Abstract
Background:
Functional neurological disorder (FND) is a prevalent neuropsychiatric condition characterized by sensorimotor difficulties. Patients with FND at times report that sensory experiences trigger and/or exacerbate their symptoms. Sensory processing difficulties are also commonly reported in other psychiatric disorders frequently comorbid in FND, suggesting that contextualizing sensory profiles in FND within a biopsychosocial model may be clinically-relevant.
Objective:
To address this literature gap, we conducted a retrospective cohort study to examine sensory processing patterns and their relationship to other neuropsychiatric characteristics in patients with FND.
Methods:
A retrospective chart review design was used to investigate sensory processing patterns, established with the Adolescent/Adult Sensory Profile self-report questionnaire, in 44 patients with FND. Univariate analyses of cross-sectional screening tests followed by multivariate linear regression analyses were performed to identify neuropsychiatric factors associated with sensory processing scores in the FND cohort.
Results:
Compared to normative data, the majority of patients with FND reported sensory processing tendencies toward low registration, sensory sensitivity, and sensation avoiding. In multivariate regression analyses, the presence of a lifetime anxiety disorder independently predicted elevated low registration scores, while female gender and number of current medications independently predicted increased sensory sensitivity scores. In uncorrected univariate analyses only, individuals with psychogenic nonepileptic seizures were more likely to report increased sensory sensitivity and elevated low registration.
Conclusion:
These preliminary findings support sensory processing difficulties in some patients with FND. Prospective and large sample size studies are needed to investigate relationships between sensory processing profiles, neuropsychiatric comorbidities, FND subtypes, and treatment outcomes.
Keywords: conversion disorder, psychogenic, dissociative seizures, functional movement disorders, sensory processing dysfunction
INTRODUCTION
Functional neurological disorder (FND), also known as conversion disorder, is a prevalent neuropsychiatric condition encountered across outpatient and inpatient clinical settings(1-3). Renewed interest in FND has emerged over the past decade, in part due to increased diagnostic specificity from “rule in” examination signs(4) and a growing repertoire of evidence-based treatments(5, 6). Foundational to developing a patient-centered treatment plan in this population is the use of the biopsychosocial model to identify predisposing vulnerabilities, acute precipitants and perpetuating factors(7, 8). Within this context, FND patients with paroxysmal or waxing and waning symptoms commonly report that sensory experiences (e.g. bright lights, loud noises, pain) trigger and/or amplify their functional neurological symptoms(9, 10). Sensory processing difficulties are also common in mood, anxiety and trauma-related disorders frequently comorbid in FND(11-16). In addition to education(17), motor retraining(5) and cognitive behavioral therapy(6, 18), occupational therapy has emerged as another potential therapeutic modality for FND(19, 20). We have previously highlighted that sensory processing difficulties in individuals with FND are an important aspect of this condition that remains poorly characterized(21). Additionally, impairments in interoception, attentional allocation and perceptual inferences are implicated in emerging conceptual models for FND(22-24). Given that sensory processing patterns in FND have yet to be well characterized using validated scales, this is an important gap in the literature that if clarified could shed light on another potential predisposing vulnerability and perpetuating factor in some patients with FND.
Sensory processing relates to the way one detects, regulates, interprets, and responds to sensory stimuli(25). Winnie Dunn developed the Four Quadrant Model of Sensory Processing(26), which proposes a relationship between neurological threshold and behavioral responses. A low neurological threshold means a person will notice and respond to stimuli easily, while a high neurological threshold indicates a need for stronger stimuli to activate a response. When a person attempts to self-regulate to a given sensory experience, they may utilize either passive or active behavioral strategies. Individuals employing active behavioral strategies control the type and amount of sensory input received (approach or avoidance), whereas those using a passive approach do not seek to modulate stimuli(27). Within this model, there are 4 core sensory processing patterns: sensory seeking (high neurological threshold, active responses), low registration (high neurological threshold, passive responses), sensory avoiding (low neurological threshold, active responses), and sensory sensitivity (low neurological threshold, passive responses)(26, 28). A main principle of Dunn’s Four Quadrant Model of Sensory Processing(28) is that in order to effectively modulate sensation for adaptive behavioral responses, there must be an appropriate balance between habituation and sensitization(28). People with sensory sensitivity tend to be hyper-focused on sensory experiences from the body and environment(29), creating a sustained state of hyperarousal, emotional dysregulation and hypervigilance. An individual can be described as sensory defensive when the nervous system is easily triggered so that sensory stimuli are abnormally perceived as harmful or threatening, eliciting the flight or fight sympathetic nervous system response(30). Extreme sensory processing patterns, or sensory processing dysfunction, is broadly characterized by an inability to modulate responses to sensory stimuli.
The Adolescent / Adult Sensory Profile (AASP) is a widely used and validated self-report measure of sensory processing in the occupational therapy literature with good psychometric properties(31). The AASP measures an individual’s sensory processing and concurrent behavioral responses based on the Four Quadrant Model of Sensory Processing(29). Notably, sensory processing dysfunction has been characterized in anxiety, depression, and trauma-related disorders(11-16). Sensory processing dysfunction in these psychiatric populations is associated with childhood abuse burden(16), psychological distress(32), poor stress coping(33), interpersonal difficulties(34, 35), and alexithymia(14, 16, 32), which themselves are predisposing vulnerabilities for the development of FND(36-38).
In this retrospective, cross-sectional cohort study, we first describe AASP data in 44 patients with FND. Thereafter, univariate screenings followed by multivariate regression analyses were performed to evaluate how baseline neuropsychiatric factors related to the four sensory processing patterns as measured by the AASP: low registration, sensation seeking, sensory sensitive and sensation avoiding. We hypothesized that individuals with psychogenic nonepileptic (dissociative) seizures (PNES), given the inherent paroxysmal nature of events, would be associated with prominent sensory avoiding and sensory sensitivity tendencies (low neurological thresholds coupled with either active or passive behavioral strategies). We also hypothesized that those with childhood physical / sexual abuse, post-traumatic stress disorder (PTSD) and comorbid pain would be more likely to exhibit sensory processing difficulties(13, 16).
METHODS
Participants
This study was approved by the Partners Healthcare institutional review board, and individual informed consent was not required. The retrospective chart review included 44 consecutive individuals (34 women, 10 men; mean age=42.6±15.4) diagnosed with FND who had available baseline AASP data obtained from an initial outpatient occupational therapy assessment. These individuals were evaluated in the Massachusetts General Hospital FND clinic between Jan 2018 and June 2019, and were diagnosed with FND using DSM-5 diagnostic criteria, emphasizing physical examination signs and/or semiological features(4, 39). This transdiagnostic cohort included individuals across the motor spectrum, including clinically-established functional movement disorders (N=34), functional weakness/paresis (N=11), and PNES (N=10). Diagnoses were not mutually exclusive as 10 of 44 individuals had two or more functional motor symptoms. Seven also had major neurologic comorbidities, including: cerebral palsy with lower extremity spasticity; mild generalized volume loss with concurrent peripheral neuropathy; mild generalized volume loss with a right frontal developmental venous anomaly; Parkinson disease; cerebellar arteriovenous malformation; left frontal arteriovenous malformation status post resection; and autism spectrum disorder with comorbid Tourette syndrome. An additional 8 individuals with FND evaluated during the same time period had missing AASP data and were thus excluded. See Table 1 for additional details regarding patient characteristics.
Table 1.
Univariate associations between sensory patterns and baseline neuropsychiatric factors in patients with functional neurological disorder. Test statistic is Mann-Whitney U or Spearman correlation coefficient. Test statistic values in bold are statistically significant at an uncorrected p-value<0.05. The college graduate variable had missing data in 3 subjects. Variable displayed in italics are shown for descriptive purposes only.
| Total Cohort (n=44) |
Low Registration |
Sensation Seeking |
Sensory Sensitivity |
Sensation Avoiding |
|
|---|---|---|---|---|---|
| n (%) or mean (SD) |
Test- Statistic |
Test- Statistic |
Test- Statistic |
Test- Statistic |
|
| Age in years | 42.6 (±15.4) | −0.085 | 0.289 | −0.063 | −0.123 |
| Gender | 34 F /10 M | 111.5 | 148.5 | 69.0 | 114.5 |
| Illness duration in years | 3.9 (±6.1) | −0.069 | 0.172 | −0.013 | −0.082 |
| Race | 37 W / 7 NW | 114.5 | 113.0 | 115.0 | 124.5 |
| Married | 22 (50%) | 211.0 | 165.0 | 224.0 | 235.0 |
| Completed college (≥16 years)* | 21 (48%) | 145.0 | 110.5 | 213.0 | 176.5 |
| Employed full time (or full-time student) | 13 (30%) | 156.0 | 168.0 | 162.5 | 168.0 |
| On or applying for work disability | 17 (39%) | 177.5 | 208.5 | 201.5 | 222.0 |
| Psychogenic nonepileptic seizures | 10 (23%) | 84.5 | 129.0 | 101.0 | 93.5 |
| Comorbid major neurological disorder** | 7 (16%) | 116.0 | 98.0 | 72.0 | 54.0 |
| Current cognitive symptoms | 31 (70%) | 130.5 | 172.5 | 136.0 | 155.5 |
| Current pain | 37 (84%) | 100.5 | 78.5 | 81.0 | 64.5 |
| H/O migraine | 17 (39%) | 225.5 | 201.0 | 210.5 | 206.0 |
| H/O head trauma | 24 (55%) | 179.5 | 229.0 | 191.5 | 172.5 |
| H/O functional somatic disorder | 12 (27%) | 151.5 | 140.0 | 157.0 | 171.0 |
| Fibromyalgia | 5 (11%) | - | - | - | - |
| Chronic fatigue | 3 (7%) | - | - | - | - |
| Irritable bowel syndrome | 9 (20%) | - | - | - | - |
| Lifetime depression | 33 (75%) | 133.0 | 164.0 | 122.5 | 147.5 |
| Lifetime anxiety | 36 (82%) | 50.0 | 137.5 | 93.0 | 109.5 |
| Lifetime post-traumatic stress disorder | 15 (34%) | 166.0 | 204.5 | 192.5 | 194.0 |
| Childhood physical and/or sexual abuse | 20 (45%) | 147.5 | 205.0 | 189.5 | 161.5 |
| Childhood verbal and/or emotional abuse | 16 (36%) | 169.5 | 185.0 | 183.0 | 160.5 |
| Lifetime alcohol/drug misuse | 4 (9%) | 77.5 | 58.5 | 70.5 | 78.0 |
| Number of current medications | 7.5 (±4.8) | 0.206 | 0.070 | 0.321 | 0.172 |
| Number of medication allergies | 2.1 (±2.9) | −0.133 | 0.040 | 0.002 | −0.053 |
| On SSRI/SNRI | 17 (39%) | 142.0 | 196.0 | 177.5 | 219.0 |
The following major neurological comorbidities were present in 7 patients: cerebral palsy with lower extremity spasticity; mild generalized volume loss with concurrent peripheral neuropathy; mild generalized volume loss with a right frontal developmental venous anomaly; Parkinson disease; cerebellar arteriovenous malformation; left frontal arteriovenous malformation status post resection; and autism spectrum disorder with comorbid Tourette syndrome.
Chart Reviews
Baseline demographic, neuropsychiatric, and psychosocial data were collected through medical chart review focusing on available data from the initial FND clinic evaluation and outpatient OT encounters. Two researchers not involved with patient care (P.R.A. and O.C.) independently performed chart reviews, and any differences were reconciled by group discussion with a double boarded neurologist-psychiatrist (D.L.P.).
Primary Measure of Interest
The AASP was administered during initial occupational therapy evaluations(11, 29). This 60-item self-report scale evaluates behavioral responses to everyday sensory experiences. Questions are organized according to the sensory processing categories of taste/smell, visual, touch, auditory, movement (vestibular) and activity (e.g., “I don’t like strong mints or candy,” “I keep the shades down during the day when I am at home”). A five-point Likert scale (1=almost never, 5=almost always) is used to rate each item. Scores for each item are sorted into four sensory processing patterns (quadrants) that cut across sensory categories as follows: low registration – individuals that fail to detect stimuli that others notice and use of passive behavioral strategies; sensation seeking – those that experience pleasure from enriched sensory environments along with use of sensation creating behaviors; sensory sensitivity – individuals feeling discomfort from sensory experiences and employing passive behavioral strategies; and sensation avoiding – those showing a tendency to limit sensory exposures while using active behavioral strategies to do so. Of note, the four quadrants of sensory processing are independent though not mutually exclusive. As such, some sensory processing patterns may seem contradictory (e.g. high scores in low registration and sensory sensitivity), although individuals can vary in their neurological thresholds and behavioral responses in the course of their daily experiences. Scores are interpreted using age-based normative data(29), and based on this, patients can be divided descriptively into 5 categories: similar to, more than, much more than, less than, or much less than most people. For descriptive purposes in this study and consistent with similar published approaches(22), the scores were reported as 3 categories: more than (combining more than and much more than), similar to and less than (combining less than and much less than) most people.
Statistical Analysis
To evaluate for potential relationships between all baseline neuropsychiatric characteristics and each sensory processing pattern used as a continuous score, nonparametric univariate screening tests were first performed (Mann-Whitney U and Spearman correlation). For statistically significant univariate relationships (p<0.05), second-level multivariate linear regression analyses were performed to evaluate for independent predictors of specific sensory processing patterns. All statistical analyses were performed using IBM SPSS 24.
RESULTS
Descriptive AASP findings
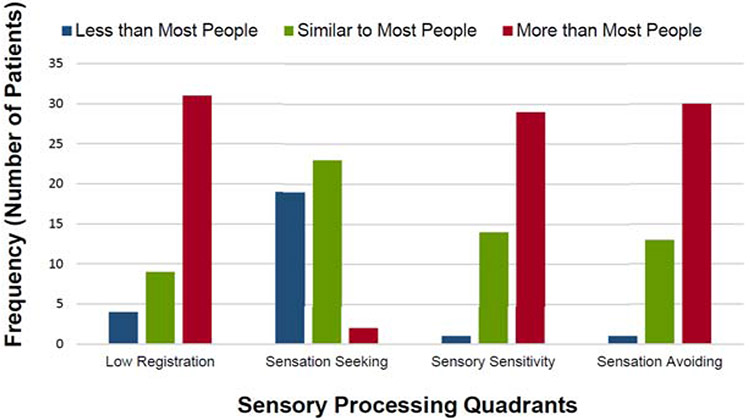
As shown in Figure 1, the majority of patients with FND reported elevated scores on 3 of the 4 sensory processing patterns: low registration (mean=40.0±10.6; 70.5% scored “more than most people”), sensory sensitivity (mean=46.3±11.5; 65.9% scored “more than most people”), and sensation avoiding (mean=45.5±10.7; 68.2% scored “more than most people”). Within the sensation seeking pattern, most individuals with FND in this cohort reported normal levels (mean=43.2±8.3; 52.3% scored “similar to most people”), although many also reported a lower level of sensation seeking tendencies (43.2% scored “less than most people”). See Supplementary Figure 1 for sensory processing scores displayed separately for those with and without a diagnosis of PNES.
Figure 1.
Characterization of sensory processing patterns in patients with functional neurological disorders (n=44) compared to normative data.
Low Registration (high neurological threshold, passive strategies)
Univariate analyses indicated that four variables were associated with elevated low registration scores: a diagnosis of PNES (p=0.015), lifetime anxiety disorder (p=0.003), childhood physical and/or sexual abuse (p=0.029), and current use of selective serotonin reuptake inhibitors (SSRIs) / serotonin norepinephrine reuptake inhibitors (SNRIs) (p=0.035) (See Table 1). A multivariate linear regression analysis showed that a lifetime anxiety disorder independently predicted having an elevated low registration sensory processing score (p=0.020) in patients with FND (See Table 2).
Table 2.
Linear regression analyses of baseline clinical factors and specific sensory processing patterns in functional neurological disorder. SSRI/SNRIs indicates selective serotonin reuptake inhibitors / serotonin norepinephrine reuptake inhibitors.
| Sensory Processing Patterns |
Variables identified by univariate screening | Unstandardized beta |
Standardized beta |
p- value |
|---|---|---|---|---|
| Low Registration | On SSRI/SNRIs | 4.264 | 0.198 | 0.154 |
| Childhood physical/sexual abuse | 3.152 | 0.150 | 0.287 | |
| Lifetime anxiety disorder | 8.896 | 0.327 | 0.020 | |
| Psychogenic nonepileptic seizure diagnosis | 6.066 | 0.243 | 0.092 | |
| Sensory Sensitivity | Female | 9.370 | 0.344 | 0.017 |
| Number of current medications | 0.713 | 0.295 | 0.039 | |
| Sensation Avoiding | Psychogenic nonepileptic seizure diagnosis | 6.171 | 0.245 | 0.098 |
| Comorbid major neurologic disorder | −7.247 | −0.252 | 0.099 | |
| Current pain | 4.705 | 0.163 | 0.291 |
Sensation Seeking (high neurological threshold, active strategies)
Graduating college (p=0.006) was the only variable in univariate analyses associated with elevated sensation seeking scores in our patient cohort. As such, a second-level multivariate regression analysis was not performed.
Sensory Sensitivity (low neurological threshold, passive strategies)
Univariate analyses indicated that female gender (p=0.005) and greater number of current medications (p=.034) correlated with elevated sensory sensitivity scores in patients with FND. Both female gender (p=0.017) and number of current medications at assessment (p=0.039) remained independent predictors of sensory sensitivity in a multivariate regression analysis.
Sensation Avoiding (low neurological threshold, active strategies)
Univariate analyses indicated that elevated sensation avoiding scores were associated with three variables: a diagnosis of PNES (p=.032), ongoing pain (p=0.037), and a lack of comorbid major neurological disorder (p=0.015). In multivariate analyses, none of the variables independently predicted sensation avoiding scores in patients with FND.
DISCUSSION
In this retrospective, cross-sectional cohort study, we characterized sensory processing patterns in 44 individuals with FND using the AASP. Compared to normative data, the majority of patients with FND reported sensory processing tendencies toward low registration, sensory sensitivity, and sensory avoiding. These sensory processing patterns suggests that some sensory experiences in patients with FND were perceived at low neurological thresholds and coupled with either passive (sensory sensitive) or active (sensory avoiding) behavioral strategies at frequencies much more common than the general population, confirming the existence of sensory processing difficulties in some patients with FND. Additionally, individuals with FND also reported other instances of high neurological thresholds for sensory perceptions coupled with passive behavioral responses (low registration). Overall, these findings suggest dysregulation in the ability to process a variety of bodily and environmental sensations in patients with FND, combined with use of poor coping strategies (both passive and active elements) for managing the emotional, behavioral and physiological responses to sensory experiences.
Lifetime Anxiety and Low Registration in FND
The sensory processing patterns detected in this FND cohort correspond well with previously published literature in other clinical and non-clinical populations. Specifically, multivariate analyses in our study found a relationship between lifetime anxiety disorders and elevated low registration scores in patients with FND. Similarly, Engel-Yeger and Dunn(11) found that healthy individuals with elevated low registration and sensory sensitivity scores showed heightened state and trait anxiety; sensory avoiding behaviors also correlated with state anxiety in this healthy subject cohort(11). In another study conducted in healthy subjects, negative affect correlated with elevated low registration, sensory sensitivity, and sensory avoiding scores, while positive affect correlated with active self-regulation strategies(40). In 231 adults with heterogenous psychiatric conditions recruited from the community, sensory processing difficulties correlated with lifetime anxiety disorders(41). In euthymic individuals with a history of mood disorders, low registration, sensory sensitivity and sensory avoiding correlated with alexithymia and impulsivity(27). Extreme sensory processing patterns were also identified in patients with non-specific chronic low back pain and central sensitization(42). Notably, mood, anxiety and pain comorbidities have prognostic implications for FND(20, 43, 44). While speculative, it is possible that sensory processing difficulties may represent a shared predisposing vulnerability for FND, mood/anxiety and trauma-related disorders, which along with other shared risk factors (e.g., childhood maltreatment(45)), may help explain the high psychiatric comorbidity rates in some individuals with FND.
Gender Differences and Sensory Sensitivity in FND
Our study also found that women compared to men with FND reported elevated sensory sensitivity. This can be contextualized using the literature on gender differences in sensory sensitivity and FND. In one study, healthy women scored higher than men in sensory sensitivity, low registration, and sensation seeking patterns(46), suggesting that the identified gender difference in our FND cohort may be indicative of a broader association between gender and specific sensory processing patterns. Notably, women with FND also report higher rates of childhood sexual abuse and anxiety disorders than men with FND(47, 48), and women are two to three times more likely than men to be diagnosed with FND(49). Thus, more work is needed to understand the intersection of FND, gender differences and other shared risk factors including sensory processing difficulties. This includes investigating if sensory processing difficulties may help mediate links between female gender, childhood maltreatment and the later-life development of FND.
PNES, Low Registration and Sensation Avoiding
The preliminary univariate finding that patients with PNES exhibited elevated sensation avoiding tendencies compared to others with FND is consistent with study hypotheses, and warrants additional inquiry in large patient samples. Clinically, we have encountered several patients with PNES who report that bright lights, loud noises or other sensory perceptions are experienced as noxious and triggering. This is similar to clinical observations that sensory experiences can precipitate headache in patients with a post-concussive syndrome. Interestingly, an association between mild traumatic brain injury, concussion and PNES has been described(50), including links between head trauma and poor prognosis in this population(51). While there are many overlapping characteristics between patients with PNES and those with other motor FND subtypes(52), individuals with PNES have been identified to potentially experience higher rates of adverse life events, dissociation and psychiatric hospitalizations compared to other FND populations(53). More research is needed to understand if the PNES subtype of FND is associated with specific abnormal sensory processing difficulties.
Potential Treatment Implications of Sensory Profiles in FND
Given the associations between discrete sensory processing difficulties, lifetime anxiety disorders and the PNES subtype of FND, the potential to provide sensory modulation training to patients with FND as an adjunctive treatment warrants research inquiry. The association between the number of currently prescribed medications and elevated sensory sensitivity scores observed in this study may also suggest that patients (and prescribers) are potentially turning to medications in part in an attempt to alleviate distressing sensory experiences in this population. From a non-pharmacologic perspective, sensory-based treatment for individuals experiencing sensory perceptions as distressing has produced positive results in the literature(33). Kinnealey, et al.(54) and Pfeiffer(55) proposed a model for treating adults with sensory processing dysfunction that is based on the core assumptions that people are experts on themselves and must be the agent of change in his or her own life. The model advocates five major components of intervention 1) education and insight, 2) self-advocacy, 3) sensory diet or regular, daily sensory input, 4) environmental adaptation, and 5) social supports(56). This treatment approach significantly improved anxiety symptoms and sensation tolerance in adults with sensory processing dysfunction and anxiety(57). Champagne(58) outlined the Sensory Modulation Program, a sensory-based treatment model emphasizing sensory diets and sensory-based modalities for self-awareness and self-regulation. In a case study examining the efficacy of the Sensory Modulation Program for an individual with PTSD and sensory processing difficulties (i.e. elevated sensory sensitive, low registration and sensation avoiding tendencies), one month of outpatient treatment led to decreased PTSD symptoms and more adaptive sensory responses(58). In addition to occupational therapy-based sensory modulation training, other psychotherapeutic modalities such as sensorimotor psychotherapy may also warrant consideration in patients with FND with abnormal sensory processing. For example, sensorimotor psychotherapy has been shown to address physiologic arousal, alexithymia, and emotional numbing in patients with PTSD, depression and anxiety through techniques that help patients to modulate autonomic arousal and reinstate adaptive responses(59). Our group is currently developing pilot observational studies to begin evaluating the tolerability and potential efficacy of occupational therapy-based interventions and related adjunctive psychotherapy treatments in patients with FND.
Limitations
The study limitations include the retrospective design, modest sample size, mixed FND population, presence of psychiatric comorbidities, concurrent psychotropic medication use, and lack of additional psychometric questionnaires to characterize the complete spectrum of predisposing vulnerabilities for the development of FND (e.g., childhood trauma questionnaire). While the AASP is a widely used self-report measure in the literature, several limitations including that the four dimensional patterns are not mutually exclusive and that the sensory processing patterns cut across sensory categories suggest that other self-report sensory processing questionnaires should also be piloted in FND populations(60). Experimental paradigms characterizing sensory perceptions and related behavioral responses would also augment the patient-reported data described here. While this study used a within-group design to characterize relationships between sensory processing patterns and other baseline variables, better powered studies are needed to evaluate if there are also between-group categorical distinctions in FND populations with and without sensory processing difficulties. On a related point, while we generally feel that dimensional consideration of sensory processing patterns has potential clinical and mechanistic utility warranting further inquiry, we are not necessarily advocating for a categorical framing of “sensory processing disorder” comorbidity in patients with FND. Future studies with prospective and longitudinal data collection are needed to investigate the potential clinical and neurobiological relevance of sensory processing difficulties in FND, including relationships with interoception, predictive coding(23), alexithymia, attachment styles(37), personality profiles(61) and clinical outcomes among other factors.
Conclusions
This study provides preliminary evidence that some individuals with FND report sensory processing difficulties as characterized by the AASP. Among other findings, FND patients with a lifetime history of anxiety were more likely to report sensory processing dysfunction. In addition to replicating our findings, future efforts are needed to determine the potential utility of occupational therapy and psychotherapy approaches aimed at adaptively modifying sensory processing in FND populations.
Supplementary Material
Sensory processing patterns in psychogenic nonepileptic seizures (panel A) (n=10). Sensory processing patterns in other motor functional neurological disorders (panel B) (n=34).
Acknowledgments
Funding:
D.L.P. received funding from the Massachusetts General Hospital Physician-Scientist Developmental Award, the Sidney R. Baer Jr. Foundation and the NIMH K23MH111983-03.
Footnotes
Disclosures:
D.L.P. has received honoraria for continuing medical education lectures in functional neurological disorder.
W.C.L. has served on the editorial boards of Epilepsia, Epilepsy & Behavior; Journal of Neurology, Neurosurgery and Psychiatry, and Journal of Neuropsychiatry and Clinical Neurosciences; receives editor’s royalties from the publication of Gates and Rowan’s Nonepileptic Seizures, 3rd ed. (Cambridge University Press, 2010) and 4th ed. (2018); author’s royalties for Taking Control of Your Seizures: Workbook and Therapist Guide (Oxford University Press, 2015); has received research support from the Department of Defense (DoD W81XWH-17-0169), NIH (NINDS 5K23NS45902 [PI]), Providence VAMC, Center for Neurorestoration and Neurotechnology, Rhode Island Hospital, the American Epilepsy Society (AES), the Epilepsy Foundation (EF), Brown University and the Siravo Foundation; serves on the Epilepsy Foundation New England Professional Advisory Board; received honoraria for the American Academy of Neurology Meeting Annual Course; served as a clinic development consultant at University of Colorado Denver, Cleveland Clinic, Spectrum Health, Emory University and Oregon Health Sciences University; and provided medicolegal expert testimony.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKee K, Glass S, Adams C, Stephen CD, King F, Parlman K, et al. The Inpatient Assessment and Management of Motor Functional Neurological Disorders: An Interdisciplinary Perspective. Psychosomatics. 2018;59(4):358–68. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR, Nakhate V, Stephen CD, Perez DL. Functional (Psychogenic) Neurological Disorders: Assessment and Acute Management in the Emergency Department. Semin Neurol. 2019;39(1):102–14. [DOI] [PubMed] [Google Scholar]
- 3.Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, et al. Who is referred to neurology clinics?--the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112(9):747–51. [DOI] [PubMed] [Google Scholar]
- 4.Daum C, Hubschmid M, Aybek S. The value of 'positive' clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180–90. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen G, Buszewicz M, Stevenson F, Hunter R, Holt K, Dudziec M, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry. 2017;88(6):484–90. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe M, Walker J, Williams C, Stone J, Cavanagh J, Murray G, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pick S, Goldstein LH, Perez DL, Nicholson TR. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry. 2019;90(6):704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuber M, Howlett S, Khan A, Grunewald RA. Non-epileptic seizures and other functional neurological symptoms: predisposing, precipitating, and perpetuating factors. Psychosomatics. 2007;48(3):230–8. [DOI] [PubMed] [Google Scholar]
- 9.Stone J, Carson A, Aditya H, Prescott R, Zaubi M, Warlow C, et al. The role of physical injury in motor and sensory conversion symptoms: a systematic and narrative review. J Psychosom Res. 2009;66(5):383–90. [DOI] [PubMed] [Google Scholar]
- 10.Parees I, Kojovic M, Pires C, Rubio-Agusti I, Saifee TA, Sadnicka A, et al. Physical precipitating factors in functional movement disorders. J Neurol Sci. 2014;338(1-2):174–7. [DOI] [PubMed] [Google Scholar]
- 11.Engel-Yeger B, Dunn W. The relationship between sensory processing difficulties and anxiety level of healthy adults. British Journal of Occupational Therapy. 2011;74:210–6. [Google Scholar]
- 12.Engel-Yeger B, Gonda X, Canepa G, Pompili M, Rihmer Z, Amore M, et al. Sensory profiles as potential mediators of the association between hypomania and hopelessness in 488 major affective outpatients. J Affect Disord. 2018;225:466–73. [DOI] [PubMed] [Google Scholar]
- 13.Engel-Yeger B, Palgy-Levin D, Lev-Wiesel R. The sensory profile of people with post-traumatic stress symptoms. Occupational Therapy in Mental Health. 2013;29:266–78. [Google Scholar]
- 14.Kinnealey M, Koenig KP, Smith S. Relationships between sensory modulation and social supports and health-related quality of life. Am J Occup Ther. 2011;65(3):320–7 [DOI] [PubMed] [Google Scholar]
- 15.Kinnealy M, Fuiek M. The relationship between sensory defensiveness, anxiety, depression and perception of pain in adults. Occupational Therapy International. 1999;6:195–206. [Google Scholar]
- 16.Serafini G, Gonda X, Pompili M, Rihmer Z, Amore M, Engel-Yeger B. The relationship between sensory processing patterns, alexithymia, traumatic childhood experiences, and quality of life among patients with unipolar and bipolar disorders. Child Abuse Negl. 2016;62:39–50. [DOI] [PubMed] [Google Scholar]
- 17.Carson A, Lehn A, Ludwig L, Stone J. Explaining functional disorders in the neurology clinic: a photo story. Pract Neurol. 2016;16(1):56–61. [DOI] [PubMed] [Google Scholar]
- 18.LaFrance WC Jr., Baird GL, Barry JJ, Blum AS, Frank Webb A, Keitner GI, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997–1005. [DOI] [PubMed] [Google Scholar]
- 19.Gardiner P, MacGregor L, Carson A, Stone J. Occupational therapy for functional neurological disorders: a scoping review and agenda for research. CNS Spectr. 2017:1–8. [DOI] [PubMed] [Google Scholar]
- 20.Jalilianhasanpour R, Ospina JP, Williams B, Mello J, MacLean J, Ranford J, et al. Secure Attachment and Depression Predict 6-Month Outcome in Motor Functional Neurological Disorders: A Prospective Pilot Study. Psychosomatics. 2019;60(4):365–75. [DOI] [PubMed] [Google Scholar]
- 21.Ranford J, Perez DL, MacLean J. Additional occupational therapy considerations for functional neurological disorders: a potential role for sensory processing. CNS Spectr. 2018;23(3):194–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricciardi L, Demartini B, Crucianelli L, Krahé C, Edwards MJ, Fotopoulou A. Interoceptive awareness in patients with functional neurological symptoms. Biological psychology. 2016;113:68–74. [DOI] [PubMed] [Google Scholar]
- 23.Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of 'hysteria'. Brain. 2012;135(Pt 11):3495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadnicka A, Daum C, Meppelink AM, Manohar S, Edwards M. Reduced drift rate: a biomarker of impaired information processing in functional movement disorders. Brain. 2019. [DOI] [PubMed]
- 25.Dunn W The sensations of everyday life: empirical, theoretical, and pragmatic considerations. Am J Occup Ther. 2001;55(6):608–20. [DOI] [PubMed] [Google Scholar]
- 26.Brown C, Tollefson N, Dunn W, Cromwell R, Filion D. The Adult Sensory Profile: measuring patterns of sensory processing. American Journal of Occupational Therapy. 2001;55:75–82. [DOI] [PubMed] [Google Scholar]
- 27.Serafini G, Gonda X, Canepa G, Pompili M, Rihmer Z, Amore M, et al. Extreme sensory processing patterns show a complex association with depression, and impulsivity, alexithymia, and hopelessness. J Affect Disord. 2017;210:249–57. [DOI] [PubMed] [Google Scholar]
- 28.Dunn W The impact of sensory processing abilities on the daily life of young children and families: a conceptual model. Infants and Young Children. 1997;9:23–5. [Google Scholar]
- 29.Brown C, & Dunn W. Adolescent/Adult Sensory Profile Manual. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- 30.Bundy AC, Lane SJ, Murray EA. Sensory Integration: Theory and Practice. 2nd ed2002. [Google Scholar]
- 31.Metz A, Boling D, DeVore A, Holladay H, Liao J, Vander Vultch K. Dunn's model of sensory processing: an investigation of the axes of the four-quadrant model in healthy adults. Brain Sciences. 2019;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar-Shalita T, Cermak SA. Atypical Sensory Modulation and Psychological Distress in the General Population. Am J Occup Ther. 2016;70(4):1–9. [DOI] [PubMed] [Google Scholar]
- 33.Abernathy H The assessment and treatment of sensory defensiveness in adult mental health: a literature review. . British Journal of Occupational Therapy. 2010;73:210–8. [Google Scholar]
- 34.Ben-Avi N, Almagor M, Engel-Yeger B. Sensory processing difficulties and interpersonal relationships in adults: an exploratory study. Psychology. 2012;3:70–7. [Google Scholar]
- 35.Jerome E, Liss M. Relationships between sensory processing style, adult attachment and coping. Personality and Individual Differences. 2005;38:1341–52. [Google Scholar]
- 36.Jalilianhasanpour R, Williams B, Gilman I, Burke MJ, Glass S, Fricchione GL, et al. Resilience linked to personality dimensions, alexithymia and affective symptoms in motor functional neurological disorders. J Psychosom Res. 2018;107:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams B, Ospina JP, Jalilianhasanpour R, Fricchione GL, Perez DL. Fearful Attachment Linked to Childhood Abuse, Alexithymia, and Depression in Motor Functional Neurological Disorders. J Neuropsychiatry Clin Neurosci. 2019;31(1):65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levita L, Mayberry E, Mehmood A, Reuber M. Evaluation of LiNES: A New Measure of Trauma, Negative Affect, and Relationship Insecurity Over the Life Span in Persons With FND. J Neuropsychiatry Clin Neurosci. 2020;32(1):43–49. [DOI] [PubMed] [Google Scholar]
- 39.Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719–25. [DOI] [PubMed] [Google Scholar]
- 40.Engel-Yeger B, Dunn W. Exporing the relationship between affect and sensory processing patterns in adults. British Journal of Occupational Therapy. 2011;74:456–64. [Google Scholar]
- 41.McMahon K, Anand D, Morris-Jones M, Rosenthal MZ. A Path From Childhood Sensory Processing Disorder to Anxiety Disorders: The Mediating Role of Emotion Dysregulation and Adult Sensory Processing Disorder Symptoms. Front Integr Neurosci. 2019;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark JR, Yeowell G, Goodwin PC. Trait anxiety and sensory processing profile characteristics in patients with non-specific chronic low back pain and central sensitisation - A pilot observational study. J Bodyw Mov Ther. 2018;22(4):909–16. [DOI] [PubMed] [Google Scholar]
- 43.Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85(2):220–6. [DOI] [PubMed] [Google Scholar]
- 44.Gelauff JM, Carson A, Ludwig L, Tijssen MAJ, Stone J. The prognosis of functional limb weakness: a 14-year case-control study. Brain. 2019;142(7):2137–48. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. 2018;5(4):307–20. [DOI] [PubMed] [Google Scholar]
- 46.Gandara-Gafo B, Santos-Del Riego S, Muniz J. Reference Values for the Adolescent/Adult Sensory Profile in Spain. Am J Occup Ther. 2019;73(5):7305205040p1–p8. [DOI] [PubMed] [Google Scholar]
- 47.Matin N, Young SS, Williams B, LaFrance WCJ, King JN, Caplan D, et al. Neuropsychiatric Associations with Gender, Illness Duration, Work Disability and Motor Subtype in a US Functional Neurological Disorders Clinic Population J Neuropsychiatry Clin Neurosci. 2017;29(4):375–82. [DOI] [PubMed] [Google Scholar]
- 48.Asadi-Pooya AA, Myers L, Valente K, Restrepo AD, L DA, Sawchuk T, et al. Sex differences in demographic and clinical characteristics of psychogenic nonepileptic seizures: A retrospective multicenter international study. Epilepsy Behav. 2019;97:154–7. [DOI] [PubMed] [Google Scholar]
- 49.Stone J, Carson A, Duncan R, Coleman R, Roberts R, Warlow C, et al. Symptoms 'unexplained by organic disease' in 1144 new neurology out-patients: how often does the diagnosis change at follow-up? Brain. 2009;132(Pt 10):2878–88. [DOI] [PubMed] [Google Scholar]
- 50.Popkirov S, Carson AJ, Stone J. Scared or scarred: Could 'dissociogenic' lesions predispose to nonepileptic seizures after head trauma? Seizure. 2018;58:127–32. [DOI] [PubMed] [Google Scholar]
- 51.LaFrance WC Jr., Deluca M, Machan JT, Fava JL. Traumatic brain injury and psychogenic nonepileptic seizures yield worse outcomes. Epilepsia. 2013;54(4):718–25. [DOI] [PubMed] [Google Scholar]
- 52.Perez DL, Dworetzky BA, Dickerson BC, Leung L, Cohn R, Baslet G, et al. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci. 2015;46(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanaan RAA, Duncan R, Goldstein LH, Jankovic J, Cavanna AE. Are psychogenic non-epileptic seizures just another symptom of conversion disorder? J Neurol Neurosurg Psychiatry. 2017;88(5):425–9. [DOI] [PubMed] [Google Scholar]
- 54.Kinnealey M, Oliver B, Wilbarger P. A phenomenological study of sensory defensiveness in adults. Am J Occup Ther. 1995;49(5):444–51. [DOI] [PubMed] [Google Scholar]
- 55.Pfeiffer B Sensory hypersensitivity and anxiety: the chicken or the egg? Sensory Integration Special Interest Section Quarterly. 2012;35:1–4. [Google Scholar]
- 56.May-Benson T, Kinnealey M. An approach to assessment of and intervention for adults with sensory processing disorders. OT Practice. 2012;17:17–23. [Google Scholar]
- 57.Pfeiffer B, Kinnealey M. Treatment of sensory defensiveness in adults. Occup Ther Int. 2003;10(3):175–84. [DOI] [PubMed] [Google Scholar]
- 58.Champagne T The influence of posttraumatic stress disorder, depression, and sensory processing patterns on occupational engagement: a case study. Work. 2011;38(1):67–75. [DOI] [PubMed] [Google Scholar]
- 59.Fisher J Sensorimotor approaches to trauma treatment. Advances in psychiatric treatment. 2011;17(3):171–7. [Google Scholar]
- 60.DuBois D, Lymer E, Gibson BE, Desarkar P, Nalder E. Assessing Sensory Processing Dysfunction in Adults and Adolescents with Autism Spectrum Disorder: A Scoping Review. Brain Sci. 2017;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ekanayake V, Kranick S, LaFaver K, Naz A, Frank Webb A, LaFrance WC Jr., et al. Personality traits in psychogenic nonepileptic seizures (PNES) and psychogenic movement disorder (PMD): Neuroticism and perfectionism. J Psychosom Res. 2017;97:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensory processing patterns in psychogenic nonepileptic seizures (panel A) (n=10). Sensory processing patterns in other motor functional neurological disorders (panel B) (n=34).