Abstract
Background.
Mesenchymal stem cell (MSC)-derived exosomes play a critical role in regenerative medicine.
Objective.
This study was to determine the dose- and time-dependent efficacy of exosomes for treatment of traumatic brain injury (TBI).
Methods.
Male rats were subjected to a unilateral moderate cortical contusion. In the dose response study, animals received a single intravenous injection of exosomes (50, 100, 200 μg/rat) or vehicle, with treatment initiated at 1 day after injury. In the therapeutic window study, animals received a single intravenous injection of 100 μg exosomes or vehicle starting at 1, 4, or 7 days after injury. Neurological functional tests were performed weekly after TBI for 5 weeks. Spatial learning was measured on days 31-35 after TBI using the Morris water maze test.
Results.
Compared to the vehicle, regardless of the dose and delay in treatment, exosome treatment significantly improved sensorimotor and cognitive function, reduced hippocampal neuronal cell loss, promoted angiogenesis and neurogenesis, and reduced neuroinflammation. Exosome treatment at 100 μg/rat exhibited a significant therapeutic effect compared to the 50 μg or 200 μg exosome groups. The time dependent exosome treatment data demonstrated that exosome treatment starting at 1 day post TBI provided a significantly greater improvement on functional and histological outcome than exosome treatments at the other two delayed treatments.
Conclusions:
These results indicate that exosomes have a wide range of effective doses for treatment of TBI with a therapeutic window of at least 7 days post injury. Exosomes may provide a novel therapeutic intervention in TBI.
Keywords: cell therapy, exosomes, functional outcome, neuroinflammation, neurovascular remodeling, rat, traumatic brain injury
Introduction
Traumatic brain injury (TBI) affects over 10 million people annually leading to mortality and hospitalization worldwide 1. To date, no effective neuroprotective agent has been identified from clinical TBI trials 2. Multipotent mesenchymal stromal cells (MSCs) are self-renewing stem/progenitor cells from bone marrow, adipose tissue, skin, umbilical cord blood, and peripheral blood as well as other organs 3. Previous studies show that a small proportion of transplanted MSCs migrate into injured brain, of which only a small number displayed neural-like markers, indicating functional recovery is not linked to neural differentiation from the transplanted MSCs 4, 5. MSC-induced brain remodeling and functional recovery are associated with their secretion-based paracrine role 6, 7. These therapeutic paracrine effects are likely mediated by MSC generated exosomes 8. In contrast to MSCs, nano-sized exosomes can easily pass the blood brain barrier and deliver their cargos including genetic materials, lipids, and proteins to recipient cells 9, without vascular obstructive effect and risks of tumor formation 10.
MSC-derived exosomes significantly improves functional recovery in rats after TBI 11. However, the dose and time-dependent effects of MSC-derived exosomes on functional recovery after TBI remain elusive, which ultimately will determine their clinical utility. The present study was designed to investigate the dose- and time-dependent efficacy of exosomes after TBI in improving cognitive and sensorimotor functional recovery, amplifying neurovascular remodeling, and reducing neuroinflammation after TBI.
Materials and Methods
Animals
Adult (3-month-old) male Wistar rats weighing 339.2 ± 13.6 g (Charles River, Wilmington, MA) were used. All experimental procedures were approved by the Henry Ford Health System Institutional Animal Care and Use Committee. The persons who performed the experiments, collected data, and assessed outcome were blinded throughout the course of the experiments (see Supplemental Materials for details).
Exosome isolation and characterization
Human MSCs were purchased from Theradigm (Bethesda, MD), and expanded, as previously described 12. MSC-derived exosomes were collected according to the published methods 13. Exosome size and particle number were characterized using a NanoSight (Merkel Technologies) instrument. Exosome morphology and exosomal markers (Alix and Hsp70) were examined by means of transmission electron microscopy and Western blot, respectively. Please see Methods in the Supplemental Materials for details.
Animal model of TBI
A well-established controlled cortical impact (CCI) rat model of TBI was utilized for the present study. Animals were anesthetized intraperitoneally (ip) with ketamine (80 mg/kg) and xylazine (13 mg/kg). Rectal temperature was maintained at 37 ± 0.5°C using a feedback-regulated water-heating pad. Rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The second craniotomy was bilaterally symmetrical to the first and performed in the contralateral skull allowing for lateral movement of cortical tissue. The dura mater was kept intact over the cortex.
Cortical injury was delivered by impacting the left parietal cortex (ipsilateral cortex) with a pneumatic piston of a 6-mm-diameter tip at a rate of 4 m/s and 2.5 mm of compression, as described in our previous study 11. The cranial defect was not filled after the impact. This moderate TBI induced by CCI targets the parietal cortex, and causes consistent cortical tissue loss (lesion cavity) and significant neuronal cell loss in the ipsilateral hippocampus as well as white matter damage 2.
Experimental groups
In the dose response experiment, the study animals were randomly divided into 5 groups (n = 8/group): 1) Sham; 2) TBI + Vehicle phosphate-buffered saline (PBS); 3) TBI + 50 μg exosomes/rat (Exo-50); 4) TBI+100 μg exosomes/rat (Exo-100); and 5) TBI + 200 μg exosomes/rat (Exo-200). Exosomes generated from MSCs (50, 100, and 200 μg total protein of exosome precipitate in 0.5 ml PBS/rat or an equal volume of PBS (0.5 ml) were administered intravenously over 5 min via tail vein, starting 1 day after injury. TBI animals treated with PBS served as a treatment control group. Sham animals underwent surgery without injury and treatment.
In the therapeutic window experiment (n=8/group), TBI rats received a single dose of 100 μg total protein of exosome precipitate in 0.5 ml PBS/rat via tail vein, starting at 1, 4, or 7 days post injury, respectively (designated as Group D1, D4, D7). TBI animals receiving 0.5 ml PBS as a control group. Sham animals underwent surgery without injury and treatment.
For labeling proliferating cells, 5-bromo-2’-deoxyuridine (BrdU, 100 mg/kg) was injected ip into all animals daily for 10 days, starting 1 day after TBI, as described in our previous study 14.
Evaluation of neurological outcome
Modified neurological severity score (mNSS) 15 and foot faults 16 were performed on all rats preinjury and at 1, 7, 14, 21, 28 and 35 days after TBI. To measure spatial learning and memory impairments, the Morris water maze (MWM) test was performed for 5 consecutive days on Days 31-35 after TBI, as described in our previous TBI study 11. All neurological functional tests were performed by observers blinded to the treatment groups. Please see Methods in the Supplemental Materials for details.
Tissue preparation, measurement of lesion volume and immunohistochemistry
On day 35 after TBI, anesthetized rats were sacrificed for collecting brains. Brains were embedded in paraffin and a series of 6-μm-thick slides were cut. The cortical lesion volume was measured. CD 68-(marker for microglia/macrophages), glial fibrillary acidic protein (GFAP, marker for astrocytes) and neuronal nuclei (NeuN, for neurons)-immunostainings were performed. Please see Methods in our previous study 12 and the Supplemental Materials for details.
Immunofluorescent staining and cell counting
Newly generated endothelial cells and newborn mature neurons 35 days after TBI were identified by double labeling for BrdU with endothelial barrier antigen (EBA) or neuronal nuclei (NeuN), respectively 11. EBA+ endothelial cells, CD68+ microglia/macrophages, GFAP+ astrocytes, and EBA/BrdU-colabeled cells were counted in the lesion boundary zone (LBZ) and the dentate gyrus (DG) 11. The LBZ is defined as the region of cortical tissue between the lesion cavity and the intact tissue (S1 Fig). For analysis of neurogenesis, we counted NeuN/BrdU-colabeled cells in the subgranular zone and granular cell layer of the DG. The fields of interest were digitized under the light microscope (Nikon, Eclipse 80i) at a magnification of either 200 or 400 using CoolSNAP color camera (Photometrics) interfaced with MetaMorph image analysis system (Molecular Devices). In brief, five fields of view in the LBZ from the epicenter of the injury cavity (bregma −3.3 mm), and 9 fields of view in the ipsilateral DG were counted in each section, as described in detail previously 17. Please see Methods in the Supplemental Materials for details.
Data analysis
Data are presented as the means with standard deviations. Data were tested for normality. When data were not normally distributed, ranked data or a nonparametric Kruskal-Wallis test was used. Analysis of variance (ANOVA) was used for repeated measurements of spatial performance and sensorimotor function. For cell counting, a one-way ANOVA followed by post hoc Tukey’s tests was used to compare the differences between the exosome-treated, PBS-treated and sham groups. Differences were considered significant if the P value was <0.05.
Results
Identification of exosomes from MSC culture medium
Using a qNano nanopore-based exosome detection system, we demonstrated that MSCs generate exosome-enriched particles (147.4 ± 62.6 nm in diameter, with a mode diameter at 122 nm in size, which is consistent with MSC exosomes identified with the transmission electron microscopy (S2 Fig A). Western blot confirmed that exosomes contained high levels of exosome proteins markers Alix and Hsp70 compared to the supernatant (S2 Fig B).
MSC exosome administration significantly enhances spatial learning in rats after TBI
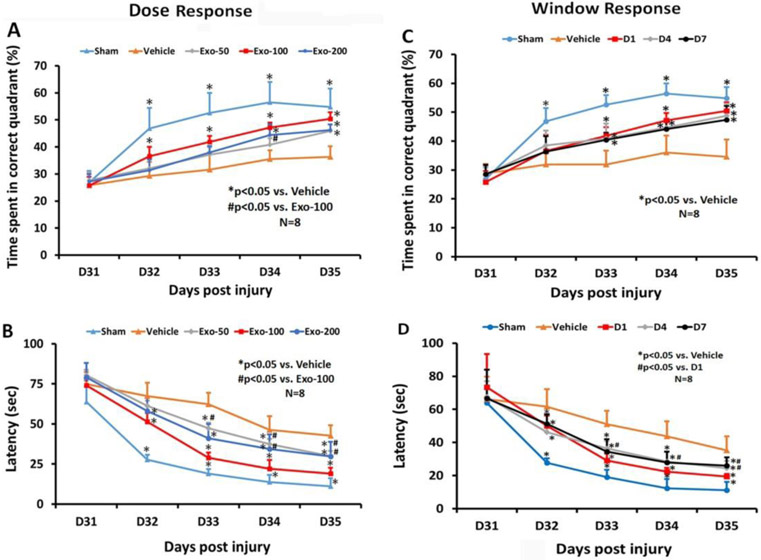
In the dose response study (Fig 1A), compared to the PBS group, post-hoc Tukey’s testing demonstrated significantly increased time spent by TBI rats treated with exosomes in the correct quadrant of the MWM test in the 50 μg exosome group at Day 35 (p < 0.05), 100 μg exosome group at Day 32-35 (p < 0.05) and 200 μg exosome group at Day 34 and 35 (p < 0.05). There was a statistically significant improved therapeutic effect with 100 μg than with the 50 μg at Day 34 (p < 0.05). There was a significantly reduced latency for TBI rats treated with exosomes in the correct quadrant in the 50 μg exosome group at Day 33-35 (Fig 1B, p < 0.05), 100 μg exosome group at Day 32-35 (p < 0.05) and 200 μg exosome group at Day 32-35 (p < 0.05). There was a statistically significant reduction in latency with 100 μg than 50 μg at Day 32–35 (p < 0.05), 100 μg than in 200 μg at Day 33–35 (p < 0.05). There was no significant difference in latency between 50 and 200 μg groups at all time points (p > 0.05).
Fig 1.
Treatment with exosomes significantly improves spatial learning after TBI. The dose response (A, B) and window response (C, D) studies show the percent time spent by animals in the correct quadrant where the hidden platform was located (A, C), and latency for animals to find the hidden platform (B, D). Data represent mean ± SD. N = 8/group.
In the therapeutic window study (Fig 1C), among the 5 groups, a statistically significant between-group effect on the time spent in the correct quadrant was noted in the MWM test at Day 32 to 35 (p < 0.001). Relative to the PBS group, there was a significantly increased time spent in the correct quadrant by TBI rats treated with exosomes in the D1, D4, and D7 exosome treatment groups at Day 33-35 (p < 0.05). There was no statistically significant difference in improvement among the 3 exosome groups (D1, D4, D7) from Day 31 to Day 35 (p>0,05). A statistically significant between-group effect on the latency was noted in the MWM test at Day 32 to 35 (Fig 1D, p < 0.001). Relative to the PBS group, at Day 32-35, a significantly reduced latency in the correct quadrant was detected in TBI rats treated with exosomes at different time points (D1, D4, D7) (p < 0.05). A significant difference in reducing latency was detected between D1 and D4 treatments at Days 33 and 34, between D1 and D4 treatments at Day 35, as well as between D1 and D7 treatment groups at Day 35 (p < 0.05), suggesting that the D1 treatment is most effective. There was no significance difference in reducing latency between D4 and D7 treatment groups (p > 0.05).
MSC exosome significantly promotes sensorimotor functional recovery in rats after TBI
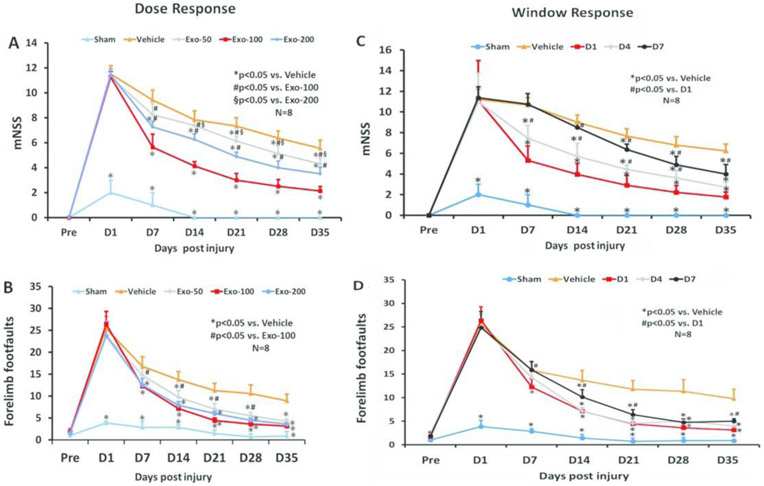
The mNSS score was identical in the PBS and exosome groups on Day 1 post TBI, indicating neurological functional deficits were comparable in all TBI rats before treatment (Fig 2A, F3,28=0.24, p = 0.87). A significant reduction in the mNSS score was found over time in the PBS-treated animals starting from Day 7 and up to Day 35 compared to Day 1 post injury (F5, 42 = 76.37, p < 0.01), suggesting a significant spontaneous sensorimotor functional recovery occurring after TBI. In the dose response study (Fig 2A), compared to the PBS treatment, functional recovery was significantly improved in the 3 exosome-treated groups (50, 100, 200 μg/rat) on Days 7-35 after TBI (p < 0.01, post-hoc Tukey’s test). There was a significant reduction in mNSS in the 100 μg group than in the 50 μg and 200 μg groups at Day 7-35 (p < 0.05). There was a significant effect of 200 μg treatment on mNSS compared with the 50 μg treatment group at Day 14-35 (p < 0.05). There was no significant difference in footfault tests among TBI groups at Day 1 post injury before assignment of treatments (PBS or exosomes). A significant reduction in the footfault number was found over time in the PBS-treated animals starting from Day 7 and up to Day 35 compared to Day 1 post injury (F5, 42 = 88.95, p < 0.01), suggesting the presence of a significant spontaneous recovery in footfault after TBI. Exosome treatment (50-200 μg/rat) also significantly reduced the frequency of forelimb footfault occurrence as compared to PBS controls (Fig 2B, at Day 7 to 35, p < 0.01). There was a significant reduction in footfault in the 100 μg group than in the 50 μg group at Day 7-28 (p < 0.05).
Fig 2.
Treatment with exosomes significantly improves sensorimotor function after TBI. The dose response (A, B) and window response (C, D) studies show the mNSS score (A, C) and frequency of foot-fault occurrence (B, D). Data represent mean ± SD. N = 8/group.
In the therapeutic window study, a statistically significant between-group effect on reducing mNSS was noted at Day 7 to 35 (Fig 2C, p < 0.001). Relative to the PBS group, a significantly reduced mNSS was found in TBI rats treated with 100 μg/rat exosomes (D1, D4, and D7 groups) at Day 21-35 (p < 0.05). In addition, early treatments (D1 and D4 groups) significantly reduced mNSS at Day 7 and 14 post injury compared to the PBS group (p < 0.05). There was no statistically significant effect on mNSS in the D7 exosome group at Day 14 compared to the PBS groups (p > 0.05). The earlier the exosome treatment started, the better effect on reducing mNSS was observed (D1>D4>D7 groups, p<0.05). There was a statistically significant between-group effect on footfaults was noted at Day 7 to 35 (p<0.05). Relative to the PBS group, at Day 14-35, a significantly reduced footfault occurrence was found in TBI rats treated with exosomes at different time points (D1, D4, D7) (p < 0.05). There was no significantly improved therapeutic effect on footfault occurrence between D1 and D4 groups (p > 0.05). However, the D1 and D4 groups had a significant reduction in footfault occurrence than D7 group at Day 14 (p < 0.05).
MSC exosome administration significantly reduces hippocampal neuronal cell loss in rats after TBI
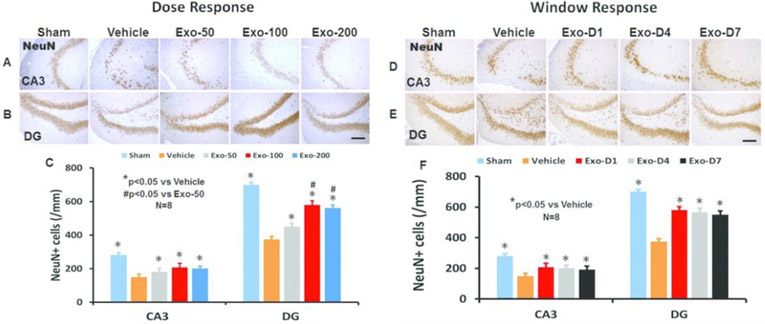
In the dose response study (Fig 3A), compared to the PBS treatment, exosome treatments (50, 100, 200 μg/rat) significantly reduced the NeuN+ neuronal cell loss in the CA3 (F4,35 = 47.00, p < 0.05) and DG region (F4,35 = 328.91, p < 0.05) detected at Day 35 post injury. A statistically significant effect with higher doses of exosomes on reducing neuronal cell loss was found in the DG (100 μg vs 50 μg, 200 μg vs 50 μg, p < 0.05). There was no significant effect on neuronal cell number in the CA3 among 3 exosomes doses (p > 0.05).
Fig 3.
Treatment with exosomes significantly reduces hippocampal neuronal cell loss after TBI. NeuN staining was performed for detection of mature neurons at day 35 after TBI in the CA3 region and dentate gyrus (DG). Representative images show NeuN+ cells in the dose response (A, B) and window response (D, E) studies. Scale bars = 100 μm. Quantitative data shown in bar graphs (C, F) represent mean ± SD. N = 8/group.
In the window study (Fig 3B), compared to the PBS treatment, exosome treatments (D1, D4, and D7) significantly reduced the NeuN+ neuronal cell loss in the CA3 (F4,35 = 49.05, p < 0.05) and DG region (F4,35 = 235.01, p < 0.05) detected at Day 35 post injury. No statistically significant difference in the effect of exosomes on reducing neuronal cell loss in the DG and CA3 regions among the 3 exosome treatment groups was detected (p > 0.05).
MSC exosome administration significantly increases vascular density and angiogenesis in rats after TBI
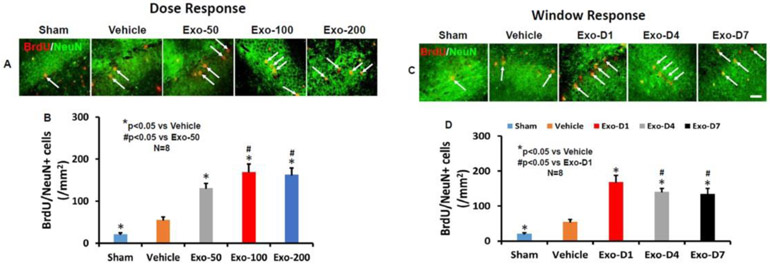
TBI alone significantly increased the density of vessels measured at Day 35 post injury in the LBZ (Fig 4, F4,35 = 363.55, p < 0.001) and DG (F4,35 = 219.12, p < 0.001) of the ipsilateral hemisphere compared to sham controls. Exosome treatments significantly increased the vascular density in the LBZ and DG compared to the PBS treatment (Fig 4A and 4C, p < 0.01). Exosome treatment at 50-200 μg/rat significantly increased angiogenesis identified by EBA/BrdU+ double labeling for newborn endothelial cells in the LBZ (Fig 4B and 4D, F4,35 = 264.91, p < 0.001) and DG (F4,35 = 300.63, p < 0.001) compared to the PBS treatment. No significant increases in vascular density and angiogenesis in the injured brain were detected between 100 and 200 μg groups (p > 0.05). However, the 100 and 200 μg treatment groups exhibited significantly increased vascular density and angiogenesis compared with the 50 μg group (p < 0.05).
Fig 4.
Treatment with exosomes significantly increases vascular density and angiogenesis after TBI. EBA staining was performed for detection of mature vasculature at day 35 after TBI in the lesion boundary zone (LBZ) and DG. Representative images from injured brain show EBA+ endothelial cells in the dose response (A) and window response (E) studies. Scale bars = 50 μm. Representative images show BrdU+/EBA+ newly generated endothelial cells in the dose response (B) and window response (F) studies. Scale bars = 25 μm. Quantitative data on vascular density (C, G) and angiogenesis (D, H) shown in bar graphs represent mean ± SD. N = 8/group.
In the therapeutic window study (Fig 4E-4H), exosome treatment initiated at Day 1, 4, and 7 post injury at 100 μg/rat significantly increased the vascular density (the number of EBA+ cells) in the LBZ (Fig 4E and 4G, F4,35 = 423.47, p < 0.001) and DG (F4,35 = 385.53, p < 0.001) compared to the PBS treatment. Exosome treatments (Day 1, 4, and 7) significantly increased angiogenesis identified by EBA/BrdU+ cells in the LBZ (Fig 4F and 4H, F4,35 = 332.00, p < 0.001) and DG (F4,35 = 146.57, p < 0.001) compared to the PBS treatment (p < 0.01). There was a significant increase in both vascular density and angiogenesis in the LBZ and DG in the D1 group compared to the D4 or D7 groups, p < 0.05). There were no significant differences in the vascular density and angiogenesis in the LBZ and DG between the D4 and D7 exosome groups (p > 0.05).
MSC exosome administration significantly increases neurogenesis in the dentate gyrus in rats after TBI
TBI alone significantly increased the number of newborn mature neurons in the DG of the ipsilateral hemisphere compared to sham controls measured at Day 35 post injury (Fig 5, F4,35 = 230.50, p < 0.001). Co-localization between BrdU and NeuN was verified using a Zeiss LSM 510 META confocal laser scanning microscope (S3 Fig). However, exosome treatments at doses of 50, 100 and 200 μg/rat significantly increased the number of newborn neurons compared to the PBS control (Fig 5A and 5B, p < 0.01). A significant increase in neurogenesis was detected in the 100 and 200 μg groups compared to the 50 μg group (p < 0.05). There was no significant difference between the 100 and 200 μg groups (p > 0.05).
Fig 5.
Treatment with exosomes significantly increases neurogenesis 35 days after TBI. Double staining with BrdU (red)/NeuN (green) was performed to identify newborn mature neurons in the DG (yellow arrows). Scale bar = 25 μm. Representative images show BrdU+/NeuN+ newly generated neurons in the dose response (A) and window response (C) studies. Scale bars = 25 μm. Quantitative data shown in bar graphs (B, D) represent mean ± SD. N = 8/group.
In the therapeutic window study, exosome treatments at Day 1, 4, and 7 post injury significantly increased the number of newborn neurons compared to the PBS control (Fig 5C and 5D, F4,35 = 209.54, p < 0.01). A significantly increased effect was detected in the D1 group compared to the D4 or D7 group, (p < 0.05). There was no significant difference between the D4 and D7 groups (p > 0.05).
MSC exosome administration significantly reduces brain inflammation in rats after TBI
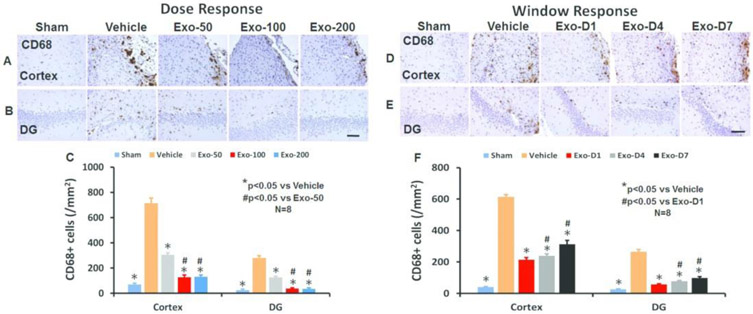
TBI alone significantly increased the density of CD68+ macrophages/microglia in the LBZ (Fig 6A and 6C, F4,35 =1100.10, p < 0.01) and DG (Fig 6B and 6C, F4,35 = 751.50, p < 0.01) of the ipsilateral hemisphere compared to sham controls. Exosome treatments at doses of 50, 100 and 200 μg/rat significantly reduced the CD68+ cell density in the LBZ and DG compared to the PBS treatment (Fig 6C, p < 0.01). Compared to the 50 μg group, the 100 and 200 μg groups exhibited significantly reduced CD68+ cell number in the LBZ and DG area (p < 0.01). There were no significant differences detected in CD68+ cells between 100 and 200 μg groups (p > 0.05). In the therapeutic window study, exosome treatments at Day 1, 4, and 7 post injury significantly reduced the number of CD68+ cells (F4,35 = 826.72 for cortex and 1023.30 for DG, p < 0.01) compared to the PBS controls (Fig 6D-F). The D1 and D4 groups exhibited a significant reduction of CD68+ cells in compared to the D7 group in the LBZ and DG (p < 0.05, with ANOVA followed by post-hoc Tukey’s test).
Fig 6.
Treatment with exosomes significantly reduces the number of activated CD68+ microglia/macrophages after TBI. CD68 staining was performed to identify activated microglia/macrophages in the dose response (A for cortex, B for DG) and window response (D for cortex, E for DG) studies. Scale bar = 100 μm. Quantitative data shown in bar graphs (C, F) represent mean ± SD. N = 8/group.
TBI also significantly increased the density of GFAP+ astrocytes compared to sham controls (S4 Fig A-C, p < 0.01). Exosome treatments (50-200 μg/rat) significantly reduced the GFAP+ astrocyte density in the LBZ and DG compared to the PBS treatment (S4 Fig A-C, p < 0.01). The 100 and 200 μg treatment groups exhibited significantly reduced GFAP+ cell number in the injured brain compared to the 50 μg treatment group (p < 0.05). There was no significant difference between the 100 and 200 μg treatment groups. There was no significant difference in GFAP+ cells among the D1, D4 and D7 groups (S4 Fig D-F, p > 0.05).
Exosome administration does not alter the volume of total brain tissue loss in rats after TBI
No significant differences in the lesion volume were observed 35 days post injury (S5 Fig A-D, p > 0.05).
Discussion
The present study demonstrates that intravenous administration of MSC-derived exosomes significantly: 1) improves cognitive and sensorimotor functional recovery in rats after TBI; 2) increases the number of BrdU+/NeuN+ newborn mature neurons in the DG (neurogenesis); 3) increases the number of BrdU+/EBA+ newborn endothelial cells in the LBZ and in the DG (angiogenesis); 4) reduces neuronal cell loss in the CA3 and in the DG (neuroprotection); and 5) reduces the number of CD68+ microglia/macrophages and GFAP+ astrocytes in the LBZ and DG (neuroinflammation). The levels of neuroprotection and neurorestoration are affected in a dose response manner with a greater effect detected at 100 μg/rat exosomes administered 1 day after injury. In addition, the therapeutic window of exosomes can be extended up to at least 7 days post TBI. These therapeutic effects of exosomes are not confined to a single cell type or region of the injured brain after TBI. Exosomes showed significant effects in both the injured cortex and hippocampus in terms of neuroinflammation and angiogenesis and neurogenesis in the DG.
Among stem cells, MSCs a mixed cell population of stem and progenitor cells, are a promising source of cell-based therapy for TBI because they can be easily isolated from many tissues and expanded in culture from patients without ethical and immune rejection problems 7, 18. TBI induces acute and chronic immune response in the brain.8 Our recent studies show that intravenous injection of 100 μg total protein of exosomes collected from culture media of approximately 2 × 106 MSCs improves functional recovery in rats with stroke 19 and TBI 11. Treatment with exosomes derived from MSCs improve functional recovery and neuroplasticity by reducing long-term neuroinflammation in TBI rats 11, 12, 20. In this study, we first determined a dose-response efficacy for this novel mode of exosome treatment for TBI. Our data indicate that a higher dose of exosomes (200 μg/rat) does not provide additional beneficial effects compared to 100 μg/rat group. However, a lower dose of exosomes (50 μg/rat) provides less beneficial effects that 100 μg/rat. Our dose response study is in line with a recent report showing that intravenous administration of exosomes from adipose tissue derived human mesenchymal stem cells (50 μg, 100 μg and 200 μg/rat, 1 day post injury) improves functional recovery, brain repair, and neural connectivity in rats with subcortical stroke induced by injecting endothelin-1 21. Our data suggest that 50-200 μg of exosomes enhance brain repair and recovery in TBI rats, whereas 100 μg of exosomes is the most effective dose when treatment is initiated 1 day post CCI-TBI.
Moderate TBI induced by CCI not only causes cortical tissue loss but also neuronal cell loss in the CA3 region and DG of hippocampus 11. The hippocampus is an important brain structure for learning and memory 11. Therefore, we analyzed cell loss, angiogenesis and neurogenesis and inflammation in the hippocampus in addition to the LBZ. Emerging preclinical studies indicate that posttraumatic prolonged and progressive neuroinflammation is associated neurodegeneration which may be treatable long after the initiating brain injury 22. Neuroinflammation is a key component of the secondary injury cascades following TBI 22. In the present study, exosomes significantly suppressed activation of GFAP+ astrocytes and CD68+ microglia/macrophages compared to the PBS control. This anti-inflammatory effect is similar to that of MSC therapy in animal models of stroke 23, 24 and TBI 25. One of main functions for astrocytes and microglia is to monitor and sustain neuronal health by releasing pro and anti-inflammatory cytokines, free radicals, anti-oxidants, and neurotrophic factors 26. Activated astrocytes and microglia contribute to both neuronal death and survival in neurodegeneration 27 and TBI 28, 29. Our data support that suppression of activated microglia/macrophages by exosomes may contribute to increased angiogenesis and neurogenesis, and subsequent improvement in functional recovery after TBI.
To explore the therapeutic window for MSC derived exosome treatment of TBI, onset of exosome treatment was initiated at different times (1 day, 4 days and 7 days post injury). There was a significant time effect on reducing neuroinflammation and neurovascular remodeling in both the injured cortex and hippocampus. Our present data demonstrate that MSC-derived exosomes improve functional recovery in rodents with a wide range of therapeutic window from 1 day to 7 days at a dose of 100 μg after TBI, which is consistent with the therapeutic window of parental MSCs for treatment of TBI rats 30-35. A recent study in a large animal model of TBI and hemorrhagic shock demonstrates that early ( 1 hour post injury) treatment with a single dose of MSC-derived exosomes significantly reduces brain swelling and lesion size, lower levels of blood-based TBI biomarkers, and improves blood brain barrier integrity 36. However, we note, that we do not exclude the possibility of therapeutic effects with exosome treatments initiated beyond 7 days post TBI.
Exosomes contain complex molecular cargo 37, 38. The benefit and potential strength of exosome treatment, as with stem cell therapy, result from targeting multiple injury and repair mechanisms mediated by delivery of exosomal complex cargo to recipient cells. One single microRNA can regulate many genes at posttranscriptional level 39. Exosome therapy may transfer miRNA to elicit a multitargeted effect, rather than the traditional, single molecular pathway approach 8, 40, 41. The precise mechanisms of exosome therapeutic effects on functional recovery after TBI are not clear. Exosomes can incorporate into recipient parenchymal cells in rats with stroke 42 and TBI 8. Intravenous administration of cell-free MSC-generated exosomes improves functional recovery and enhances neurite remodeling, neurogenesis, and angiogenesis after brain injuries in rats 11, 12, 19, pigs 43, and monkeys 44. Further studies are warranted to identify the molecular constituents of the exosomes, including specific miRNAs and growth factors that promote angiogenesis and neurogenesis, and reduce neuroinflammation after TBI. When the specific molecules necessary for a therapeutic effect are identified, selective manipulation of expression of those molecules in the parent MSCs may lead to an enhancement of the therapeutic efficiency of isolated exosomes.
Neurovascular units (NVUs) in the central nervous system consists of endothelial cells, pericytes, neurons, glial cells and extracellular matrix proteins 45. The generation of new vasculature facilitates highly coupled neurorestorative processes including neurogenesis and synaptogenesis, which in turn lead to improved functional recovery 45. New neurons are generated from neural stem/progenitor cells occurring in mammals during adulthood and in the pathology of different neurological disorders, and thus neurogenesis may be a potential target area for treatments 46. Exosome-induced angiogenesis may contribute to motor functional recovery by promoting neurite growth and synaptogenesis in the injured brain 19. Our data in the DG suggest that exosome-induced angiogenesis and neurogenesis may play an important role in improving learning and memory after brain injury, which is consistent with previous studies 47-50. In addition to exosomes, MSCs also release other extracellular particles and soluble factors, which may contribute to therapeutic effects underlying MSC therapy 37, 38. We do not exclude their effect in MSC-mediating TBI recovery.
In the present study, the cell counting was performed using a profile-based method on a computer monitor to improve visualization and in one focal plane to avoid oversampling 51, 52. This method, although suboptimal, provides a meaningful comparison of differences in cell numbers among groups after brain injuries 51, 52. Since many investigators seek to determine relative and significant differences in cell numbers between treatment and control groups rather than attempting to estimate absolute cell numbers, it has been argued that in many cases the systematic bias will be canceled out, and thus conventional methods suffice for this task 53.
Collecting comparably reliable physical dissector data is very time-consuming requiring considerably more sophisticated equipment than profile counts 54. Since we are interested in detecting a significant difference in relative cell numbers among groups, the profile counting is the more rational choice. It should be emphasized that when information about absolute cell numbers is required, major biases should be ruled out by calibration or the unbiased stereology method should be employed.
Conclusions
MSC derived exosomes administered intravenously following moderate CCI-TBI in rats improve function recovery with a wide therapeutic window (at least up to 7 days) and offer substantial neuroprotective and neurorestorative effects by reducing neuronal cell loss, neuroinflammation, and amplifying angiogenesis and neurogenesis. Our data and literature suggest that intravenous administration of exosomes may represent a novel therapeutic approach for treatment of acute and subacute TBI.
Supplementary Material
Acknowledgements
The authors thank Sutapa Santra and Qinge Lu for their technical assistance.
Source of funding: This work was supported by National Institutes of Health Grant 1R01NS100710-01A1 to YX.
Abbreviations
- ANOVA
analysis of variance
- BrdU
5-bromo-2’-deoxyuridine
- CCI
controlled cortical impact
- DG
dentate gyrus
- EBA
endothelial barrier antigen
- FITC
Fluorescein isothiocyanate
- MSC
mesenchymal stem cell
- GFAP
glial fibrillary acidic protein
- ip
intraperitoneally
- LBZ
lesion boundary zone
- mNSS
modified neurological severity score
- MWM
Morris water maze
- NTA
nanoparticle tracking analysis
- NeuN
neuronal nuclei
- NVU
Neurovascular unit
- PBS
phosphate-buffered saline
- TEM
transmission electron microscopy
- TBI
traumatic brain injury
Footnotes
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Runyan DK. The challenges of assessing the incidence of inflicted traumatic brain injury: a world perspective. Am J Prev Med. 2008;34:S112–5. [DOI] [PubMed] [Google Scholar]
- 2.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–30. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–72. [DOI] [PubMed] [Google Scholar]
- 5.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–63. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol 2002;1:92–100. [DOI] [PubMed] [Google Scholar]
- 8.Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo SA, Sugimoto H, O'Connell JT, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Chopp M, Zhang ZG, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin H, Li Y, Buller B, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Y, Mahmood A, Meng Y, et al. Treatment of traumatic brain injury with thymosin beta(4) in rats. J Neurosurg. 2011;114:102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–8. [DOI] [PubMed] [Google Scholar]
- 16.Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Chopp M, Meng Y, et al. Improvement in functional recovery with administration of Cerebrolysin after experimental closed head injury. J Neurosurg. 2013;118:1343–55. [DOI] [PubMed] [Google Scholar]
- 18.Hasan A, Deeb G, Rahal R, et al. Mesenchymal Stem Cells in the Treatment of Traumatic Brain Injury. Front Neurol. 2017;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez-Fernãndez M, Laso-García F, Frutos MCG-d, et al. Abstract TMP31: Exosomes Role in Brain Repair and Recovery in Stroke. A Dose Response Study. Stroke. 2018;49:ATMP31–ATMP31. [Google Scholar]
- 22.Xiong Y, Mahmood A, Chopp M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin J Traumatol. 2018;21:137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin H, Chopp M, Shen LH, et al. Multipotent mesenchymal stromal cells decrease transforming growth factor beta1 expression in microglia/macrophages and down-regulate plasminogen activator inhibitor 1 expression in astrocytes after stroke. Neurosci Lett. 2013;542:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai MJ, Tsai SK, Hu BR, et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci. 2014;21:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R, Liu Y, Yan K, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S, Swarnkar S, Goswami P, Nath C. Astrocytes and microglia: responses to neuropathological conditions. Int J Neurosci. 2011;121:589–97. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Chopp M, Mahmood A, Meng Y, Qu C, Xiong Y. Impact of inhibition of erythropoietin treatment-mediated neurogenesis in the dentate gyrus of the hippocampus on restoration of spatial learning after traumatic brain injury. Exp Neurol. 2012;235:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Mahmood A, Lu D, et al. Simvastatin attenuates microglial cells and astrocyte activation and decreases interleukin-1beta level after traumatic brain injury. Neurosurgery. 2009;65:179–85; discussion 185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–31; discussion 1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmood A, Wu H, Qu C, Xiong Y, Chopp M. Effects of treating traumatic brain injury with collagen scaffolds and human bone marrow stromal cells on sprouting of corticospinal tract axons into the denervated side of the spinal cord. J Neurosurg. 2013;118:381–9. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, Hong K. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke. 2011;42:1437–44. [DOI] [PubMed] [Google Scholar]
- 34.Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. [DOI] [PubMed] [Google Scholar]
- 35.Bedi SS, Aertker BM, Liao GP, et al. Therapeutic time window of multipotent adult progenitor therapy after traumatic brain injury. J Neuroinflammation. 2018;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AM, Bhatti UF, Brown JF, et al. Early Single-Dose Treatment with Exosomes Provides Neuroprotection and Improves Blood-Brain Barrier Integrity in Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J Trauma Acute Care Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 37.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai RC, Yeo RW, Tan KH, Lim SK. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen Med. 2013;8:197–209. [DOI] [PubMed] [Google Scholar]
- 39.Lakshmipathy U, Hart RP. Concise review: MicroRNA expression in multipotent mesenchymal stromal cells. Stem Cells. 2008;26:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15:193–203. [DOI] [PubMed] [Google Scholar]
- 41.Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin H, Li Y, Liu Z, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams AM, Dennahy IS, Bhatti UF, et al. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J Neurotrauma. 2019;36:54–60. [DOI] [PubMed] [Google Scholar]
- 44.Moore TL, Bowley BGE, Pessina MA, et al. Mesenchymal derived exosomes enhance recovery of motor function in a monkey model of cortical injury. Restor Neurol Neurosci. 2019;37:347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- 46.Taupin P The therapeutic potential of adult neural stem cells. Curr Opin Mol Ther. 2006;8:225–31. [PubMed] [Google Scholar]
- 47.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276:4644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. [DOI] [PubMed] [Google Scholar]
- 49.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong Y, Zhang Y, Mahmood A, Meng Y, Qu C, Chopp M. Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Transl Stroke Res. 2011;2:619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang R, Wang Y, Zhang L, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–80. [DOI] [PubMed] [Google Scholar]
- 52.Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma. 2010;27:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Bartheld C Counting particles in tissue sections: choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–48. [DOI] [PubMed] [Google Scholar]
- 54.Farel PB. Trust, but verify: the necessity of empirical verification in quantitative neurobiology. Anat Rec. 2002;269:157–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.