Abstract
Background:
Periodontitis is the inflammation of the tooth-supporting structures and is one of the most common diseases of the oral cavity. The outcome of periodontal infections is tooth loss due to a lack of alveolar bone support. Osteoclasts are giant, multi-nucleated, and bone-resorbing cells that are central for many osteolytic diseases, including periodontitis. Receptor activator of nuclear factor-kB ligand (RANKL) is the principal factor involved in osteoclast differentiation, activation, and survival. However, under pathological conditions, a variety of pro-inflammatory cytokines secreted by activated immune cells also contribute to osteoclast differentiation and activity. Lipopolysaccharide (LPS) is a vital component of the outer membrane of the Gram-negative bacteria. It binds to the Toll-like receptors (TLRs) expressed in many cells and elicits an immune response.
Highlights:
The presence of bacterial LPS in the periodontal area stimulates the secretion of RANKL as well as other inflammatory mediators, activating the process of osteoclastogenesis. RANKL, either independently or synergistically with LPS, can regulate osteoclastogenesis, while LPS alone cannot. MicroRNA, IL-22, M1/M2 macrophages, and memory B cells have recently been shown to modulate osteoclastogenesis in periodontal diseases.
Conclusion:
In this review, we summarize the mechanism of osteoclastogenesis accompanying periodontal diseases at the cellular level. We discuss a) the effects of LPS/TLR signaling and other cytokines on RANKL-dependent and -independent mechanisms involved in osteoclastogenesis; b) the recently identified role of several endogenous factors such as miRNA, IL-22, M1/M2 macrophages, and memory B cells in regulating osteoclastogenesis during periodontal pathogenesis.
Keywords: Alveolar Bone Loss, Lipopolysaccharides, Osteoclasts, RANK Ligand
1. Introduction
Periodontal inflammation is one of the major diseases affecting the oral cavity. It is initiated by oral pathogens that exist within the periodontal tissues [1]. The disruption of the ecological homeostasis between the host defense and bacterial population can promote periodontal pathogenesis [2]. The red complex, which includes Porphyromonas gingivalis (Pg), Tannerella forsythia (formerly known as Bacteroides forsythus), and Treponema denticola, represent the essential periodontopathic bacteria that are responsible for adult periodontal diseases [3]. These bacteria are usually found in periodontal pockets and can work together or with other low-grade periodontopathic bacteria to mediate pathologic tissue loss [4]. Tissues destruction is commonly associated with periodontal diseases and disease progression ultimately leads to not only a gradual loss of alveolar bone supporting the teeth but also tooth mobility and tooth loss [5].
Among various bacterial pathogenic factors, the endotoxin lipopolysaccharide (LPS) is considered a primary agent capable of eliciting a local immune response [6]. LPS is an essential constituent of the outer membrane of Gram-negative bacteria that can induce a septic shock [7]. Structurally, bacterial LPS is made up of the following three components 1) the O-antigen (or O polysaccharide) which forms the outermost domain of the LPS molecule; 2) the core oligosaccharide which directly attaches to the innermost lipid A; 3) the innermost lipid A region which consists of hydrophobic fatty acid chains that anchor the LPS into the bacterial membrane [8]. When LPS interacts with the gingival tissue, it can mediate pathologic tissue breakdown by triggering inflammation [9]. Amongst the different LPS components, lipid A is the most biologically active and conserved region [8, 9]. Lipid A interaction with TLR4 receptors was shown to trigger innate immune responses involving transcription of inflammatory mediators. The sublingual microbial burden leads to an accumulation of LPS, which was found to be a critical molecular mediator of, not only the periodontitis but also coronary artery disease [10].
2. Toll-like receptors −2 and − 4
Toll-like receptors (TLRs) are a well-known family of proteins that trigger the immune reaction in response to microbial invasion [11]. TLRs are expressed in many cells, such as macrophages, dendritic cells, and neutrophils. They possess a unique potential to distinguish and identify highly conserved structures expressed by different pathogens. These structures are called pathogen-associated molecular patterns (PAMPs) and can be seen in the form of lipopolysaccharide (LPS), peptidoglycan, lipoprotein, bacterial DNA, or double-stranded RNA [2]. Till now, ten human TLRs have been identified, namely TLR1 through TLR10 [12,13]. TLR1 or TLR6, along with TLR2, respond to a wide variety of PAMPs such as peptidoglycans, zymosan, lipoproteins, lipoteichoic acids, and mannan [7]. TLR5 interacts with bacterial flagellin [14]. RNA from streptococcus B bacteria can be recognized by TLR7 expressed in dendritic cells [15]. Similarly, human TLR8 can detect bacterial RNA [16]. Besides, bacterial DNA which is rich in unmethylated CpG-DNA motifs is often recognized by TLR9 [17].
TLR2 and TLR4 are both cell surface receptors [11]. TLR2 can detect various molecular components of the bacterial cell wall, such as lipoproteins and peptidoglycan, whereas TLR4 mainly interacts with the bacterial LPS [13]. Nevertheless, the interaction between TLR2 and LPS remains controversial. It was believed that TLR2 can bind purified LPS, mainly the Porphyromonas gingivalis LPS (PgLPS), and directly mediate LPS-induced signaling as reported by many studies [18–20]. However, the activation of TLR2 was attributed to the insufficient purity of the isolated LPS. LPS purification from bacteria is primarily conducted via the phenol-water extraction technique. These extracts are contaminated with other bacterial components such as lipoprotein [18], which might be the underlying reason for TLR2 activation. Re-purified LPS failed to stimulate TLR2 activity, and this observation suggests that the re-purification might have eliminated the bioactive contaminants co-purified with LPS [19]. Recently Nativel et al. have determined the effects of different grades of PgLPS purity and the role of TLR2 and TLR 4 in a pro-inflammatory activity. Their results suggested that PgLPS mediates its effect exclusively through TLR 4, although it is recognized differently by TLR4 in human and mouse cells [20]. Furthermore, the induction of pro-inflammatory cytokine by PgLPS is very weak in mouse models [20]. Collectively, the above observations favor the hypothesis that LPS acts exclusively via TLR4.
During inflammatory events, the interaction of LPS/TLR can upregulate local receptor activator of nuclear factor kappa-Β ligand (RANKL) expression and therefore launch the osteoclastogenesis process [21,22]. The interplay between RANK/RANKL and LPS/TLR in osteoclast differentiation will be discussed in detail in the next section.
3. Mechanisms of osteoclastogenesis
Osteoclasts are large multinucleated cells characterized by their ability to resorb bone or dentine matrix. They are primarily derived from hematopoietic stem cells located in the bone marrow and their mononuclear precursors are typically found circulating in peripheral blood [23]. RANKL and Macrophage Colony-Stimulating Factor (M-CSF) are the central regulators of osteoclast differentiation. RANKL promotes the fusion of the mononuclear precursors to form the multinucleated osteoclasts and hence, induce the expression of the osteoclasts-specific marker genes [24]. M-CSF regulates the proliferation and survival of cells of the monocyte lineage [25]. Tartrate-resistant acid phosphatase (TRAP), calcitonin receptor, and vitronectin receptor αvβ3 are markers for mature osteoclasts, with calcitonin receptor being a specific marker for osteoclast differentiation [24]. One of the significant hallmarks of periodontal inflammation is the resorption of the alveolar bone surrounding the tooth [2]. The interaction of bacterial products, especially LPS, with the periodontal and immune cells stimulates the differentiation of the osteoclasts and thus activates pathologic bone loss [21,22]. During the active phase of periodontal disease, osteoclastogenesis occurs by different mechanisms. These mechanisms can be broadly classified into two categories: a) complete RANKL-dependent osteoclastogenesis initiated by LPS/TLR signaling; b) partial RANKL-dependent osteoclastogenesis initiated by LPS/TLR signaling, promoted with RANKL, and sustained by other pro-inflammatory cytokines (e.g. interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and other cytokines). We have also discussed here whether LPS alone could mediate osteoclastogenesis in a completely RANKL independent manner.
3.1. RANKL-dependent osteoclastogenesis
During periodontal pathogenesis, the periodontopathic bacteria utilize a unique mechanism to induce RANKL expression. The LPS released by these Gram-negative bacteria interacts with the TLR4 on the innate immune cells, including macrophages and dendritic cells [26,27], and thus, promote the secretion of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6 [13,28]. These cytokines can stimulate RANKL expression in osteoblasts [29,30]. Also, the secreted TNF-α can stimulate T and B cells to produce RANKL [31,32]. LPS can also interact with the osteoblasts through TLR4 and enhance the expression of RANKL [33]. Moreover, periodontal ligament fibroblasts can further augment the secretion of RANKL upon exposure to the bacterial LPS [34,35]. RANKL produced during periodontal pathogenesis binds receptor activator of nuclear factor kappa-Β (RANK), a receptor expressed in osteoclast precursor cells. The RANKL/RANK signaling pathway regulates osteoclast differentiation and activation [29] (Figure 1).
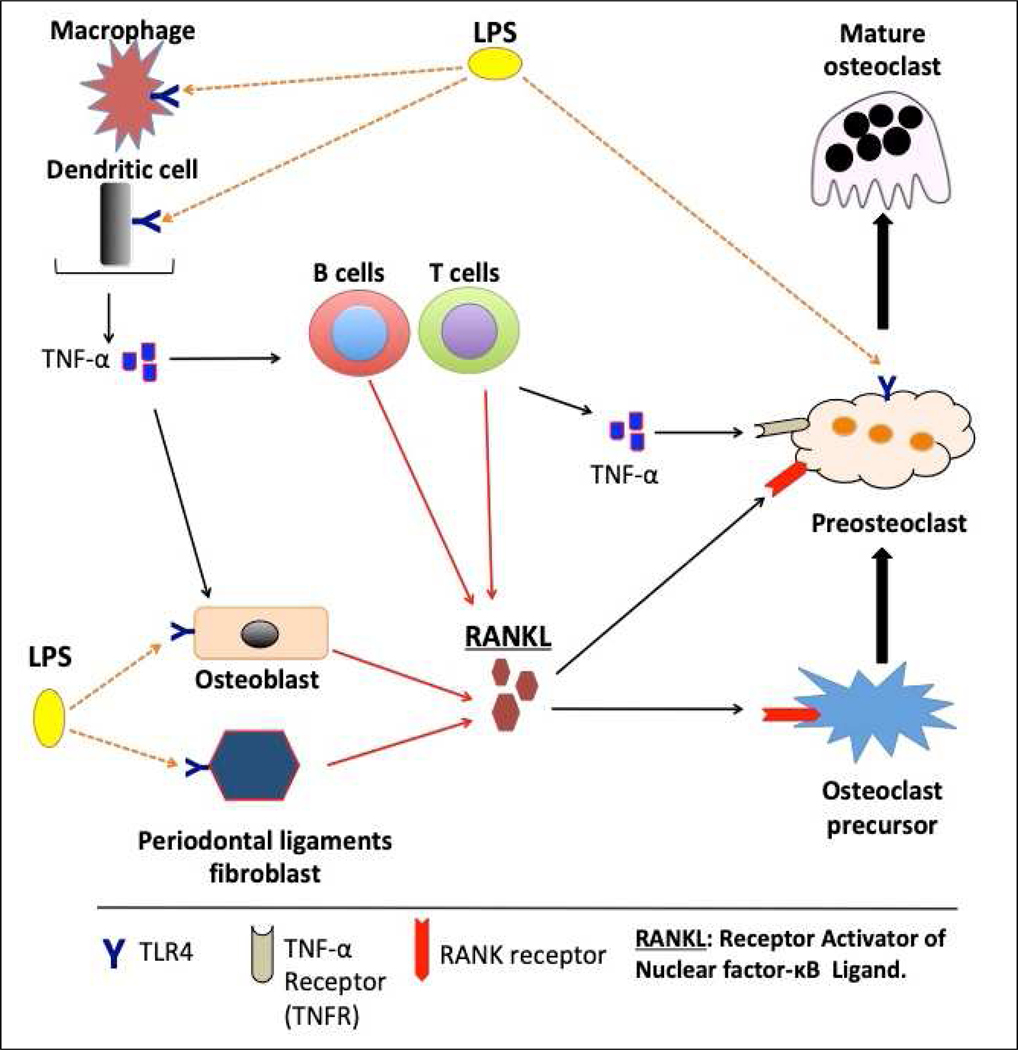
Figure 1.
Schematic illustration of inflammation-induced osteoclastogenesis in periodontal diseases. Lipopolysaccharide (LPS) originates from bacteria in the oral biofilm. LPS can initiate osteoclastogenesis upon binding TLR4 (orange dotted arrows) that is expressed in macrophages, dendritic cells, osteoblasts, and periodontal ligament fibroblasts. Osteoblasts, as well as gingival fibroblasts, and periodontal ligament fibroblasts, can produce RANK ligand (RANKL) in response to LPS stimulation (red arrows). RANKL secretion by other cells (immune cells such as B cells, T cells, and osteoblasts) can also be induced by TNF-α produced by macrophages and dendritic cells (red arrows). RANKL then binds to its receptor RANK to stimulate osteoclast differentiation and mediates the regulation of corresponding signaling pathways. The preosteoclasts can be differentiated into fully mature osteoclasts either by continuous exposure to RANKL, TNF-α, or both. Mature osteoclasts can utilize an alternative functional cycle of adhesion, resorption, and migration on the alveolar bone surface to efficiently perform their functions. Preosteoclasts express TLR4 and the direct interaction of LPS with preosteoclasts through TLR4 (orange dotted arrow) promotes TNF-α mediated events (osteoclast differentiation and activity).
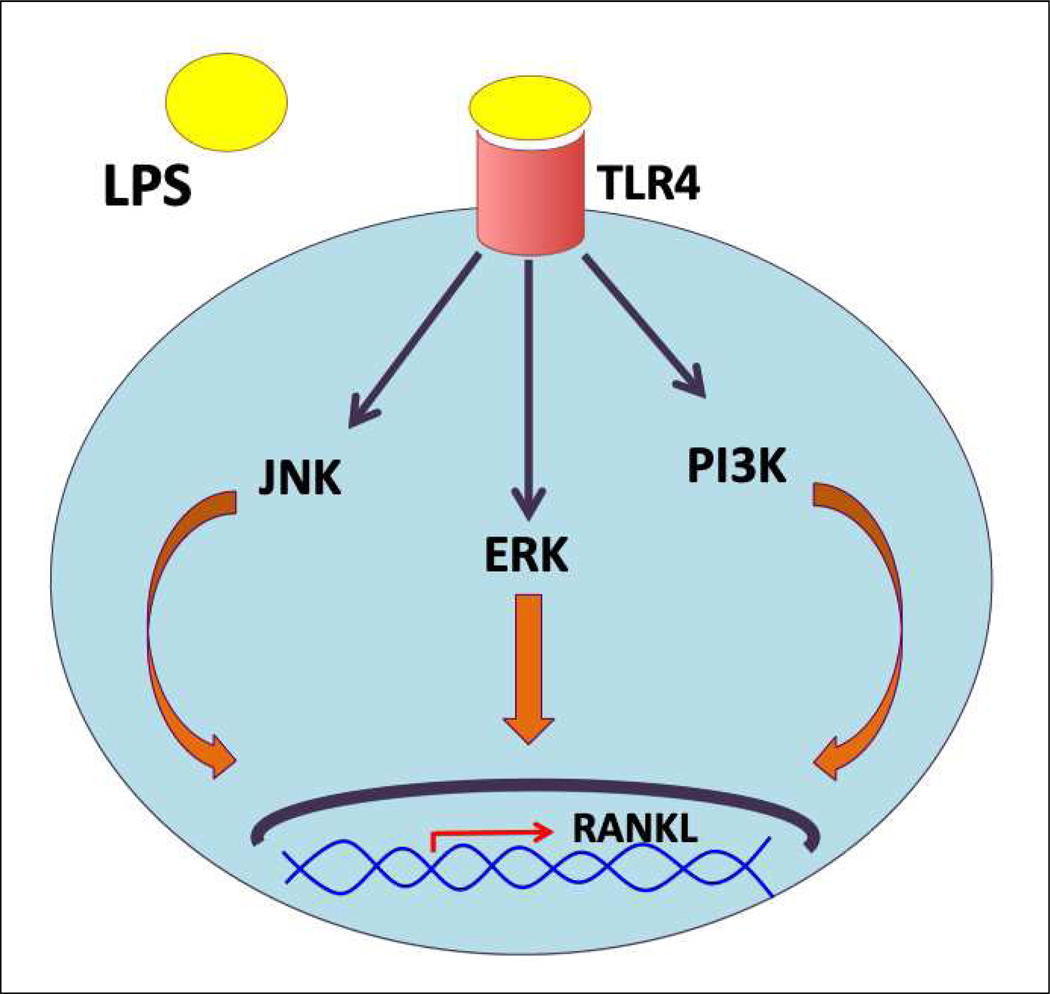
In line with these observations, Tang et al. showed that after inhibiting the expression of TLR4 and TLR2 in mouse osteoblast-derived MC3T3-E1 cells, the level of RANKL was markedly decreased upon exposure to LPS [36]. On the contrary, incubating the primary murine osteoblastic cells with a TLR2 agonist results in an amplification of RANKL gene expression [37]. The signaling pathway that regulates LPS-mediated RANKL expression in osteoblasts is entirely dependent on the bacteria from where the LPS originated and its binding to the toll-like receptor, as shown in Figure 2. For example, LPS derived from Porphyromonas endodontalis induces RANKL expression in osteoblasts through the c-Jun N-terminal kinase (JNK) pathway [36]. Similarly, P.gingivalis-infection resulted in the upregulation of RANKL expression via activation of JNK and activator protein 1 (AP-1) transcription factor in osteoblasts [38]. In contrast, E.coli LPS seems to induce the expression of RANKL via different pathways, which involves the activation of extracellular-signal-regulated kinase (ERK) or phosphoinositide 3-kinase (PI3K) signaling molecules, as indicated in Figure 2 [36]. Activation of nuclear factor-kB (NF-kB) is not required for RANKL secretion [36], which is consistent with the observation that the promoter of the mouse RANKL gene has no NF-kB binding motifs [39]. The above studies have shown that inflammatory mediators can trigger osteoclastogenesis. However, all these different mechanisms indeed highlight the role of RANKL as the sole factor orchestrating osteoclast differentiation.
Figure 2.
Schematic illustration of the different signaling pathways involved in LPS-induced RANKL expression in osteoblasts.
3.2. Synergistic effect of TNF-α and permissive levels of RANKL on osteoclastogenesis
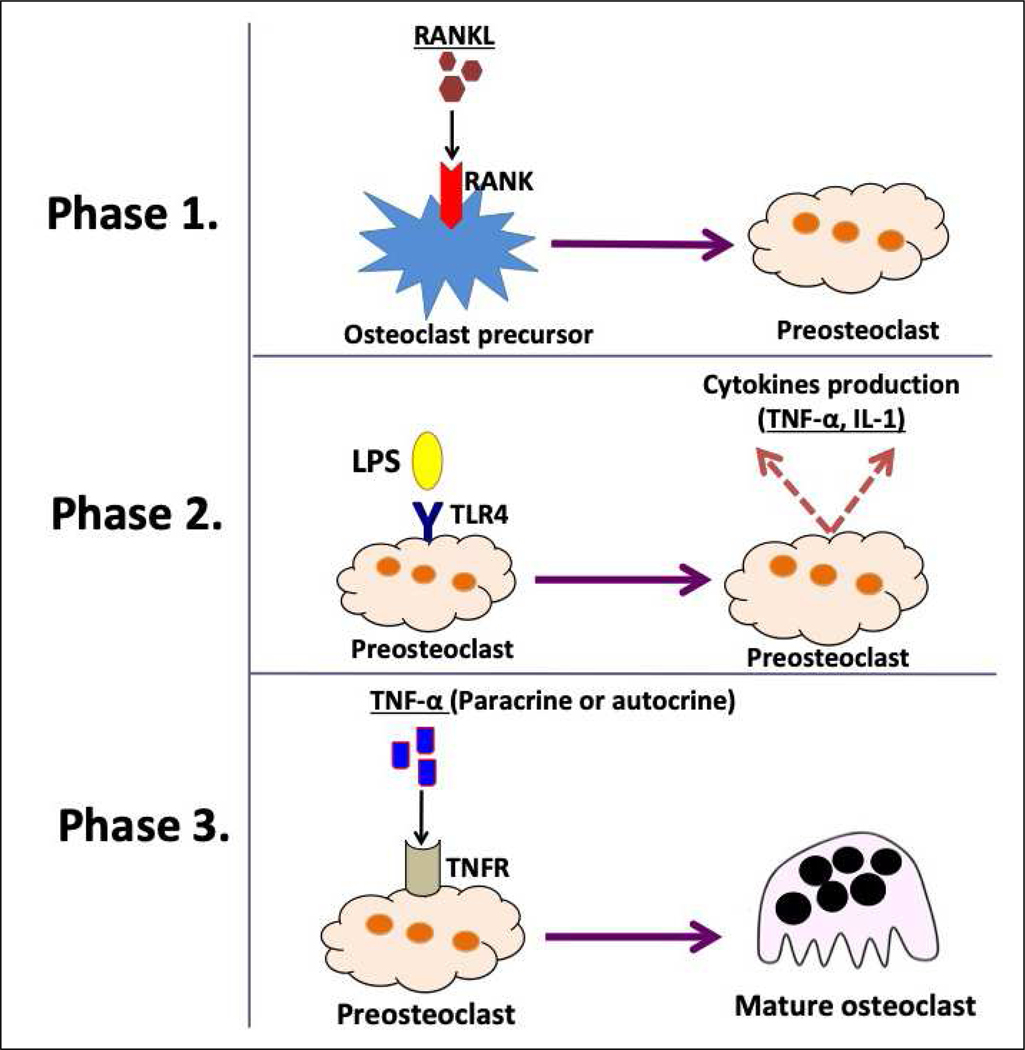
A key concept in this area states that during osteolytic inflammatory disease, pro-inflammatory cytokines, such as TNF-α, can act as possible osteoclast-differentiating factors [40–43]. It is well-known that TNF-α is released in response to LPS stimulation [2]. Therefore, the LPS-TLR4 interaction can be translated as a potent enhancer of the osteoclastogenesis process [2]. Briefly, this process occurs in three phases (Figure 3). The first phase involves the commitment of cells to the osteoclastic phenotype after exposure to RANKL [44]. In the second phase, exposure of these cells to bacterial virulence factors such as LPS results in the production of cytokines. The third phase represents the final stage in which TNF-α induces osteoclastogenesis in an autocrine/paracrine manner independent of RANKL [45]. Concordantly, Lam et al. showed that TNF-α induced-osteoclast formation was entirely dependent on the presence of permissive levels of RANKL. They also showed a complete abrogation of osteoclastogenesis upon addition of osteoprotegerin (OPG), the decoy receptor of RANKL, indicating the inability of TNF-α alone to regulate osteoclastogenesis. However, when macrophages were primed with RANKL and then treated with TNF-α, robust osteoclast generation was observed [46]. Some culture systems contain contaminant stromal cells that can produce RANKL. This RANKL may prime macrophages to differentiate into mature osteoclasts [46]. However, OPG fails to suppress LPS-induced osteoclastogenesis in RANKL-primed cells [47], which confirms the theory that osteoclastogenesis is mediated through the TNF-α/TNFR axis and not by a contaminant RANKL.
Figure 3.
Schematic illustration of the consecutive phases of osteoclast precursors undergoing TNF-α mediated osteoclastogenesis.
Moreover, osteoclast progenitors from tumor necrosis factor receptor (TNFR)-knockout mice fail to generate osteoclasts after stimulation with TNF-α but not RANKL, emphasizing a unique regulatory role of the TNF-α/TNFR axis in osteoclastogenesis. Consistently, neutralizing antibody against TNFR has markedly reduced the osteoclastogenic process [41]. These observations suggest a synergistic role of RANKL and TNF-α, in which RANKL is only needed to commit the cells into the osteoclastic lineage [43]. Subsequently, TNF-α takes the lead and directly induces osteoclastogenesis through TNFR signaling (Figure 1).
The RANKL-induced osteoclast formation is initially mediated through the recruitment of adaptor proteins such as TNF receptor-associated factor (TRAF) [48]. Among different TRAF family members, only TRAF6 can transmit the RANKL signal [49,50] and induce osteoclastogenesis through activation of the NF-κB and mitogen-activated protein kinase (MAPK) pathways [51,52]. However, TNF-α mediated osteoclastogenesis is not entirely regulated by TRAF6. The use of TRAF6 −/− osteoclast precursors reduced RANKL but not TNF-mediated osteoclast differentiation. TRAF3, on the other hand, inhibits the formation of TNF-induced osteoclasts. The generation of TNF-induced osteoclasts was significantly enhanced in myeloid lineage cells with a conditional deletion of TRAF3, suggesting that TRAF3 is a significant regulator of TNF-α-induced osteoclastogenesis [53].
3.3. Can LPS mediate RANKL-independent osteoclastogenesis?
Over the last decade, the concept of RANKL-independent osteoclastogenesis has given rise to many scientific conflicts. Can LPS exclusively induce osteoclastogenesis without the involvement of RANKL? In a study conducted by Liu et al., freshly isolated bone marrow macrophages (BMMs) from long bones of mice were stimulated with LPS alone, but osteoclast differentiation failed. The osteoclastogenesis by LPS was reinstated when BMMs were pre-treated with RANKL. In contrast, the LPS pre-treated BMMs failed to undergo osteoclast differentiation after treatment with increasing doses of RANKL. This was due to the ability of LPS to downregulate RANKL-induced nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) expression, a master regulator of osteoclastogenesis [44]. Takami et al. demonstrated almost all TLRs members are expressed on murine osteoclast precursors. Like the previous study, TLR4 activation by LPS reduced the RANKL-mediated osteoclast differentiation of these precursors. The other interesting observation is that TLR4 is not the only TLR family member that could suppress the osteoclastogenic ability of the precursors upon ligand stimulation. When TLR2, TLR3, and TLR9, that are expressed on osteoclast precursors, were stimulated with their respective microbial ligand, RANKL-induced osteoclast differentiation was inhibited [54].
Furthermore, Porphyromonas gingivalis (Pg), a well-known periodontal pathogen, failed to induce osteoclast differentiation when cultured with BMMs from C57BL/6 mice. However, when these BMMs were pre-treated with RANKL and then incubated with Pg, a dramatic increase in osteoclast differentiation was noticed. On the other hand, when BMMs co-treated with Pg and RANKL at the same time, Pg completely suppressed the RANKL-induced osteoclastogenesis [55]. This microorganism can further promote RANKL-induced osteoclastogenesis in an LPS-independent manner. A recent in vitro study demonstrated a unique property of Pg to secrete cell-permeable ceramides called phosphoglycerol dihydroceramide, which stimulates osteoclast fusion [56]. In vivo, Lin et al. used a mouse model that utilized a Pg-associated ligature to induce periodontal disease. They showed a significant reduction in alveolar bone resorption in mice treated with a RANKL antibody compared to the untreated group (both groups had been ligated and treated with Pg) [57]. Collectively, these observations rule out the possibility that LPS alone can induce osteoclastogenesis and instead confirm the vital role of RANKL as a prerequisite for osteoclast differentiation. Also, LPS pre-treatment blocks the effect of RANKL in terms of osteoclast differentiation in vitro. Many studies, however, used LPS alone to induce osteoclast differentiation [58–61]. Although the purpose of these studies was not meant to specifically investigate RANKL independent LPS-induced osteoclastogenesis, nevertheless, their methodology, signaling mechanisms, as well as data in terms of osteoclast differentiation, were not sufficiently persuasive to support such a claim. Therefore, to address this matter, it would be useful in the future to use a RANK knock out animal model or RANK knockdown culture system combined with LPS treatment to discern this dichotomy.
4. Other possible regulators of osteoclastogenesis in periodontal diseases
RANKL, LPS, and TNF-α have all been extensively studied for their involvement in osteoclastogenesis. Recent studies have shed light on other factors such as miRNA, IL-22, M1/M2 macrophages, and memory B cells, which may regulate osteoclastogenesis in periodontal diseases. Here we will discuss each one of them and their impact on osteoclastogenesis and the bone resorption process accompanying periodontal infections. (Table. 1)
Table 1.
Summary of the possible effect of different factors on osteoclastogenesis
4.1. miRNA
Micro RNAs (miRNA) are small, single-stranded, non-coding RNA molecules that regulate gene expression at the post-transcriptional level [62]. MicroRNAs are involved in the modulation of a variety of biological processes including inflammatory reactions, cancer development, cellular differentiation and apoptosis [63]. Lately, the role of miRNA in periodontal pathogenesis and bone resorption has been documented [64–66]. MicroRNAs can play a preventive role in this disease, for example, miRNA-218 was found to orchestrate an inhibitory effect on osteoclast differentiation and bone resorption in vitro and in a rat periodontitis model through the regulation of matrix metalloproteinase 9 (MMP9) [67]. Moreover, miRNA-34a showed an ability to suppress osteoclastogenesis, supported by the observation that knockdown of miRNA-34a led to increased osteoclast differentiation, while overexpression of miRNA-34a attenuated this process [68]. Similar observations were obtained with miRNA-124. During mouse osteoclast differentiation, the miRNA-124 expression decreased in a time-dependent manner. Moreover, osteoclast differentiation was inhibited upon ectopic expression of miRNA-124, which supports its negative regulatory role [63,69]. On the other hand, miRNAs can also promote inflammatory bone loss. For instance, during osteoclast differentiation, miRNA-31 expression was highly upregulated. Additionally, miRNA-31 was also able to control the formation of the actin ring in osteoclasts by regulating RhoA expression [70]. Lastly, the combined treatment with RANKL and TNF-α or RANKL alone was associated with the upregulation of miRNA-21 and miRNA-125a during osteoclastogenesis [71]. Thus, miRNAs exert a dual function when it comes to inflammatory bone loss, which makes these tiny non-coding RNAs a potential target for therapy.
4.2. IL-22
IL-22 is a recently identified cytokine that is involved in several diseases such as rheumatoid arthritis [72], peri-implantitis [73], and diabetes [74]. This cytokine is produced by CD4+ T-helper subtypes, mainly by T-helper 17 [75], and can activate host innate immune response [74]. The role of IL-22 in periodontal inflammations was unclear, until recently, when a couple of studies investigated the involvement of IL-22 in periodontal inflammation. Firstly, higher levels of IL-22 were found in periodontitis patients compared to both gingivitis patients and healthy individuals. This finding correlated with increased osteoclastogenesis and bone resorption observed in periodontitis patients. Briefly, when murine osteoclast precursors were exposed to homogenates obtained from periodontitis patients, the number of osteoclasts as well as their resorptive activity were dramatically increased compared to the cells exposed to homogenates taken from healthy individuals. The key factor underlying this difference was the addition of IL-22 to both groups [76]. Moreover, Pan et al., 2017 found a differential temporal expression of IL-22 during experimental periodontitis in rats, which may explain the involvement of IL-22 in different phases of periodontal pathogenesis [77]. Based on these observations, future studies investigating the molecular mechanisms of IL-22 - associated periodontal pathogenesis are imperative. Besides its pro-inflammatory function, IL-22 also has some biological impact on periodontal tissues. It induces Runt-related transcription factor 2 (RUNX2) and osteocalcin gene expression and facilitates the precipitation of mineralized nodule formation within the periodontal ligament cells. These findings suggest a potential mineralizing effect of IL-22 that could be considered in developing regenerative therapies [78].
4.3. M1/M2 macrophages
Macrophages are one of the most important immune cells that initiate a host response during periodontal pathology [79]. Due to their high plasticity, macrophages can exhibit a unique functional diversity depending on the surrounding microenvironment [80]. Based on that, macrophages can be broadly classified into two functional classes, the classically-activated macrophages (M1) and the alternatively-activated macrophages (M2). M1 macrophages are mainly involved in pro-inflammatory activity, while M2 macrophages demonstrate pro-healing and anti-inflammatory properties [80]. Recently, the role of M1 and M2 macrophages in the modulation of osteoclastogenesis and alveolar bone resorption has been elucidated. Generally, the ratio of M1/M2 macrophages is positively correlated with the progression of periodontal diseases [81,82]. Therefore, some studies deliberately induced a shift towards M2 macrophages and monitored the changes in the inflammatory status. As anticipated, M2 activation reduces the TNF-α secretion in RAW 264.7 cells and decreases the alveolar bone resorption in a murine periodontitis model. Fewer osteoclasts were observed in the group with the activated M2 profile compared to the control [83]. Furthermore, the induction of M2 macrophages not only reduced bone loss during inflammation but also led to increased bone formation during healing [84]. The in vitro stimulation of BMMs with IL-4 shifts them into the M2 phenotype. The supernatant of IL-4 treated BMMs significantly stimulated mineral deposition in cells of the osteoblastic lineage and inhibited osteoclast formation. In vivo, the activation of M2 macrophages with peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists, such as rosiglitazone, contributes to the increased bone formation during healing. It is hypothesized that the M2-macrophages secrete Cystatin C, a cysteine proteinase inhibitor constitutively present in all tissues and body fluids, which is partly responsible for the reduced catabolic and enhanced anabolic action of M2 macrophages [84].
Another protein that has been shown to regulate M1/M2 polarization is Sprouty2 [85]. Atomura et al. demonstrated that knockdown of Sprouty2 in LPS and IFN-γ stimulated macrophages triggered a transition from M1 to M2 phenotype. Furthermore, Sprouty2 depletion reduced the secretion of pro-inflammatory cytokines and enhanced the release of anti-inflammatory mediators from LPS and IFN-γ treated macrophages, which suggests a potential anti-osteoclastic role of Sprouty2 inhibitors in osteolytic inflammatory disorders [85]. Despite the ability of M1 macrophages to promote inflammation, Yamaguchi et al. unearthed an inhibitory effect of M1 macrophages on osteoclastogenesis. Interestingly, the addition of M1 macrophages to osteoclast precursors in vitro significantly reduces the number of osteoclasts compared to the addition of M2 or non-stimulated macrophages (M0). These results were confirmed in an in vivo ligature-induced periodontitis model, in which alveolar bone resorption and osteoclast number were significantly reduced upon transfer of M1 macrophages. The underlying molecular mechanism of such an inhibition can be attributed to IFN-γ and IL-12 produced by M1 macrophages. IFN-γ can downregulate NFATc1 expression, whereas IL-12 induces apoptosis in preosteoclasts [86]. Although these observations may contradict each other and appear to be controversial, they all underscore the vital role of M1/M2 macrophages in modulating periodontal diseases. Therefore, it is possible the therapeutic manipulation of the M1/M2 ratio could interrupt the periodontal inflammation and favor subsequent healing.
4.4. Memory B cells
The infiltration of B cells, especially plasma cells, in the vicinity of the periodontium is one of the most distinctive features of periodontal inflammation. The assessment of plasma cells derived from periodontitis tissue samples revealed the presence of surface and intracellular RANKL [87,88]. B cells produced more RANKL than T cells and other lymphocytes during periodontal diseases [89]. Among different subsets of B cells, the memory B cells were, interestingly, secreted the most RANKL [89]. Accordingly, memory B cells were purified from healthy and periodontitis animals and co-cultured with bone marrow mononuclear cells to evaluate their osteoclastogenic effect [88]. The number of osteoclasts, as well as gene expression of RANK, NFATc1, and c-Src, was remarkably higher in the co-cultures of periodontitis plasma cells than co-cultures of healthy plasma cells. Moreover, the adoptive transfer of pathogen sensitized memory B cells into periodontitis rats increased the extent of alveolar bone resorption compared to the control periodontitis rats [88]. Furthermore, memory B cell subsets obtained from periodontitis animals induced more osteoclast differentiation in vitro than other B cell subsets, when co-cultured with osteoclast precursors. Anti-RANKL treatment completely inhibited the osteoclastogenesis process in these cultures, suggesting that the osteoclastogenic effect of memory B cells is entirely dependent on RANKL [89]. Interestingly, LPS-TLR4 interaction can induce memory B cells to differentiate into plasma cells [90]. Moreover, LPS signaling through TLR4 was shown to have a critical role in promoting memory B cell survival through Syk tyrosine kinase [91,92]. Thus, LPS can indirectly sustain osteoclastogenesis during inflammation.
5. Conclusions
Many efforts have been made over the last few decades to elucidate the mechanisms of bone resorption in periodontal diseases. It is vital to fully understand the cellular and molecular mechanisms of osteoclast differentiation as it represents the cornerstone of osteolytic diseases. The well-known RANKL is the key osteoclast differentiating factor, not only in the pathologic events but also during normal physiological activities such as bone remodeling. However, other co-differentiating factors can synergize with RANKL, especially under inflammatory conditions, to further augment osteoclastogenesis. Plenty of recent reports have discovered the role of certain endogenous factors in regulating osteoclast differentiation. Hence, targeting these co-differentiating factors and the regulators could mediate a promising pharmacological outcome that can ultimately inhibit the development and consequences of osteolytic inflammatory diseases.
Acknowledgments
This work was supported by a research grant to MAC from the National Institute of Health - National Institute of Arthritis and Musculoskeletal and Skin Diseases (5R01AR066044) and by the support funding provided by the University of Maryland, School of Dentistry to MAC.
Footnotes
Ethical approval
Ethical approval was not required for this review.
Conflict of interest
The authors have no potential conflict of interest relevant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Petersen PE, Ogawa H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontol 2000 2012;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- [2].Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol 1998;25:134–44. [DOI] [PubMed] [Google Scholar]
- [4].Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000 1997;14:12–32. [DOI] [PubMed] [Google Scholar]
- [5].Van Dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med 2017;58:21–36. doi: 10.1016/j.mam.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch Oral Biol 2014;59:167–75. doi: 10.1016/j.archoralbio.2013.11.008. [DOI] [PubMed] [Google Scholar]
- [7].Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat Immunol 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- [8].Ogawa T, Yagi T. Bioactive mechanism of porphyromonas gingivalis lipid A. Periodontol 2000 2010;54:71–7. doi: 10.1111/j.1600-0757.2009.00343.x. [DOI] [PubMed] [Google Scholar]
- [9].Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol 2012;10:717–25. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Patel PN, Shah RY, Ferguson JF, Reilly MP. Human experimental endotoxemia in modeling the pathophysiology, genomics, and therapeutics of innate immunity in complex cardiometabolic diseases. Arterioscler Thromb Vasc Biol 2015;35:525–34. doi: 10.1161/ATVBAHA.114.304455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kawasaki T, Kawai T. Toll-Like Receptor Signaling Pathways. Front Immunol 2014;5. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Estornes Y, Bertrand MJM. IAPs, regulators of innate immunity and inflammation. Semin Cell Dev Biol 2015;39:106–14. doi: 10.1016/j.semcdb.2014.03.035. [DOI] [PubMed] [Google Scholar]
- [13].Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, Hernández M, Gamonal J. Host response mechanisms in periodontal diseases. J Appl Oral Sci 2015;23:329–55. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Akira S, Uematsu S, Takeuchi O. Pathogen Recognition and Innate Immunity. Cell 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- [15].Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol 2009;10:587–94. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- [16].Guiducci C, Gong M, Cepika A-M, Xu Z, Tripodo C, Bennett L, Crain C, Quartier P, Cush JJ, Pascual V, Coffman RL, Barrat FJ. RNA recognition by human TLR8 can lead to autoimmune inflammation. J Exp Med 2013;210:2903–19. doi: 10.1084/jem.20131044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Coban C, Igari Y, Yagi M, Reimer T, Koyama S, Aoshi T, Ohata K, Tsukui T, Takeshita F, Sakurai K, Ikegami T, Nakagawa A, Horii T, Nuñez G, Ishii KJ, Akira S. Immunogenicity of Whole-Parasite Vaccines against Plasmodium falciparum Involves Malarial Hemozoin and Host TLR9. Cell Host Microbe 2010;7:50–61. doi: 10.1016/j.chom.2009.12.003. [DOI] [PubMed] [Google Scholar]
- [18].Lee H-K, Lee J, Tobias PS. Two lipoproteins extracted from Escherichia coli K-12 LCD25 lipopolysaccharide are the major components responsible for Toll-like receptor 2-mediated signaling. J Immunol 2002;168:4012–7. doi: 10.4049/jimmunol.168.8.4012. [DOI] [PubMed] [Google Scholar]
- [19].Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting Edge: Repurification of Lipopolysaccharide Eliminates Signaling Through Both Human and Murine Toll-Like Receptor 2. J Immunol 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- [20].Nativel B, Couret D, Giraud P, Meilhac O, D’Hellencourt CL, Viranaïcken W, Da Silva CR. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci Rep 2017;7. doi: 10.1038/s41598-017-16190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Teramachi J, Inagaki Y, Shinohara H, Okamura H, Yang D, Ochiai K, Baba R, Morimoto H, Nagata T, Haneji T. PKR regulates LPS-induced osteoclast formation and bone destruction in vitro and in vivo. Oral Dis 2017;23:181–8. doi: 10.1111/odi.12592. [DOI] [PubMed] [Google Scholar]
- [22].Gao A, Wang X, Yu H, Li N, Hou Y, Yu W. Effect of Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) on the expression of EphA2 in osteoblasts and osteoclasts. Vitr Cell Dev Biol - Anim 2016;52:228–34. doi: 10.1007/s11626-015-9965-0. [DOI] [PubMed] [Google Scholar]
- [23].Väänänen K Mechanism of osteoclast mediated bone resorption - Rationale for the design of new therapeutics. Adv Drug Deliv Rev 2005;57:959–71. doi: 10.1016/j.addr.2004.12.018. [DOI] [PubMed] [Google Scholar]
- [24].Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- [25].Kim W-S, Kim H, Jeong EM, Kim HJ, Lee ZH, Kim I-G, Kim H-H. Transglutaminase 2 regulates osteoclast differentiation via a Blimp1-dependent pathway. Sci Rep 2017;7:10626. doi: 10.1038/s41598-017-11246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fujihara M, Muroi M, Tanamoto KI, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol Ther 2003;100:171–94. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- [27].Díaz-Zúñiga J, Monasterio G, Alvarez C, Melgar-Rodríguez S, Benítez A, Ciuchi P, García M, Arias J, Sanz M, Vernal R. Variability of the Dendritic Cell Response Triggered by Different Serotypes of Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis Is Toll-Like Receptor 2 (TLR2) or TLR4 Dependent. J Periodontol 2015. doi: 10.1902/jop.2014.140326. [DOI] [PubMed] [Google Scholar]
- [28].Watanabe K, Iizuka T, Adeleke A, Pham L, Shlimon AE, Yasin M, Horvath P, Unterman TG. Involvement of toll-like receptor 4 in alveolar bone loss and glucose homeostasis in experimental periodontitis. J Periodontal Res 2011;46:21–30. doi: 10.1111/j.1600-0765.2010.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taubman M a, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol 2005;76:2033–41. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- [30].Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1β and tumor necrosis factor-α, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 1999. doi: 10.1016/S8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- [31].Algate K, Haynes DR, Bartold PM, Crotti TN, Cantley MD. The effects of tumour necrosis factor-α on bone cells involved in periodontal alveolar bone loss; osteoclasts, osteoblasts and osteocytes. J Periodontal Res 2016;51:549–66. doi: 10.1111/jre.12339. [DOI] [PubMed] [Google Scholar]
- [32].Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CWO, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 2006;169:987–98. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kikuchi T, Matsuguchi T, Tsuboi N, Mitani a, Tanaka S, Matsuoka M, Yamamoto G, Hishikawa T, Noguchi T, Yoshikai Y. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J Immunol 2001;166:3574–9. doi: 10.4049/jimmunol.166.5.3574. [DOI] [PubMed] [Google Scholar]
- [34].Nagasawa T, Kiji M, Yashiro R, Hormdee D, Lu H, Kunze M, Suda T, Koshy G, Kobayashi H, Oda S, Nitta H, Ishikawa I. Roles of receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin in periodontal health and disease. Periodontol 2000 2007;43:65–84. doi: 10.1111/j.1600-0757.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- [35].Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res 2015;2015:1–10. doi: 10.1155/2015/615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tang Y, Sun F, Li X, Zhou Y, Yin S, Zhou X. Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of escherichia coli lipopolysaccharide. J Endod 2011. doi: 10.1016/j.joen.2011.08.015. [DOI] [PubMed] [Google Scholar]
- [37].Matsumoto C, Oda T, Yokoyama S, Tominari T, Hirata M, Miyaura C, Inada M. Toll-like receptor 2 heterodimers, TLR2/6 and TLR2/1 induce prostaglandin E production by osteoblasts, osteoclast formation and inflammatory periodontitis. Biochem Biophys Res Commun 2012. doi: 10.1016/j.bbrc.2012.10.016. [DOI] [PubMed] [Google Scholar]
- [38].Okahashi N, Inaba H, Nakagawa I, Yamamura T, Kuboniwa M, Nakayama K, Hamada S, Amano A. Porphyromonas gingivalis induces receptor activator of NF-kappaB ligand expression in osteoblasts through the activator protein 1 pathway. Infect Immun 2004;72:1706–14. doi: 10.1128/iai.72.3.1706-1714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kitazawa R, Kitazawa S, Maeda S. Promoter structure of mouse RANKL/TRANCE/OPGL/ODF gene. Biochim Biophys Acta 1999;1445:134–41. doi: 10.1016/s0167-4781(99)00032-9. [DOI] [PubMed] [Google Scholar]
- [40].Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem 2000;275:4858–64. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- [41].Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor Necrosis Factor α Stimulates Osteoclast Differentiation by a Mechanism Independent of the Odf/Rankl-Rank Interaction. J Exp Med 2000;191:275–86. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA. Proinflammatory cytokine (TNFα/IL-Iα) induction of human osteoclast formation. J Pathol 2002;198:220–7. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- [43].Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFα potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 2002;143:1108–18. doi: 10.1210/en.143.3.1108. [DOI] [PubMed] [Google Scholar]
- [44].Liu J, Wang S, Zhang P, Said-Al-Naief N, Michalek SM, Feng X. Molecular Mechanism of the Bifunctional Role of Lipopolysaccharide in Osteoclastogenesis. J Biol Chem 2009;284:12512–23. doi: 10.1074/jbc.M809789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nason R, Jung JY, Chole RA. Lipopolysaccharide-induced osteoclastogenesis from mononuclear precursors: a mechanism for osteolysis in chronic otitis. J Assoc Res Otolaryngol 2009;10:151–60. doi: 10.1007/s10162-008-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000;106:1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res 2002;17:1211–8. doi: 10.1359/jbmr.2002.17.7.1211. [DOI] [PubMed] [Google Scholar]
- [48].Gravallese EM, Galson DL, Goldring SR, Auron PE. The role of TNF-receptor family members and other TRAF-dependent receptors in bone resorption. Arthritis Res 2001;3:6. doi: 10.1186/ar134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF Family of Signal Transducers Mediates NF-κB Activation by the TRANCE Receptor. J Biol Chem 1998;273:28355–9. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- [50].Lomaga MA, Yeh W-C, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 1999;13:1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 2007;26:3214–26. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- [52].Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu L-C, Cao Y, Schett G, Wagner EF, Karin M. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med 2005;201:1677–87. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yao Z, Lei W, Duan R, Li Y, Luo L, Boyce BF. RANKL cytokine enhances TNF-induced osteoclastogenesis independently of TNF receptor associated factor (TRAF) 6 by degrading TRAF3 in osteoclast precursors. J Biol Chem 2017;292:10169–79. doi: 10.1074/jbc.M116.771816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Takami M, Kim N, Rho J, Choi Y. Stimulation by Toll-Like Receptors Inhibits Osteoclast Differentiation. J Immunol 2002;169:1516–23. doi: 10.4049/JIMMUNOL.169.3.1516. [DOI] [PubMed] [Google Scholar]
- [55].Zhang P, Liu J, Xu Q, Harber G, Feng X, Michalek SM, Katz J. TLR2-dependent modulation of osteoclastogenesis by Porphyromonas gingivalis through differential induction of NFATc1 and NF-κB. J Biol Chem 2011;286:24159–69. doi: 10.1074/jbc.M110.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kanzaki H, Movila A, Kayal R, Napimoga MH, Egashira K, Dewhirst F, Sasaki H, Howait M, Al-Dharrab A, Mira A, Han X, Taubman MA, Nichols FC, Kawai T. Phosphoglycerol dihydroceramide, a distinctive ceramide produced by Porphyromonas gingivalis, promotes RANKL-induced osteoclastogenesis by acting on non-muscle myosin II-A (Myh9), an osteoclast cell fusion regulatory factor. Biochim Biophys Acta Mol Cell Biol Lipids 2017;1862:452–62. doi: 10.1016/j.bbalip.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lin J, Bi L, Yu X, Kawai T, Taubman MA, Shen B, Han X. Porphyromonas gingivalis exacerbates ligature-induced, RANKL dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect Immun 2014;82:4127–34. doi: 10.1128/IAI.02084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J Clin Invest 1997;100:1557–65. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].HOU G-Q, GUO C, SONG G-H, FANG N, FAN W-J, CHEN X-D, YUAN L, WANG Z-Q. Lipopolysaccharide (LPS) promotes osteoclast differentiation and activation by enhancing the MAPK pathway and COX-2 expression in RAW264.7 cells. Int J Mol Med 2013;32:503–10. doi: 10.3892/ijmm.2013.1406. [DOI] [PubMed] [Google Scholar]
- [60].Nakanishi-Matsui M, Yano S, Matsumoto N, Futai M. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem Biophys Res Commun 2012;425:144–9. doi: 10.1016/j.bbrc.2012.07.050. [DOI] [PubMed] [Google Scholar]
- [61].Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, Okahashi N, Nishihara T, Takahashi N. Lipopolysaccharide Promotes the Survival of Osteoclasts Via Toll-Like Receptor 4, but Cytokine Production of Osteoclasts in Response to Lipopolysaccharide Is Different from That of Macrophages. J Immunol 2003;170:3688–95. doi: 10.4049/jimmunol.170.7.3688. [DOI] [PubMed] [Google Scholar]
- [62].Gao Y, Wang B, Shen C, Xin W. Overexpression of miR‑146a blocks the effect of LPS on RANKL‑induced osteoclast differentiation. Mol Med Rep 2018;18:5481–8. doi: 10.3892/mmr.2018.9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kagiya T. MicroRNAs: Potential biomarkers and therapeutic targets for alveolar bone loss in periodontal disease. Int J Mol Sci 2016. doi: 10.3390/ijms17081317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Perri R, Nares S, Zhang S, Barros SP, Offenbacher S. MicroRNA modulation in obesity and periodontitis. J Dent Res 2012. doi: 10.1177/0022034511425045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lee YH, Na HS, Jeong SY, Jeong SH, Park HR, Chung J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell 2011. [PubMed] [Google Scholar]
- [66].Ogata Y, Matsui S, Kato A, Zhou L, Nakayama Y, Takai H. MicroRNA expression in inflamed and noninflamed gingival tissues from Japanese patients. J Oral Sci 2014. [DOI] [PubMed] [Google Scholar]
- [67].Guo J, Zeng X, Miao J, Liu C, Wei F, Liu D, Zheng Z, Ting K, Wang C, Liu Y. MiRNA-218 regulates osteoclast differentiation and inflammation response in periodontitis rats through Mmp9. Cell Microbiol 2019:e12979. doi: 10.1111/cmi.12979. [DOI] [PubMed] [Google Scholar]
- [68].Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, Sood AK, Mendell JT, Wan Y. MiR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 2014. doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [69].Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ, Kim JY, Kim HH. MicroRNA-124 regulates osteoclast differentiation. Bone 2013. doi: 10.1016/j.bone.2013.07.007. [DOI] [PubMed] [Google Scholar]
- [70].Mizoguchi F, Murakami Y, Saito T, Miyasaka N, Kohsaka H. MiR-31 controls osteoclast formation and bone resorption by targeting RhoA. Arthritis Res Ther 2013. doi: 10.1186/ar4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kagiya T, Nakamura S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J Periodontal Res 2013. doi: 10.1111/jre.12017. [DOI] [PubMed] [Google Scholar]
- [72].Zhu J, Jia E, Zhou Y, Xu J, Feng Z, Wang H, Chen X, Li J. Interleukin-22 secreted by NKp44 natural killer cells promotes proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Med (United States) 2015. doi: 10.1097/MD.0000000000002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Luo Z, Wang H, Sun Z, Luo W, Wu Y. Expression of IL-22, IL-22R and IL-23 in the peri-implant soft tissues of patients with peri-implantitis. Arch Oral Biol 2013. doi: 10.1016/j.archoralbio.2012.08.006. [DOI] [PubMed] [Google Scholar]
- [74].Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, Van Bruggen N, Kolumam G, Ouyang W. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- [75].Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu Rev Immunol 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- [76].Díaz-Zúñiga J, Melgar-Rodríguez S, Rojas L, Alvarez C, Monasterio G, Carvajal P, Vernal R. Increased levels of the T-helper 22-associated cytokine (interleukin-22) and transcription factor (aryl hydrocarbon receptor) in patients with periodontitis are associated with osteoclast resorptive activity and severity of the disease. J Periodontal Res 2017;52:893–902. doi: 10.1111/jre.12461. [DOI] [PubMed] [Google Scholar]
- [77].Pan S, Yang D, Zhang J, Zhang Z, Zhang H, Liu X, Li C. Temporal expression of interleukin-22, interleukin-22 receptor 1 and interleukin-22-binding protein during experimental periodontitis in rats. J Periodontal Res 2017. doi: 10.1111/jre.12512. [DOI] [PubMed] [Google Scholar]
- [78].Kato-Kogoe N, Nishioka T, Kawabe M, Kataoka F, Yamanegi K, Yamada N, Hata M, Yamamoto T, Nakasho K, Urade M, Terada N, Ohyama H. The promotional effect of IL-22 on mineralization activity of periodontal ligament cells. Cytokine 2012;59:41–8. doi: 10.1016/j.cyto.2012.03.024. [DOI] [PubMed] [Google Scholar]
- [79].Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2014. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sima C, Glogauer M. Macrophage subsets and osteoimmunology: Tuning of the immunological recognition and effector systems that maintain alveolar bone. Periodontol 2000. 2013. doi: 10.1111/prd.12032. [DOI] [PubMed] [Google Scholar]
- [81].Yu T, Zhao L, Huang X, Ma C, Wang Y, Zhang J, Xuan D. Enhanced Activity of the Macrophage M1/M2 Phenotypes and Phenotypic Switch to M1 in Periodontal Infection. J Periodontol 2016;87:1092–102. doi: 10.1902/jop.2016.160081. [DOI] [PubMed] [Google Scholar]
- [82].Yang J, Zhu Y, Duan D, Wang P, Xin Y, Bai L, Liu Y, Xu Y. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Arch Oral Biol 2018;96:234–42. doi: 10.1016/j.archoralbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- [83].Zhuang Z, Yoshizawa-Smith S, Glowacki A, Maltos K, Pacheco C, Shehabeldin M, Mulkeen M, Myers N, Chong R, Verdelis K, Garlet GP, Little S, Sfeir C. Induction of M2 Macrophages Prevents Bone Loss in Murine Periodontitis Models. J Dent Res 2019;98:200–8. doi: 10.1177/0022034518805984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Viniegra A, Goldberg H, Çil, Fine N, Sheikh Z, Galli M, Freire M, Wang Y, Van Dyke TE, Glogauer M, Sima C. Resolving Macrophages Counter Osteolysis by Anabolic Actions on Bone Cells. J Dent Res 2018. doi: 10.1177/0022034518777973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Atomura R, Sanui T, Fukuda T, Tanaka U, Toyoda K, Taketomi T, Yamamichi K, Akiyama H, Nishimura F. Inhibition of Sprouty2 polarizes macrophages toward an M2 phenotype by stimulation with interferon γ and Porphyromonas gingivalis lipopolysaccharide. Immunity, Inflamm Dis 2016;4:98–110. doi: 10.1002/iid3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yamaguchi T, Movila A, Kataoka S, Wisitrasameewong W, Ruiz Torruella M, Murakoshi M, Murakami S, Kawai T. Proinflammatory M1 Macrophages Inhibit RANKL-Induced Osteoclastogenesis. Infect Immun 2016;84:2802–12. doi: 10.1128/IAI.00461-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mahanonda R, Champaiboon C, Subbalekha K, Sa-Ard-Iam N, Rattanathammatada W, Thawanaphong S, Rerkyen P, Yoshimura F, Nagano K, Lang NP, Pichyangkul S. Human Memory B Cells in Healthy Gingiva, Gingivitis, and Periodontitis. J Immunol 2016;197:715–25. doi: 10.4049/JIMMUNOL.1600540. [DOI] [PubMed] [Google Scholar]
- [88].Han YK, Jin Y, Miao YB, Shi T, Lin XP. Improved RANKL production by memory B cells: A way for B cells promote alveolar bone destruction during periodontitis. Int Immunopharmacol 2018. doi: 10.1016/j.intimp.2018.08.033. [DOI] [PubMed] [Google Scholar]
- [89].Han Y, Jin Y, Miao Y, Shi T, Lin X. Improved RANKL expression and osteoclastogenesis induction of CD27+CD38− memory B cells: A link between B cells and alveolar bone damage in periodontitis. J Periodontal Res 2019;54:73–80. doi: 10.1111/jre.12606. [DOI] [PubMed] [Google Scholar]
- [90].Richard K, Pierce SK, Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J Immunol 2008;181:1746–52. doi: 10.4049/jimmunol.181.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ackermann JA, Nys J, Schweighoffer E, McCleary S, Smithers N, Tybulewicz VLJ. Syk Tyrosine Kinase Is Critical for B Cell Antibody Responses and Memory B Cell Survival. J Immunol 2015;194:4650–6. doi: 10.4049/jimmunol.1500461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schweighoffer E, Nys J, Vanes L, Smithers N, Tybulewicz VLJ. TLR4 signals in B lymphocytes are transduced via the B cell antigen receptor and SYK. J Exp Med 2017;214:1269–80. doi: 10.1084/jem.20161117. [DOI] [PMC free article] [PubMed] [Google Scholar]