) to lower the impact on protein dynamics in contrast with conventional labeling through covalent attachment of bulky fluorescent proteins. While both K and E coils display negligible homodimerization [7], there is higher affinity within a heterodimeric K/E complex that depends on the number of heptad repeats and amino acid sequence of each heptade (for example, the presence of Ile or Val at the a position). This affinity can be fine-tuned in the nM–μM range (Supplementary Table S1). So far, protein labeling with K/E coils has shown promising results in labeling of membrane proteins (reviewed in Ref [10].) with exogenously supplied synthetic peptide conjugated with chemical fluorophores. We expected that certain combinations of K and E coils would provide both sufficient imaging contrast and a high fluorophore exchange rate (Fig. 1b) for imaging of intracellular proteins with a fully genetically encoded reporter system based on fluorescent proteins.
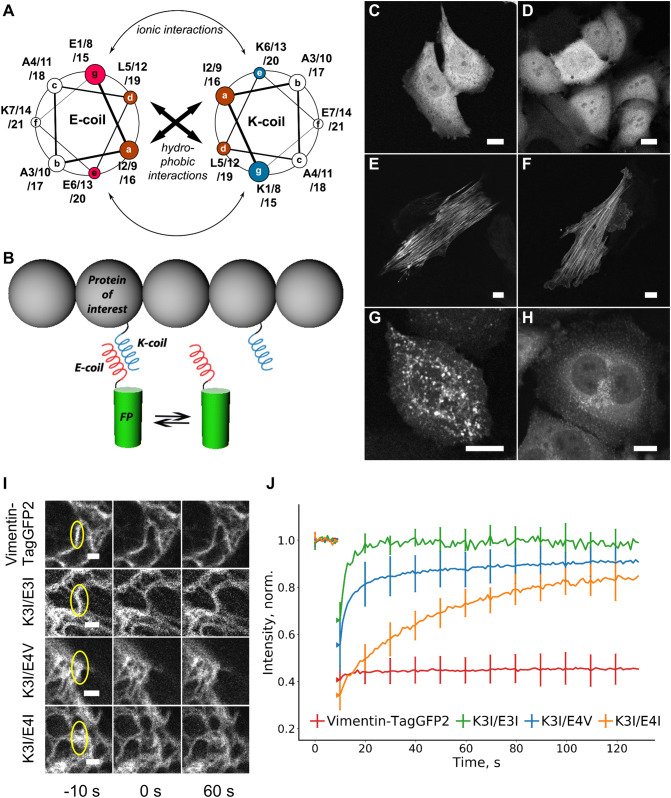
Fig. 1.
Live-cell protein labeling with K/E coils. a A helical wheel representation of the K/E heterodimer; letters in circles (a–g) correspond to the relative positions of amino acid residues within a heptade. The name of each coil is composed from: (1) amino acid residues in e and g positions (E for glutamate and K for lysine); (2) number of heptades (3 or 4) and (3) amino acid residue in a position (I for isoleucine and V for valine). b Schematic representation of the transient labeling methodology: a constant exchange of the fluorescent label on the protein of interest with the cytosolic label pool provides higher effective photostability in comparison with a conventional covalent fusion. c, d Intracellular distribution of the E4V–TagGFP2 (c) and K4I–TagGFP2 (d) tags expressed alone in HeLa Kyoto cells. e H9c2 cells co-expressing E4V–TagGFP2 with FusionRed–myosin–K4I. f H9c2 cells co-expressing E4V–TagGFP2 with K3I–actin. g HeLa Kyoto cells co-expressing K4V–Dendra2 with caveolin-E3I. h HeLa Kyoto cells co-expressing K4V–Dendra2 with clathrin–E3I. Scale bars are 10 μm. i FRAP analysis in HeLa Kyoto cells transiently expressing vimentin–TagGFP2, or co-expressing vimentin–K3I with E3I–TagGFP2 (K3I/E3I), E4V–TagGFP2 (K3I/E4V) or E4I–TagGFP2 (K3I/E4I) probes. Yellow ellipses are indicating vimentin fibers that are photobleached 10 s before photobleaching, 0 s and 60 s after photobleaching. Scale bars are 2 μm. j The corresponding averaged fluorescence recovery curves. Data are shown as mean (solid line) ± SD (whiskers); n = 11–26 cells per curve
Results and discussion
The proposed strategy of genetically encoded labeling with K/E coils (KECs labeling) involves fusing one coil (whether K or E coils) to the protein of interest and another, complementary coil to a fluorescent protein. We expected that as far as the target protein contacts with cytoplasm or nucleoplasm and localizes to a certain pattern, the fluorescent protein would stick to it and highlight the pattern of its intracellular localization (Fig. 1b).
We first expressed fluorescent proteins that contain a single coil structure in their N or C termini in cultured mammalian cells. This resulted in an even distribution of the fluorescent signal throughout the cytoplasm and the nucleus of transfected cells without any visible preference for specific cellular structures or compartments (Fig. 1c, d). We next co-expressed these probes with several proteins representing various intracellular structures, each fused with a complementary coil to evaluate the ability of various K/E coil pairs to heterodimerize. We focused on the coils variants from Yano et al. [7] (E3I, K3I, E4I, K4I in combinations E3I/K3I, E4I/K4I and E4I/K3I) as well as some variants from Litowski and Hodges [11] (E3V, K3V, E4V, K4V in combinations E3V/K3V, E4V/K4V, and E4V/K3I). The fluorescent signal localization for each heterodimeric (K + E) combination of these coils was well consistent with the expected localization of each targeted protein, with a varying degree of contrast between the cytoplasm and the structure of interest (Fig. 1e–h, Fig. S1–S5, Table S2). Specifically, fluorescent labeling of F-actin and myosin was restricted to intracellular filamentous structures, whereas labeling of caveolin and clathrin displayed bright dots consistent with membranous structures. We also tested the KEC probe mCherry–K4V for non-specific interaction with proteins, carrying natural coiled coils (such as vimentin), and did not observe any colocalization of mCherry–K4V with vimentin or actin fibers. In fact, we did not observe any specific targeting of mCherry–K4V to any other intracellular structures as it only was only a diffuse signal (Fig S6).
Litowski and Hodges [11] previously reported that KECs with four heptad repeats (such as K4V, K4I, and E4I) can homodimerize at high concentrations (> 300 μM) in vitro in the absence of their coiled-coil partner. Although typical expression levels of transgenes even with strong promoters rarely exceed concentrations in the micromolar range [12], we tested K4V and E4I in living cells with the OSER (organazed smooth endoplasmic reticulum) assay, commonly used for assessing the oligomerization state of fluorescent proteins [13]. Indeed, we observe some tendency for homodimerization for E4I coil tagged with fluorescent protein mScarlet, in the range similar to fluorescent protein EGFP [14]. K4V behaved indistinguishably from the monomeric fluorescent protein mScarlet (Table S3). However, as a precaution, we refrained from placing E4I on proteins of interest.
For further experiments, we selected three K/E pairs that showed superior imaging contrast, which span a range of affinities (60–4 nM) [7, 11]: K3I/E3I, K3I/E4V, and K3I/E4I. We first tested whether the helices within the K + E complex exchange efficiently with the cytosolic pool of tags by measuring fluorescence recovery after photobleaching (FRAP) [15] for a fluorescent protein fused with various E coils and co-expressed with vimentin-K3I fusion constructs. The recovery of fluorescence for K3I/E3I and K3I/E4V pairs occurred rapidly on a timescale of several seconds, whereas the recovery of the K3I/E4I pair was an order of magnitude slower (Fig. 1i–j, Fig. S7, Table S4). In contrast, the control vimentin–TagGFP2 fusion construct showed essentially no recovery under these conditions.
These FRAP experiments demonstrate that the fluorescent tag bound to a targeted protein molecule is continuously exchanged and replenished by the cytosolic pool of soluble fluorescent coils. As a result, the ability to image the targeted molecule is preserved much longer with continuously exchanged KEC than with a single covalently attached fluorescent tag. Ultimately, the duration of imaging is determined by the ratio between the volume of the cell undergoing imaging/bleaching and the total cell volume. This principle is similar to point accumulation for imaging in nanoscale topography (PAINT) [16] or Protein-PAINT [3], but extends it to genetically encoded protein detection systems.
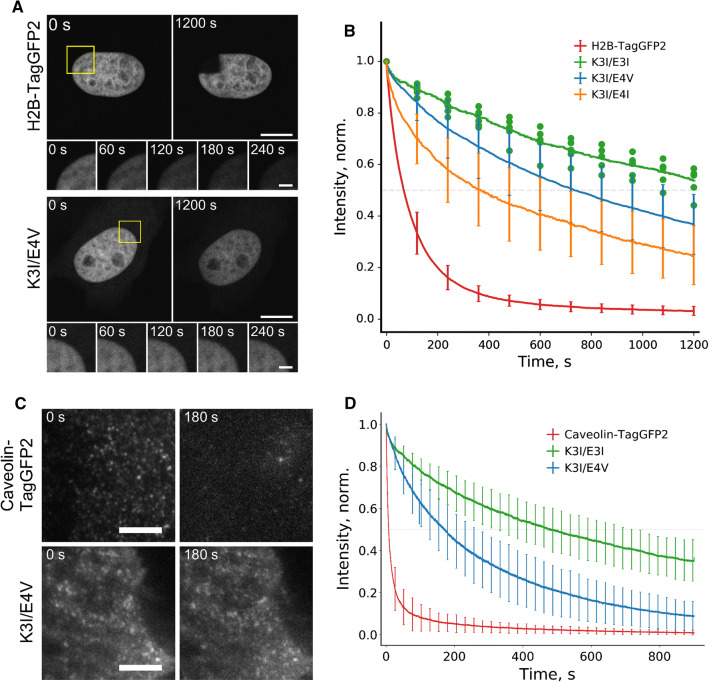
We next compared the photobleaching curves of histone H2B labeled with either KEC or a fused fluorescent protein in the confocal mode (Fig. 2a, b). By limiting the region of interest to ~ 15% of the nucleus, we reached more than tenfold improvement in labeling photostability with KEC (Table S5). Even after 20 min of continuous laser scanning, the nucleus labeled with KEC was still visible, while the labeling with fluorescent protein was gone.
Fig. 2.
Photostability of KEC labeling with illumination restricted either to a subcellular region (in confocal mode) or by light penetration depth (in TIRF regime). a HeLa Kyoto cells transiently expressed either H2B–TagGFP2 or H2B–K3I with TagGFP2–E4V (K3I/E4V). Yellow squares designate the bleached regions (showed as rows of insets). Images were acquired using confocal microscope. Scale bars are 10 μm. b Corresponding averaged curves of H2B photobleaching (K3I/E3I and K3I/E4I refer to transient co-expression of H2B–K3I with E3I–TagGFP2 or with E4I–TagGFP2, respectively). Data are shown as mean ± SD (for K3I/E3I pair individual data points are shown); n = 6–15 cells per curve. c HeLa Kyoto cells expressed either caveolin–TagGFP2 or caveolin–K3I with TagGFP2–E4V (K3I/E4V). Cells were pretreated with 10 μM PP2 for 15 min to fix the caveolae on the membrane (see “Materials and methods” for details). The entire cells were illuminated in TIRF regime (6 mW of 488 laser at the objective plane, 30 ms exposure time, 30 fps). Scale bars are 5 μm. d Corresponding averaged curves of caveolae photobleaching (K3I/E3I refers to transient co-expression of caveolin–K3I with E3I–TagGFP2). Data are shown as mean (solid line) ± SD (whiskers); n = 90–130 caveolae per curve
The resistance of KEC labeling to photobleaching is also beneficial for conducting total internal reflection fluorescence microscopy (TIRF), in which sample excitation is limited to the penetration depth of the evanescent field. Therefore, most of the cell volume is not illuminated and serves as a large pool of undamaged labels. Indeed, caveolin labeled by KEC remained fluorescent even after 15 min of high-intensity laser exposure, in contrast to caveolin fused to a conventional GFP probe (Fig. 2c, d, Table S5).
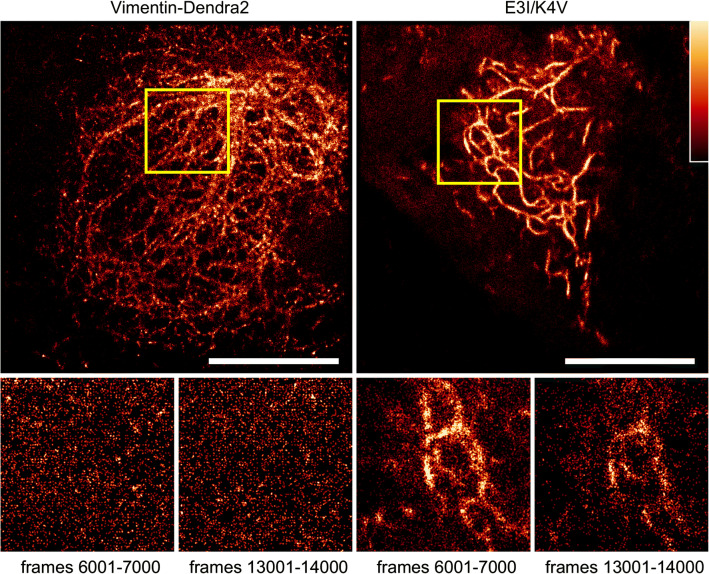
The TIRF setup is widely utilized in single-molecule localization microscopy, for example, in photoactivated localization microscopy (PALM). Typically, thousands of frames are acquired in high-power illumination regime, and the quality of image reconstruction declines over time. We tested whether labeling with exchangeable KEC in living cells would provide an improvement for super-resolution imaging, similar to that described in fixed cells with low-affinity protein tags (probes for integrating exchangeable single-molecule localization (IRIS)) [2]. We labeled vimentin by either KEC or covalent fusion to the photoactivatable fluorescent protein Dendra2. We then repeated multiple cycles of photoactivation by 403 nm laser pulsing followed by image acquisition. The reconstructed image from vimentin-Dendra2 deteriorated quickly, in approximately 1000 frames. In contrast, KEC-labeled vimentin filaments could be observed even after 10 000 frames (Fig. 3). Live-cell PALM facilitated by KEC labeling also allows for improved spatial resolution in comparison with wide-field imaging (Fig. S8). As an example, we can capture the temporal dynamics of the vimentin cytoskeleton in PALM mode for ~ 8 min of continuous imaging (Fig. S9). The ability to conduct prolonged super-resolution imaging is crucial for capturing temporal dynamics of target proteins in living cells and KEC can serve as invaluable tools for conducting these studies.
Fig. 3.
Live-cell PALM microscopy with KEC. HeLa Kyoto cells transiently expressed either vimentin-Dendra2 (a) or vimentin–E3I along with K4V–Dendra2 (E3I/K4V). b Images were reconstructed from frames 1–1000 using the Haar wavelet kernel (HAWK) algorithm designed for a high-density labeling regime. The bottom row shows magnified regions from (a) and (b), marked with yellow squares. Each image was rendered from 1000 frames (~ 24 s). Images were acquired at 42 fps with 16 ms exposure time under excitation of 56 mW of 561 nm laser line. Scale bars are 10 μm
We then stained fixed cells that were expressing vimentin-E3I with exogenously supplied recombinant mNeonGreen–K3I (Fig. S10) and observed mNeonGreen–K3I labeling of the vimentin cytoskeleton, confirming the feasibility of using KECs as additional method of staining in multi-color labeling applications. We further tested the performance of KECs as universal IRIS probes in fixed cells for super-resolution imaging of vimentin-E3I. We observed a stable number of localizations per frame for over 10,000 frames with an exogenously supplied solution of mNeonGreen–K3I (Fig. S11). Therefore, KECs could be used as an additional tag in fixed cells making the protein of interest suitable for post-fixation staining which is compatible with super-resolution imaging.
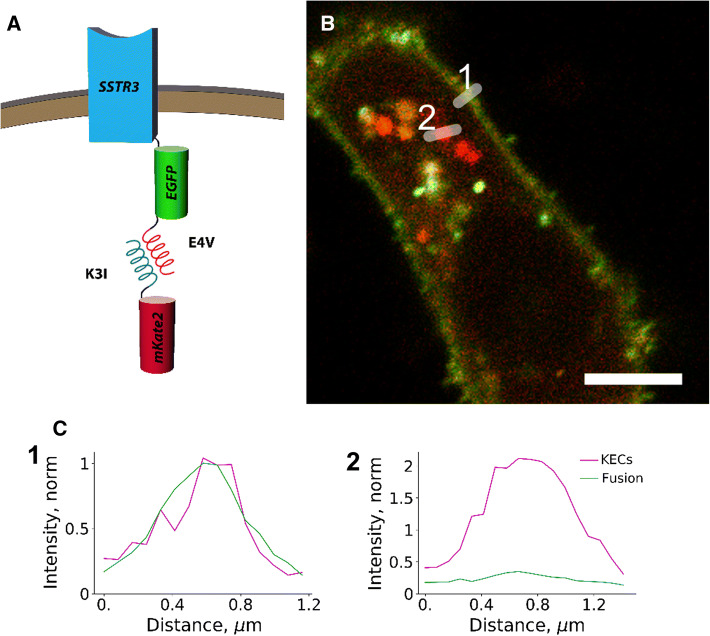
Another potential application of KEC labeling relates to the fact that self-catalyzed post-translational maturation of fluorescent protein chromophores includes a considerable delay in the appearance of fluorescence, ranging from a quarter hour to an hour [17]. This puts severe constraints to monitoring the localization of nascent proteins and early trafficking events. In contrast, small KEC tags preexisting in the cytoplasm are predicted to enable visualization of these early events by labelling nascent proteins almost immediately after their coil-tagged termini become available for interaction. Indeed, similar rapid staining (in less than 1 min) has been shown previously for receptors at cellular surface and exogenously added K/E coils [7]. We tested this idea by simultaneously labeling a transmembrane protein, somatostatin receptor 3, with EGFP and E4V KEC tags for imaging with mKate2 (Fig. 4a). We expected to detect comparable signal intensity from EGFP and KEC on the plasma membrane where the mature protein resides, and also visualize intracellular structures with lower green-to-red ratio, with the lowest ratio in the biosynthetic membranes. Indeed, we observed maximum green-to-red ratio at the plasma membrane, whereas the signal co-localizing with the Golgi marker tagBFP2-mannosidase had a much lower green component (Fig. 4b, c, Fig. S12). Also, a range of intracellular vesicle-like structures with intermediate green-to-red ratio was observed. Because a previous report showed that some fluorescent proteins can accumulate in lysosomes upon overexpression in cell culture [18], we stained live IMCD3 cells co-expressing SSTR3–EGFP–E4V and K3I–mKate2 with blue fluorescent LysoView lysosomal marker (Supplementary Fig. S13). No significant overlap between lysosomes and coil-labeled red or green puncta was observed, further suggesting that these intracellular structures are indeed biosynthetic membranes and/or transport vesicles.
Fig. 4.
Capturing early trafficking events for a transmembrane protein with KEC labeling. a Scheme of the experiment: somatostatin receptor 3 (SSTR3) was fused at the C-terminus with both static (EGFP) and exchangeable (E4V) tags, and co-expressed in mIMCD-3 cells with K3I–mKate2. b Confocal image of a representative cell co-transiently expressing SSTR3–EGFP–E4V (green) and K3I–mKate2 (red). Scale bar 5 μm. c Intensity profiles along the lines depicted in panel (b) to aid with data visualization. The intensities of red and green channels are scaled to 1 at the plasma membrane
We obtained similar results through chemical induction of caveolin synthesis using the Tet-On system [19] in HeLa cells. In this case, the expression of the protein of interest (caveolin–EGFP–E3I under inducible promoter) was triggered shortly after the fluorescence of mCherry-K4V (under constitutive promoter) was detected. Following induction, the diffuse distribution of red fluorescence signal from mCherry-K4V was replaced with patterns resembling caveolae or caveosomes, followed by a delayed appearance of the green signal from caveolin–EGFP–E3I (Fig S14, Supplementary Movie S1), consistent with the maturation time of the EGFP.
It should be noted that besides some reports indicating that, unlike covalent labels, low-affinity tags preserve natural protein dynamics [4], other studies highlight new potential sources of misinterpretation of observed protein behavior which are specific to non-covalent tags [20]. To reduce apparent photobleaching decay, excess amounts of KEC-fused fluorescent proteins and the use of fast exchangeable KEC pairs may be useful, but alternatively, signal may be blurred due to a lower labeling ratio. Care must be taken for maintaining an adequate concentration and ratio of the KEC pairs. Furthermore, labeling of low-abundant protein targets may be challenging with KECs. In this case, the efficiency of labeling can be tuned using KEC pairs with higher affinity or placing KEC fused to fluorescent protein under control of weaker promoter. In its current implementation, this technique is not compatible with simultaneous labeling of multiple protein targets due to an inevitable cross interaction between various K/E coils. However, some reports indicate [21] that certain K/E-coil combinations could be adopted for multi-target labeling.
In conclusion, we report KECs—a simple technique for live-cell labeling of intracellular proteins with K/E coils in a fully genetically encoded manner. In contrast to previously published low-affinity labeling of extracellular exposed membrane proteins with short exogenous peptides [10], KECs’ labeling is applicable to intracellular proteins. Transient labeling with KECs has two fundamental advantages over the conventional protein imaging with covalently attached fluorescent tags. First, it allows a marked improvement in the photostability of labeled molecules in zoomed confocal and TIRF microscopy regimes, which could be further extended to other microscopy techniques employing partial illumination of the sample (e.g., light-sheet microscopy) as well as scanning nanoscopy (e.g., STED and RESOLFT). Second, KECs’ labeling provides superior opportunities to visualize proteins immediately following their biosynthesis, as this technique does not rely on the requirement of fluorescent probes to undergo the lengthy process of fluorescence maturation. To the best of our knowledge, these advantages have been demonstrated for the first time in this work. We also believe that small size of the coils (compared to fluorescent protein) and transient nature of their interaction (i.e., the target protein spends significant time alone, without fluorescent protein bound) lead to less disturbance of target protein’s functioning and dynamics compared to conventional covalent tagging with fluorescent proteins. Thus, KEC tags will potentially broaden the range of protein targets accessible for genetically encoded fluorescent labeling.
Materials and methods
DNA cloning
Plasmids for expression in mammalian cell cultures were constructed using C1, N1, or similar backbone. Plasmids TagGFP2-C1, TagGFP2-N1, EGFP, mCherry-N1, mKate2-C1, Dendra2-C1, mTurquoise2, FusionRed–actin, TagGFP2–actin, FusionRed–myosin, vimentin–mKate2, H2B–mKate2, clathrin–mKate2, mKate2–fmem, and eCFP–NLS were provided by Evrogen, ClonTech or constructed in our laboratory earlier. pEGFPN3–Sstr3 was a gift from Kirk Mykytyn (Addgene plasmid # 35623; https://n2t.net/addgene:35623; RRID: Addgene 35623) [22]. mTagBFP2–MannII-N-10 was a gift from Michael Davidson (Addgene plasmid # 55309; https://n2t.net/addgene:55309; RRID: Addgene 55309) [23].
Plasmids encoding fluorescent proteins with N-terminal KEC tags (Table S2) were constructed in two steps. First, single-strand oligonucleotides that encode the whole coil (see Table S6) were annealed in ligation buffer (Evrogen, Russia) using the following program of thermal cycler: (1) 95 ℃ for 20 s; (2) 72 ℃ for 20 min. Double-strand DNA was purified using agarose gel (0.5% agarose) and CleanUp Kit (Evrogen, Russia) for extraction from the gel. Then, coil-coding double-strand DNA were inserted into corresponding vectors by NheI and AgeI restriction sites using FastDigest restriction enzymes (ThermoFischer Scientific, USA). The standard protocol of cloning was as follows: restriction in FastDigest Green Buffer followed by agarose gel purification and extraction by CleanUp; overnight ligation with T4 DNA ligase kit (Evrogen, Russia); electroporation in XL1-blue or XL Gold strains of E. coli.
For plasmids encoding fluorescent proteins with C-terminal KEC tags, similarly obtained coil-coding double-strand DNA fragments were inserted into corresponding vectors by BglII and HindIII restriction sites using standard protocol.
Plasmids E3I/K4V–mCherry–fmem and E3I/K4V–TagGFP2–fmem were constructed by replacing mKate by mCherry or TagGFP2 in vectors E4I/K4V–mKate2–fmem by AgeI and BglII restriction sites using standard protocol.
Plasmids E3I/K3I–actin and E3I/K3I–FusionRed–actin were constructed by inserting coil-coding double-strand DNA by NheI and AgeI restriction sites using standard protocol.
The plasmid E3I-clathrin was constructed by replacing mKate2 in mKate2–clathrin vector by E3I-coding double-strand oligonucleotide between NheI and AgeI using standard protocol.
The plasmid vimentin-mKate2-K3I was constructed by two-step PCR: first, vimentin–mKate2 was amplified from vector vimentin–mKate2 with primers Vimentin HindIII F and mKate2 K3I R (Table S6) that has replaced stop-codon from mKate2 with half of the first heptade of K3I. The PCR was performed with Phusion HF polymerase kit (ThermoFischer Scientific, USA) using the following program for thermal cycler: (1) 98 ℃ for 10 s; (2) 65 ℃ for 15 s; (3) 72 ℃ for 2 min; number of cycles—25. The PCR product was mixtured with coil-coding double-strand oligonucleotides, derived from anneal of K3I MfeI F and K3I MfeI R (95 ℃ for 20 s; 72 ℃ for 20 min in ligation buffer), and exposed to second PCR with Vimentin HindIII F and K3I MfeI R primers, using the same program, as in first step. This construct was inserted between HindIII and MfeI using standard protocol.
Plasmids vimentin-E3I/K3I and H2B-E3I/K3I were constructed by replacing mKate2 with corresponding coil-coding double-strand oligonucleotides in vector vimentin–mKate2 by restriction sites AgeI and MfeI using standard protocol.
Plasmids H2B–mKate2–E3V/K3V were constructed by inserting H2B and corresponding coil-coding double-strand oligonucleotides into vector mKate2-C1, using NheI+ AgeI or BglII+ SalI sites, respectively, using standard protocol.
To construct plasmids, encoding caveolin, CAV1 gene was amplified from fetal liver cDNA using nested primers (Table S6; caveolin1 for the first PCR and caveolin2 for the second PCR). The first PCR was performed with Phusion HF polymerase kit (ThermoFischer Scientific, USA) using the following program for thermal cycler: (1) 98 ℃ for 10 s; (2) 60 ℃ for 15 s; (3) 72 ℃ for 1 min; number of cycles—25. The second PCR was performed with Taq polymerase kit (Evrogen, Russia) using the following program for thermal cycler: (1) 95 ℃ for 10 s; (2) 60 ℃ for 15 s; (3) 72 ℃ for 1.5 min; number of cycles—30. Amplified gene was inserted in TA vector using TA-cloning kit (Evrogen, Russia) by overnight ligation. Then, CAV1 was inserted between NheI and AgeI into the corresponding vectors using standard protocol.
pCMV–SSTR–EGFP–E4V construct was generated using GoldenGate cloning [24] using the following primers with pCMV–SSTR3–EGFP and EGFP–E4V as templates: Backbone FWD/REV, Insert #1 FWD/REV, Insert #2 FWD/REV, Insert #3 FWD/REV (Table S6). PCR was performed with Phusion HF polymerase kit (ThermoFischer Scientific, USA) using the following program for thermal cycler: (1) 98 ℃ for 10 s; (2) 60 ℃ for 15 s; (3) 72 ℃ for 1 min; number of cycles—25. The Golden Gate reaction was performed as follows: 200 ng of each fragment was added in reaction mixture, containing DpnI 20 U, BsmBI 10 U, T4 ligase 400 U (total volume—20 µl); then, it was incubated at 37 ℃ for 5 h followed by inactivation at 50 ℃ for 10 min; 10 µl of reaction mixture was transformed by chemical transformation.
Plasmids Cyt–ERM–mScarlet, Cyt–ERM–mScarlet–K4V, Cyt–ERM–mScarlet–E4I, mNeonGreen–K4V, K4V–mNeonGreen, mCherry–K3I, mCherry–K4V, and (mCherry–K4V(hPGK promoter)–(caveolin–EGFP-E3I(pTRE3G promoter))–(tet-On 3G) were assembled into vectors for Golden Gate cloning following MoClo standard described in Ref. [25]. Each transcriptional unit contained promoter (CMV unless otherwise specified), genes, and SV40 terminator. All Golden Gate cloning reactions were performed in 1X T4 ligase buffer (SibEnzyme, Russia) containing 10U of T4 ligase, 20U of either BsaI or BpiI (ThermoFisher, USA), and 100 ng of DNA of each DNA part. Golden Gate reactions were performed using the following cycling conditions: 30 cycles between 37 °C and 16 °C (90 s at 37 °C; 180 s at 16 °C). Plasmid for protein expression of mNeonGreen-K3I was assembled into pBAD vector, optimized for MoClo standard using Golden Gate protocol as described previously.
Before mammalian cell transfection, all the plasmids were purified by Endotoxin Free Midiprep kit (Evrogen, Russia).
Labeling in living cells
HeLa Kyoto and HEK293T cell lines were obtained from established frozen stocks of laboratory. Rat cardiomyoblasts H9c2 (ATCC CRL-1446) and mouse inner medullary collecting duct mIMCD-3 (ATCC CRL-2123) cell lines were obtained from ATCC. Mouse myoblasts C2C12 were a kind gift of N. Podkuychenko (Institute of Experimental Cardiology, National Medical Research Center for Cardiology, Moscow, Russia). All cell lines were grown in an adhesion culture in Dulbecco’s Modified Essential Medium (DMEM) with 2 mM glutamine and 4.5 g/l glucose (PanEco, Russia) supplemented with 10% fetal bovine serum (HyClone, ThermoScientific, USA) and 1% Penicillin + Streptomycin in a humidified atmosphere under 37℃ and 5% CO2.
Twenty-four hours before transfection, cells were seeded on glass bottom culture dishes (Fluorodish, World Precision Instruments, USA). For transient transfection, the transfection reagent FuGene HD (Promega, USA) was used in accordance to the manufacturer’s protocol. The transfection mixture was diluted with 700 μl of OptiMEM (Gibco, ThermoScientific, USA), and incubation was continued for 16–24 h.
After pre-optimization (data not shown) for wide-field and confocal microscopy, the DNA ratio for localized KECs and cytosolic pool was 2 for better contrast, and for super-resolution microscopy, DNA ratio was 0.5.
Fluorescence imaging of transfected cultured cells
Transfected cells were imaged in Imaging Medium (IM): MEM without sodium bicarbonate (Sigma-Aldrich, USA), with addition of 20 mM HEPES (Sigma-Aldrich, USA).
Wide-field images were acquired with Leica DMI6000b inverted microscope (Leica Microsystem, Germany) equipped with HC PL Apo 100 × 1.40–0.70 oil lens and HC PL Apo 40 × 0.85 lens (Leica Microsystem, Germany), GFP filter cube (excitation filter 470/40, emission filter 525/50) (Leica Microsystem, Germany), CoolLED pE-300white (CoolLED Ltd, UK) illumination source and Andor Zyla 5.5 CL 10 Tap sCMOS camera (Andor Technology, UK), and using Micromanager software [26]. Image acquisition was performed using blue channel of CoolLEDwhite pE-300 with illumination intensity of about 2.5–5 mW for 40× lens and 0.5–1.5 mW for 100× lens.
Confocal images (Fig. 1–2, Fig. S1, S7) were acquired with TSC SP2 (Leica Microsystem, Germany) based on inverted fluorescent microscope Leica DM IRE equipped with HCX PL APO Lbd.BL 63 × 1.40 oil lens and both argon (458/488 nm) and HeNe (543 nm) lasers. Image acquisition was performed using the 458 nm line of the argon laser (≈8 µW) with the emission band of 469–491 nm for mTurquoise2 and ECFP imaging; 488 nm line of the argon laser (≈9 µW) with the emission band of 499–540 nm for EGFP/TagGFP2/green form of Dendra2 imaging; 543 nm HeNe laser (≈18 µW) with the emission band of 563–700 nm for mKate2/mCherry/FusionRed/red form of Dendra2 imaging. Images in Fig. 4, Fig. S12, and Fig. S13 were acquired with Nikon A1+ confocal microscope, equipped with (excitation with 405 nm of full power, emission 450/50 nm), FITC (excitation with 488 nm line of Ar laser, emission 525/50), TRITC (excitation with 561 nm laser, emission 595/50 nm) filter sets, and four-channel dichroic mirror (405/488/561/640 nm). All lasers were operated at 0.5% of full power. The emission was collected with a GaAsP detector.
TIRF images (Figs. 2c, 3, Fig S8, S9) were acquired with Eclipse Ti microscope with N-STORM (Nikon Instruments, Japan) equipped with Apo TIRF 100 × 1.49 Oil DIC N2 lens, diode laser of 403 nm, argon laser of 488 nm, fiber laser of 561 nm, Andor DU-897 X-6517 sCMOS camera (Andor Technology, UK), and using NiS-Elements software (Nikon Instruments, Japan). Image acquisition was performed using the 488 nm line of the argon laser (≈0.17 mW). Images in Fig. S11 were acquired with Nanoimager S (ONI, UK) equipped with UPlanSApo 100 × 1.4NA oil immersion lens, 488 nm laser, 560 nm dichroic mirror, and Scope8 sCMOS camera. Imaging of mNeonGreen was performed in TIRF mode with 65 mW 488 nm laser light intensity with 20 fps.
Signal colocalization assay
To evaluate the efficiency of labeling were transiently co-expressed in HeLa Kyoto or HEK293T cells several pairs of plasmids: H2B–mKate2–E3V + K3V–mTurquoise2; vimentin–mKate2–K3I + E3I-TagGFP2; vimentin–mKate2–K3I + E4V–TagGFP2; vimentin–mKate2–K3I + E4I–TagGFP2; NLS-ECFP–K4I + E4I–TagGFP2; K4V–TagGFP2–fmem + mKate2–E4V.
Images were acquired with confocal microscopy using two channels simultaneously. Each image comprised several cells with protein of interest, labeled in both channels. Processing of images was performed using Fiji ImageJ distribution [27]. Quantification of signal colocalization was performed by measuring the fluorescence intensity in the rectangular ROI crossing the cell in both channels. No less than 5 cells were considered.
Fluorescence recovery after photobleaching analysis
Hela Kyoto cells transiently expressing vimentin–TagGFP2, H2B–TagGFP2, or co-expressing the K-coil-labeled vimentin or H2B with E-coil-labeled TagGFP2 were imaged 24 h after transfection. Images were acquired with confocal microscopy. A defined region, containing the protein of interest, was bleached at a full laser power (≈100 μW) for 2 s using a 488 nm line from an Argon laser. Recovery of a fluorescence was monitored by scanning a defined region at low laser power (≈9 μW).
Images were processed using Fiji ImageJ. Quantification of FRAP experiments was performed in accordance with the protocol of FRAP analysis [28].
The exchange rate of K/E heterodimers was evaluated from the half-time of fluorescence recovery. For calculation of the fluorescence recovery, half-time were used FRAP curves without bleach correction. Curves were fitted by single-exponential back multiplication method according to Eq. 1:
| 1 |
where B, y0, and τ2 were obtained by single-exponential Phair’s method fitting the decay curve of total intensity of the whole cell. These three values were taken as constant values during back multiplication fitting. The half-time of fluorescence recovery (t1/2) was calculated according to Eq. 2, using τ1:
| 2 |
The estimation of fraction of exchanging labels was performed by calculation of the mobile fraction. The average intensity value of the last ten frames were subtracted from the average intensity value of first ten frames of normalized and bleach-corrected curve.
Fluorescent labeling photostability analysis
The photostability analysis was performed using both confocal and TIRF microscopy.
Hela Kyoto cells transiently expressing H2B–TagGFP2 or co-expressing the K-coil-labeled H2B-K3I with E-coil-labeled TagGFP2 were imaged 24 h after transfection using confocal microscopy. For the estimation of the photostability of KECs labeling in confocal regime, the fluorescence intensity of the small area of labeled nucleus (10–15% of all nucleus) was monitored by scanning a defined region at low laser power (≈9 μW).
For photostability analysis in TIRF regime, Hela Kyoto cells were transiently transfected by plasmid caveolin–TagGFP2 or co-transfected by plasmids caveolin–K3I with E3I–TagGFP2 or TagGFP2–E4V. Cells were pretreated with 10 μM PP2 (Sigma-Aldrich, USA), added from 5 mM DMSO stock solution, for 15 min to fix the caveolae on the membrane [29]. The fluorescence decay was monitored by imaging the whole field of view, containing one or more cells, at high laser power (≈6 mW) at 30 fps.
Images were processed using Fiji ImageJ. The photostability was evaluated by measuring the fluorescence intensity for defined area of nucleus in confocal regime or for several caveolae or the whole cell in TIRF regime for 20 min. The fluorescence intensity was normalized to the initial value. The photostability of labeling was evaluated depending on the half-time (t1/2) of fluorescence decay. For calculation of the half-time decay curves was fitted using the following equation:
| 3 |
The half-time of fluorescence recovery (t1/2) was calculated according to Eq. 4:
| 4 |
Localization microscopy
For localization microscopy, Hela Kyoto cells were transiently transfected with plasmids encoding caveolin-Dendra2, vimentin-Dendra2, or the K-coil-labeled vimentin or caveolin with E-coil-labeled Dendra2. Cells were imaged 24 h after the transfection in IM. Imaging was performed in TIRF mode.
To visualize vimentin fibers, labeled with photoconverted protein Dendra2 (Fig. 3, Fig S8, S9) 403 nm (≈1.9–3.8 mW) and 561 nm (≈56 mW) laser beams of Eclipse Ti microscope with N-STORM were used for activation or acquisition, respectively. A series of multiple images (15,000–21,000 frames) were obtained, where 1 activation frame was followed by three acquisition frames with 16 ms exposure time (42 fps).
Image processing and data analysis were performed using Fiji ImageJ platform with ThunderSTORM [30] and HAWK [31] plugins. The running median (50 frames) or HAWK filters was employed to reduce out-of-focus background before the localization [32]. Single-molecule localization and image reconstruction were performed using ThunderSTORM. For detailed information about image acquisition and processing, see Table S7.
To visualize vimentin fibers labeled with mNeonGreen (Fig S11), 488 nm laser (65 mW) of Nanoimager S was used with following light program: 10 frames with 65 mW 488 laser ON followed by 60 frames with laser OFF at 20 fps (exposure time 50 ms).
Image processing and data analysis of Fig S11 were performed using NimOS 1.6.1.9898 (ONI, UK) and custom-made python 3.6 script. Image reconstruction was performed using default parameters of initial sigma. For detailed information about image acquisition, see Table S7.
Early detection of target proteins
To test early labeling of proteins, mIMCD-3 cells were co-transfected with pCMV-SSTR-EGFP-E4V and mKate–K3I. Cells were imaged twenty-four hours after transfection. TagBFP2-mannosidase was used as a Golgi marker. LysoView 405 was used as a lysosomal marker.
Another approach was the use of inducible synthesis of target proteins. Hela Kyoto cells were transfected with tri-cistronic plasmid encoding mCherry–K4V (hPGK promoter), caveolin–EGFP–E3I or H2B–EGFP–E3I (pTRE3G tetracycline/doxycycline inducible promoter [19]), and tet3G-On (protein co-activator, CMV promoter). Sixteen hours after transfection medium was changed to IM, containing 100 ng/ml doxycycline and imaged for the next 5–20 h at room temperature.
Statistics
Statistical differences between samples were determined using a one-factor ANOVA test. P values < 0.01 were considered statistically different. Sample sizes indicated in figure legends and in Supplementary Tables S4 and S5.
All data shown in this paper represent experiments repeated at least twice in independent cellular samples with similar results obtained each time.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Nikita Podkuychenko (Institute of Experimental Cardiology, National Medical Research Center for Cardiology, Moscow, Russia) for giving the C2C12 cell culture. We thank Dr. Irina Shagina (Pirogov Russian National Research Medical University, Moscow, Russia) for providing cDNA for caveolin-1 gene cloning.
Author contributions
KL and NG conceived the idea. KL, NG, and AM designed experiments and supervised the project. MP, NG, and SK did the cloning. MP and NG performed confocal and wide-field imaging. MP, PM, and VB performed TIRF imaging. ES, TL, and VA designed and performed experiments with somatostatin receptor. MP and AM analyzed the data. MP and AM wrote the manuscript. All co-authors discussed the results and exchanged comments on the manuscript.
Funding
This work was supported by the Russian Science Foundation grant 16-14-10364 and the NIH grants EY022959 (V.Y.A.), EY05722 (V.Y.A.), and EY029929 (T.R.L.). Experiments were partially carried out using equipment provided by the IBCH core facility (CKP IBCH, supported by Russian Ministry of Science and Higher Education, grant RFMEFI62117X0018).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maxim M. Perfilov and Nadya G. Gurskaya have contributed equally to this work.
Contributor Information
Alexander S. Mishin, Email: mishin@ibch.ru
Konstantin A. Lukyanov, Email: kluk@ibch.ru
References
- 1.Spahn C, Grimm JB, Lavis LD, Lampe M, Heilemann M. Whole-cell, 3D and multi-color STED imaging with exchangeable fluorophores. Nano Lett. 2018;19:500–505. doi: 10.1021/acs.nanolett.8b04385. [DOI] [PubMed] [Google Scholar]
- 2.Kiuchi T, Higuchi M, Takamura A, Maruoka M, Watanabe N. Multitarget super-resolution microscopy with high-density labeling by exchangeable probes. Nat Methods. 2015;12:743–746. doi: 10.1038/nmeth.3466. [DOI] [PubMed] [Google Scholar]
- 3.Bozhanova NG, et al. Protein labeling for live cell fluorescence microscopy with a highly photostable renewable signal. Chem Sci. 2017;8:7138–7142. doi: 10.1039/C7SC01628J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedl J, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schell MJ, Erneux C, Irvine RF. Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus. J Biol Chem. 2001;276:37537–37546. doi: 10.1074/jbc.M104101200. [DOI] [PubMed] [Google Scholar]
- 6.Olson KR, McIntosh JR, Olmsted JB. Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J Cell Biol. 1995;130:639–650. doi: 10.1083/jcb.130.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yano Y, et al. Coiled-coil tag-probe system for quick labeling of membrane receptors in living cells. ACS Chem Biol. 2008;3:341–345. doi: 10.1021/cb8000556. [DOI] [PubMed] [Google Scholar]
- 8.Tsutsumi H, et al. Fluorogenically active leucine zipper peptides as tag-probe pairs for protein imaging in living cells. Angew Chem Int Ed Engl. 2009;48:9164–9166. doi: 10.1002/anie.200903183. [DOI] [PubMed] [Google Scholar]
- 9.Chao H, et al. Kinetic study on the formation of a de novo designed heterodimeric coiled-coil: use of surface plasmon resonance to monitor the association and dissociation of polypeptide chains. Biochemistry. 1996;35:12175–12185. doi: 10.1021/bi9530604. [DOI] [PubMed] [Google Scholar]
- 10.Yano Y, Matsuzaki K. Live-cell imaging of membrane proteins by a coiled-coil labeling method—principles and applications. Biochim Biophys Acta Biomembr. 2019;1861:1011–1018. doi: 10.1016/j.bbamem.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Litowski JR, Hodges RS. Designing heterodimeric two-stranded alpha-helical coiled-coils. Effects of hydrophobicity and alpha-helical propensity on protein folding, stability, and specificity. J Biol Chem. 2002;277:37272–37279. doi: 10.1074/jbc.M204257200. [DOI] [PubMed] [Google Scholar]
- 12.Weidemann T, et al. Counting nucleosomes in living cells with a combination of fluorescence correlation spectroscopy and confocal imaging. J Mol Biol. 2003;334:229–240. doi: 10.1016/j.jmb.2003.08.063. [DOI] [PubMed] [Google Scholar]
- 13.Costantini LM, Fossati M, Francolini M, Snapp EL. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic. 2012;13:643–649. doi: 10.1111/j.1600-0854.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cranfill PJ, et al. Quantitative assessment of fluorescent proteins. Nat Methods. 2016;13:557–562. doi: 10.1038/nmeth.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2:444–456. doi: 10.1038/35073068. [DOI] [PubMed] [Google Scholar]
- 16.Sharonov A, Hochstrasser RM. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc Natl Acad Sci USA. 2006;103:18911–18916. doi: 10.1073/pnas.0609643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balleza E, Kim JM, Cluzel P. Systematic characterization of maturation time of fluorescent proteins in living cells. Nat Methods. 2018;15:47–51. doi: 10.1038/nmeth.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama H, Yamamoto A, Mizushima N, Yoshimori T, Miyawaki A. GFP-like proteins stably accumulate in lysosomes. Cell Struct Funct. 2008;33:1–12. doi: 10.1247/csf.07011. [DOI] [PubMed] [Google Scholar]
- 19.Das AT, Tenenbaum L, Berkhout B. Tet-on systems for doxycycline-inducible gene expression. Curr Gene Ther. 2016;16:156–167. doi: 10.2174/1566523216666160524144041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashiro S. Convection-induced biased distribution of actin probes in live cells. Biophys J. 2019;116:142–150. doi: 10.1016/j.bpj.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zane HK, Doh JK, Enns CA, Beatty KE. Versatile interacting peptide (VIP) tags for labeling proteins with bright chemical reporters. ChemBioChem. 2017;18:470–474. doi: 10.1002/cbic.201600627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–1547. doi: 10.1091/mbc.e07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subach OM, Cranfill PJ, Davidson MW, Verkhusha VV. An enhanced monomeric blue fluorescent protein with the high chemical stability of the chromophore. PLoS ONE. 2011;6:e28674. doi: 10.1371/journal.pone.0028674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engler C, Kandzia R, Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE. 2011;6:e16765. doi: 10.1371/journal.pone.0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using µManager. Curr Protoc Mol Biol Chapter. 2010;14(Unit14):20. doi: 10.1002/0471142727.mb1420s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura K (2005) Analysis of FRAP curves. EMBL. https://www.embl.de//eamnet/frap/FRAP6.html. Accessed 16 Sep 2005
- 29.Banquet S. Role of Gi/o-Src kinase-PI3K/Akt pathway and caveolin-1 in β2-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell Signal. 2011;23:1136–1143. doi: 10.1016/j.cellsig.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Ovesný M, Křížek P, Borkovec J, Švindrych Z, Hagen GM. ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics. 2014;30:2389–2390. doi: 10.1093/bioinformatics/btu202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh RJ, et al. Artifact-free high-density localization microscopy analysis. Nat Methods. 2018;15:689–692. doi: 10.1038/s41592-018-0072-5. [DOI] [PubMed] [Google Scholar]
- 32.Hoogendoorn E, et al. The fidelity of stochastic single-molecule super-resolution reconstructions critically depends upon robust background estimation. Sci Rep. 2015;4:3854. doi: 10.1038/srep03854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.