Abstract
Despite the association of physical activity with improved cardiovascular outcomes and the association of high coronary artery calcification (CAC) scores with poor prognosis, elite endurance athletes have increased CAC. Yet, they nevertheless have better cardiovascular survival. We hypothesized that exercise may transform vascular calcium deposits to a more stable morphology. To test this, hyperlipidemic mice (Apoe−/−) with baseline aortic calcification were separated into 2 groups (n = 9/group) with control mice allowed to move ad-lib while the exercise group underwent a progressive treadmill regimen for 9 weeks. All mice underwent blood collections and in vivo 18F-NaF μPET/μCT imaging both at the start and end of the exercise regimen. At euthanasia, aortic root specimens were obtained for histomorphometry. Results showed that, while aortic calcification progressed similarly in both groups based on μCT, the fold change in 18F-NaF density was significantly less in the exercise group. Histomorphometric analysis of the aortic root calcium deposits showed that the exercised mice had a lower mineral surface area index than the control group. The exercise regimen also raised serum PTH levels two-fold. These findings suggest that weeks-long progressive exercise alters the microarchitecture of atherosclerotic calcium deposits by reducing mineral surface growth, potentially favoring plaque stability.
Keywords: treadmill exercise, aortic, calcification, PTH, 18F-NaF PET/CT imaging, microarchitecture, hyperlipidemia
Introduction
Regular physical exercise is widely accepted as an effective way to reduce risk of coronary heart disease [1], and the recently updated World Health Organization Physical Activity Guidelines recommend at least 150 minutes of moderate-intensity exercise weekly in the adult population in order to reduce risk of cardiovascular disease [2]. It is also widely accepted that coronary artery calcification (CAC) is a strong predictor of cardiovascular morbidity and mortality [3,4]. In two large studies of subjects without clinical coronary or cardiovascular disease, higher levels of cardiorespiratory fitness (self-reported or measured by exercise treadmill testing) were associated with better cardiovascular outcomes, whereas higher CAC scores were associated with worse cardiovascular outcomes at all levels of cardiorespiratory fitness [5,6].
Interestingly, multiple recent clinical studies have shown higher CAC scores in endurance athletes, such as veteran marathon runners and competitive cyclists, than in less active subjects [7-11]. This paradoxical high CAC is both at the level of prevalence and severity. The prevalence of CAC is greater in the highly athletic subjects than in controls matched for age and risk factors, and the CAC scores are higher in the athletes [7-10]. Nevertheless, despite their higher CAC scores, elite athletes appear to have better cardiovascular survival than their more sedentary controls [12]. These clinical findings beg the question of whether exercise is a contributing factor to CAC and/or whether the CAC in elite endurance athletes differs in some respects from the CAC in the general population, as it does not appear to impart a similar degree of risk. Microarchitectural features of calcium deposits, such as density or surface area, have been suggested to be the key parameters in determining mechanical vulnerability to plaque rupture [13-15]. Since the calcified plaque found in athletes has a greater ratio of mineral to lipid content [8], it is conceivable that this difference in composition may change density or surface area and, hence, risk of plaque rupture. Thus, in the present study, we tested whether a progressive, weeks-long exercise regimen in a mouse model of atherosclerotic calcification modulates the microarchitecture of aortic calcium deposits.
Materials and Methods
Animals and exercise regimen
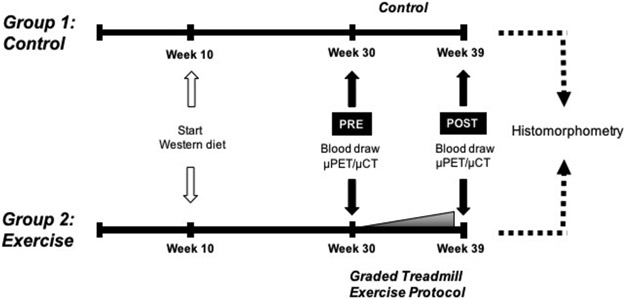
Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Los Angeles. Female apolipoprotein E (Apoe)-null mice (C57BL/6 background, Jackson Laboratory, Bar Harbor, ME) were placed on “Western” diet (21% fat and 0.2% cholesterol, Envigo) to induce baseline aortic calcification (Fig. 1), starting at 10 weeks of age. After 20 weeks on the diet, the mice were assigned to a control group (n = 9) or a treadmill group (n = 9) for an additional 9 weeks. Though starting numbers were > 9 mice/group, mice treated for ulcerative dermatitis were excluded from analyses. For the treadmill group, the mice were acclimated on a treadmill (Columbus Instruments Exer-3/6 Animal Treadmill Rodent 6-Lane) for one week and subjected to a 9-week-long exercise regimen without inclination or electric shock stimulation. The speed of exercise was increased weekly from 12.5 meters/min to 18.5 meters/min (0.5 -1.0 meters/min/wk), and duration of exercise was increased daily (5 min/day) from 30 minutes to 50 minutes. Since the exercise capacity varied somewhat among mice, the daily distance run by each mouse was recorded, and the total distance run was used as a measure of exercise capacity. Female Apoe-null mice were chosen for this study, as they have been shown to have more aortic calcification than male mice [16].
Figure 1: Experimental timeline.
Schematic of the timing of interventions for control and exercise groups. The diet was started at 10 weeks of age.
PTH measurements
Blood samples from all mice were collected once prior to the exercise regimen (week 30) and once at the end of week 39. Serum levels of PTH were measured using a PTH ELISA kit (Immutopics International), according to the manufacturer’s protocol.
Serial in vivo 18F-NaF μPET/CT imaging and analysis
Fused 18F-NaF μPET/μCT imaging was performed once prior to the exercise regimen (week 30) and once at the end of week 39. The imaging and analysis were performed as described previously [17]. Briefly, mice were injected with ~200 μCi 18F-NaF via tail vein. One hour post-injection, mice were anesthetized and imaged in the μPET scanner (Focus 200 μPET, Concorde Microsystems, Knoxville, TN) for 10 min and, subsequently, in the μCT scanner (CrumpCAT, UCLA) for 2 min in the same gantry. Images were analyzed using AMIDE software. An initial volumetric ROI was drawn to isolate parts of cardiac and aortic regions from the remainder of the body; three-dimensional isocontour ROIs were then drawn of the calcified areas within this region; and quantification of the isocontour ROIs was performed on the original images. The minimum μCT isocontour threshold for aortic calcification was 200 Hounsfield units (HU) and the minimum 18F-NaF isocontour threshold was defined as 2% injected dose per cubic centimeter (%ID/cc). The mean threshold of background 18F-NaF uptake, measured at the cardiac silhouette of four mice, was 0.8 %ID/cc.
Histomorphometric analysis
Hearts (n = 9/group) were isolated, embedded in optimal cutting temperature compound, and 10-μm cryosections were obtained. Aortic calcification was analyzed by segmenting alizarin red positive calcified regions using NIH ImageJ software. The mineral surface area index (perimeter/cross-sectional area) of individual calcium deposits was quantified using custom Matlab code (Mathworks, Natick, MA), as previously described [17].
Ex vivo μCT imaging of femurs
Femurs from each mouse were scanned utilizing a μCT scanner (μCT Skyscan 1172; Skyscan, Kontich, Belgium) at 55kV, 181uA, and 20-μm resolution. Volumetric data were converted to DICOM format and imported into Dolphin Imaging software (Chatsworth, CA) to generate 3D and multiplanar reconstructed images. Analysis was performed using CT-Analyser (CTAn) software on transaxial datasets. ROIs of distal femur metaphyses included trabecular but excluded cortical bone. ROIs of the mid-shaft included the cortical, but excluded trabecular, bone. Grayscale thresholds for quantitation of structural parameters were determined using the thresholding algorithm within CTAn.
Statistical Analysis
Values are expressed as mean ± SEM. Statistical analysis was performed with Prism software (GraphPad). For comparisons of fold-change, we used the parametric Student’s t-test (2-tailed, unpaired) where data were normally distributed per the Shapiro-Wilk test, otherwise we used the non-parametric Mann-Whitney test. For pre- and post-comparisons within the same group, we used a paired Student’s t-test (2-tailed) or the Wilcoxon matched-pairs signed rank test. Pearson’s correlation was used to test strength of association. Values of p ≤ 0.05 were considered statistically significant.
RESULTS
In vivo μCT imaging of aortic calcium deposition following the 9-week exercise regimen
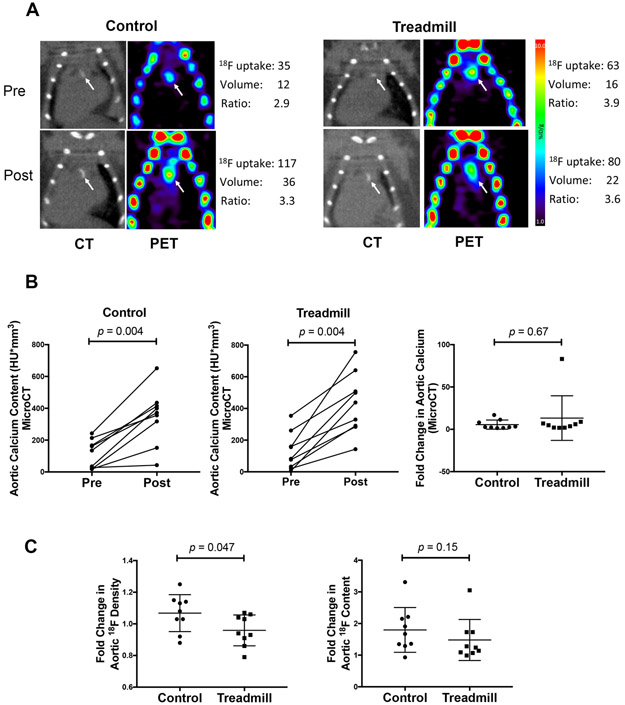
Representative images of microCT and microPET images of the control and treadmill groups are shown in Fig. 2A. In both groups, aortic calcification by microCT analysis progressed significantly with an approximately 25% increase over the study period (Fig. 2B, left and middle; p = 0.004). The fold change in the total aortic calcium content was similar between the two groups (Fig. 2B, right; p = 0.67), suggesting that exercise did not augment the progression of aortic calcification as assessed by μCT.
Figure 2: In vivo imaging analyses of the effects of exercise on aortic calcium deposits.
(A) In vivo imaging analyses of the effects of exercise on aortic calcium deposits. (A) Vascular calcium deposits (white arrows) imaged by microCT and microPET in control and exercise mice at the start (pre) and end (post) of the 9-week period. (B) MicroCT analysis of total aortic calcium content (left and middle) and fold change in aortic calcium content (right) over the intervention period. (C) MicroPET analysis of the fold change in the 18F-NaF density (left) and total 18F-NaF content (right) over the intervention period.
In vivo 18F-NaF μPET imaging of aortic calcium deposition following long-term exercise
18F-NaF tracer uptake was previously shown to adsorb onto the surface of vascular calcium deposits [18]. Thus, we analyzed 18F-NaF tracer uptake to assess exposed surface area of aortic calcium deposits in two ways: (1) normalized surface area (or 18F-NaF density), defined as 18F-NaF uptake as a percent injected dose of tracer normalized to deposit volume (%ID/cc) and (2) total surface area, defined as total 18F-NaF uptake as a percent of injected dose of tracer (%ID). Results showed that the fold change of the normalized surface area (18F-NaF density) was significantly lower over the study period in the exercise group than in the control group (Fig. 2C, left; p = 0.047), whereas the fold change in total surface area (18F-NaF content) was similar between the two groups (Fig. 2C, right; p = 0.15).
Effects of 9-week-long exercise on morphology of aortic calcium deposits by histochemical stain
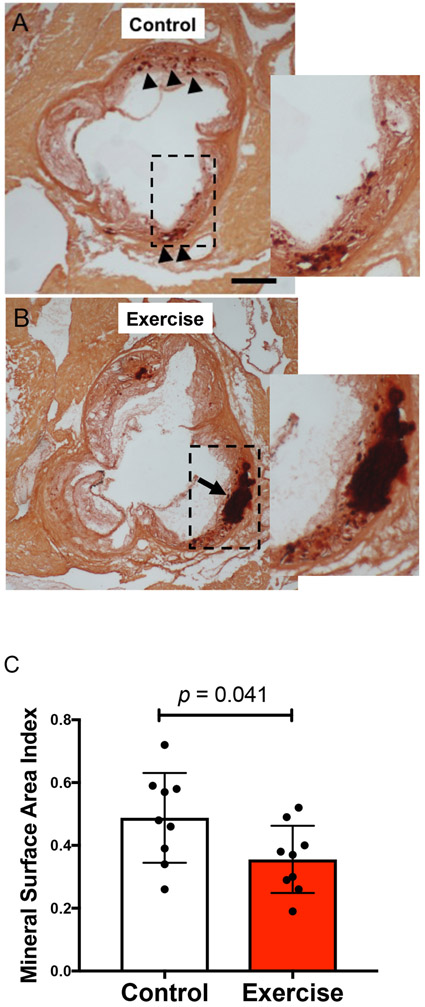
At euthanasia, we analyzed the microarchitecture of calcium deposits by histomorphometry using Alizarin red staining of aortic root sections. Deposits appeared to be somewhat more scattered (“spotty”) in aortic root sections from the control group and more coalesced in those from the exercise group (Fig. 3A-B). Quantitative analysis also showed a decrease in the exercise group of a 2-dimensional index of surface area per deposit volume, the “mineral surface area index (MSI),” defined as total perimeter/total cross-sectional area in each section (Fig. 3C; p = 0.041). This is consistent with the observed changes in “18F-NaF density,” another measure of surface area per deposit volume.
Figure 3: Histological analyses of the effects of exercise on aortic calcium deposits.
(A-B) Alizarin red staining of representative aortic root sections. Arrowheads indicate small deposits (“spotty”) of calcification, and the arrow indicates a larger coalesced deposit of calcium mineral. Scale bar, 500 μm. (C) Mineral surface area index (MSI) of the control and exercise groups.
Effect of aortic calcification on exercise capacity
Since exercise tolerance varied among the mice, we determined whether the exercise capacity, measured as total distance run, depended on the severity of aortic calcification. In the exercise group, total distance run correlated inversely with both baseline and final aortic mineral content as measured by μCT (r = −0.76; p = 0.017; r = −0.78; p = 0.013, respectively) but was not correlated with baseline or final 18F-NaF uptake (r = −0.15, p = 0.70; r = −0.23, p = 0.56, respectively).
Effects of long-term exercise on femoral trabecular bone
MicroCT analysis of femoral trabecular bone revealed that mice in the exercise group also had a lower specific bone surface compared with mice in the control group (41.9 ± 1.5 vs. 48.2 ± 2.5; p = 0.046).
Effects of the 9-week exercise regimen on serum PTH levels
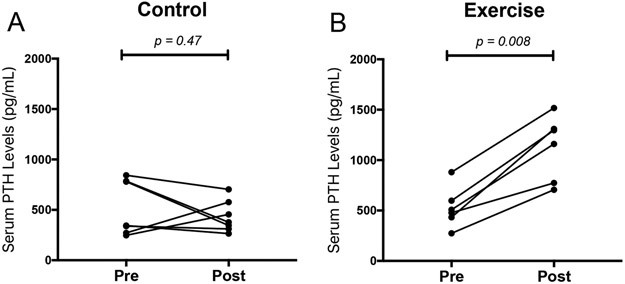
In the control group, serum PTH levels were not significantly different over the 9-week study period (Fig. 4A; p = 0.47). In the treadmill group, serum PTH levels increased significantly following the graded treadmill protocol intervention (Fig. 4B; p = 0.008).
Figure 4: Effects of exercise on serum PTH levels.
(A-B) Serum PTH was measured before (Pre) and after (Post) the intervention period.
DISCUSSION
The results of our study indicate that hyperlipidemic mice with aortic calcification subjected to a progressive exercise regimen undergo a change in the microarchitecture of their calcium deposits that may affect mechanical stability. Although the total amount of calcification, as measured by μCT, progressed similarly in the two groups, three independent measures of surface area per volume of mineral all consistently showed a decrease with exercise: 18F-NaF density in the aorta, histological MSI in the aorta, and specific bone surface in femoral bone. These findings suggest that exercise reduces the amount of exposed surface area per unit of mineral volume in both aorta and skeletal bone.
A previous theoretical finite element analysis revealed that rupture (von Mises) stress increases at the surfaces of a rigid inclusion, such as a calcium deposit, embedded in a distensible material, such as vascular tissue [13], suggesting that the amount of surface area of calcium deposits relates positively with the risk of rupture. Clinical evidence is consistent with this theoretical analysis. First, it is known that a spotty pattern of calcium deposits, which has a higher surface area than a contiguous pattern, is associated with plaque rupture vulnerability, and it is now an established marker for “high-risk” plaque [19,20]. Second, clinical PET imaging studies show that plaque rupture vulnerability is identified by 18F-NaF uptake [21], which labels the exposed mineral surface area [18].
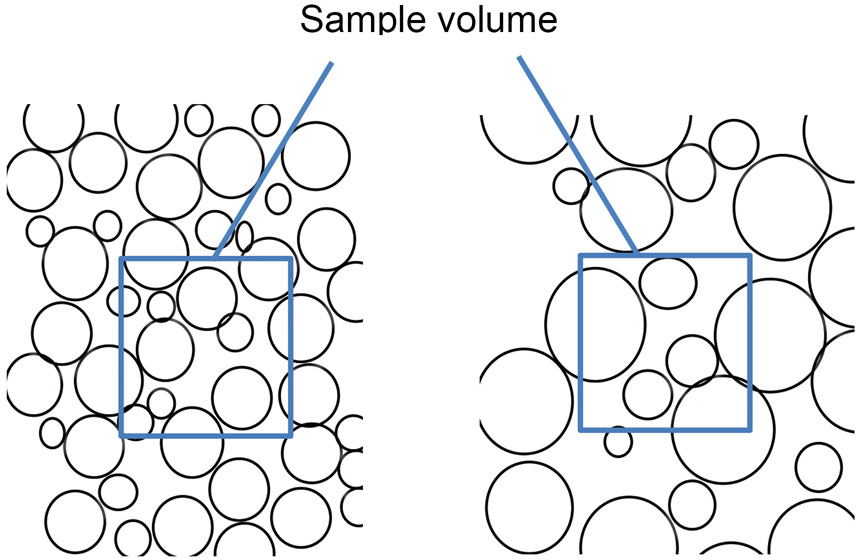
Given this evidence that mineral surface area promotes rupture risk, one would expect progression of vascular calcification, as in athletes, to increase mineral surface area and, thus, risk. Paradoxically, the athletes appear to have lower risk. One possible explanation is that progression of calcification can have opposite effects on surface area depending on whether mineral growth occurs by coalescence or by de novo formation of deposits arising at scattered sites. With coalescence, in a given unit of volume, larger mineral deposits eventually have lower surface area than smaller dispersed deposits (Fig. 5). In the present study, normalized mineral surface area increased in the control mice whereas it decreased in the exercised mice despite a similar progression of calcification based on μCT. A similar phenomenon, consisting of decreased normalized surface area despite progression of calcification based on μCT, was found in our previous study of hyperlipidemic mice treated with intermittent PTH therapy [22]. Such findings may represent evidence for coalescence of calcium deposits.
Figure 5: A diagram illustrating the concept of surface area index or 18F-NaF density.
As seen in this illustration, an increase in the size of round particles in a given space results in a local decrease in the surface area per volume of particles, i.e., surface area index (because volume increases more rapidly than area as radius increases). Conversely, a decrease in the size of particles has the opposite effect (not illustrated).
The similar findings with exercise and intermittent PTH therapy may have a biological basis. Studies in both humans and mice show that PTH levels are transiently increased with exercise, depending on duration and volume. In humans, physical activity longer than 40 minutes and with at least moderate intensity (> 45% VO2max) was found to cause a rise in serum PTH levels, whereas exercise of shorter duration or lower intensity did not [23]. In mice, a single, brief (30-minute) episode of treadmill exercise also led to a rise in serum PTH levels [24]. Consistent with this, our results show that the 9-week-long exercise regimen also increased serum PTH levels. Interestingly, Lee & Prisby found that treating mice with daily injections of PTH(1-34), an intermittent regimen, causes coalescence of mineral deposits in femoral bone marrow blood vessels [25]. Similarly, we recently reported that intermittent PTH(1-34) regimen altered the microarchitecture of aortic calcification by promoting the coalescence of calcium deposits [17].
The present study has limitations. The control mice were allowed to move freely in their cages, and the exercise regimen may not have been at an intensity comparable to that of elite athletes. It is possible that greater differences would be found between sedentary vs. athletic humans. It is also possible that coronary calcification would respond differently to exercise than aortic calcification. We chose to study calcification in the mouse aorta because it is similar in size to human coronaries; in addition, mouse coronary calcification is below the resolution of microCT and microPET imaging. Further, our measure of exercise capacity may have underestimated actual capacity for some of the mice, given that some were able to continue running at the end of some exercise sessions.
To our knowledge, this study is the first to show the effects of exercise on vascular calcification in mice. It is important to note that, even among elite athletes and subjects with high levels of exercise and fitness, CAC scores correlate with worsening cardiovascular outcomes [6,9,10]. Nevertheless, our findings suggest that exercise induces coalescence of aortic calcium deposits, which may render them less vulnerable to mechanical rupture than deposits in a “spotty” distribution, potentially through PTH.
New Knowledge Gained
Hyperlipidemic mice that underwent long-term, progressive exercise regimen on a treadmill had reduced normalized surface area of aortic calcium deposits, suggestive of a more stable microarchitecture. Total distance run correlated inversely with the severity of aortic calcium content in these mice. These findings may help explain the overall improved cardiovascular outcomes of endurance athletes, despite the signal towards a higher CAC score in this population.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge Drs. Jason Lee, Arion Chatziioannou, and Sotirios Tetradis for support with imaging, as well as the use of the Mouse Physiology Laboratory of the Department of Physiology and the Pre-Clinical Facility of the Crump Institute for Molecular Imaging at the California NanoSystems Institute.
Funding: This work was supported by the National Institutes of Health [HL12109, HL137647, AG61586, P30AG028748], and an American Heart Association Post-Doctoral Research Fellowship [18POST34030272]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- CAC
coronary artery calcification
- PET
positron emission tomography
- CT
computed tomography
- Apoe
apolipoprotein E
- NaF
sodium fluoride
- ROI
region of interest
REFERENCES
- [1].Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB, Exercise type and intensity in relation to coronary heart disease in men, JAMA. 288 (2002) 1994–2000. [DOI] [PubMed] [Google Scholar]
- [2].Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD, The Physical Activity Guidelines for Americans, JAMA. 320 (2018) 2020–2028. 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P, Coronary artery calcium score and risk classification for coronary heart disease prediction, JAMA. 303 (2010) 1610–1616. 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH, Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis, Circ Cardiovasc Imaging. 8 (2015). 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arnson Y, Rozanski A, Gransar H, Hayes SW, Friedman JD, Thomson LEJ, Berman DS, Impact of Exercise on the Relationship Between CAC Scores and All-Cause Mortality, JACC Cardiovasc Imaging. 10 (2017) 1461–1468. 10.1016/j.jcmg.2016.12.030. [DOI] [PubMed] [Google Scholar]
- [6].Radford NB, DeFina LF, Leonard D, Barlow CE, Willis BL, Gibbons LW, Gilchrist SC, Khera A, Levine BD, Cardiorespiratory Fitness, Coronary Artery Calcium, and Cardiovascular Disease Events in a Cohort of Generally Healthy Middle-Age Men: Results From the Cooper Center Longitudinal Study, Circulation. 137 (2018) 1888–1895. 10.1161/CIRCULATIONAHA.117.032708. [DOI] [PubMed] [Google Scholar]
- [7].Möhlenkamp S, Lehmann N, Breuckmann F, Bröcker-Preuss M, Nassenstein K, Halle M, Budde T, Mann K, Barkhausen J, Heusch G, Jöckel K-H, Erbel R, Marathon Study Investigators, Heinz Nixdorf Recall Study Investigators, Running: the risk of coronary events : Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners, Eur. Heart J 29 (2008) 1903–1910. 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- [8].Aengevaeren VL, Mosterd A, Braber TL, Prakken NHJ, Doevendans PA, Grobbee DE, Thompson PD, Eijsvogels TMH, Velthuis BK, Relationship Between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes, Circulation. 136 (2017) 138–148. 10.1161/CIRCULATIONAHA.117.027834. [DOI] [PubMed] [Google Scholar]
- [9].Laddu DR, Rana JS, Murillo R, Sorel ME, Quesenberry CP, Allen NB, Gabriel KP, Carnethon MR, Liu K, Reis JP, Lloyd-Jones D, Carr JJ, Sidney S, 25-Year Physical Activity Trajectories and Development of Subclinical Coronary Artery Disease as Measured by Coronary Artery Calcium: The Coronary Artery Risk Development in Young Adults (CARDIA) Study, Mayo Clin. Proc 92 (2017) 1660–1670. 10.1016/j.mayocp.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwartz RS, Kraus SM, Schwartz JG, Wickstrom KK, Peichel G, Garberich RF, Lesser JR, Oesterle SN, Knickelbine T, Harris KM, Duval S, Roberts WO, O’Keefe JH, Increased Coronary Artery Plaque Volume Among Male Marathon Runners, Mo Med. 111 (2014) 89–94. [PMC free article] [PubMed] [Google Scholar]
- [11].Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, David S, Yeo TJ, Narain R, Malhotra A, Papadakis M, Wilson MG, Tome M, AlFakih K, Moon JC, Sharma S, Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes With a Low Atherosclerotic Risk Profile, Circulation. 136 (2017) 126–137. 10.1161/CIRCULATIONAHA.116.026964. [DOI] [PubMed] [Google Scholar]
- [12].Garatachea N, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Emanuele E, Lucia A, Elite athletes live longer than the general population: a meta-analysis, Mayo Clin. Proc 89 (2014) 1195–1200. 10.1016/j.mayocp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- [13].Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL, Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability, Am. J. Physiol. Heart Circ. Physiol 297 (2009) H802–810. 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abedin M, Tintut Y, Demer LL, Vascular calcification: mechanisms and clinical ramifications, Arterioscler. Thromb. Vasc. Biol 24 (2004) 1161–1170. 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- [15].Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA, Calcium density of coronary artery plaque and risk of incident cardiovascular events, JAMA. 311 (2014) 271–278. 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marek I, Canu M, Cordasic N, Rauh M, Volkert G, Fahlbusch FB, Rascher W, Hilgers KF, Hartner A, Menendez-Castro C, Sex differences in the development of vascular and renal lesions in mice with a simultaneous deficiency of Apoe and the integrin chain Itga8, Biol Sex Differ. 8 (2017) 19. 10.1186/s13293-017-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsu JJ, Lu J, Umar S, Lee JT, Kulkarni RP, Ding Y, Chang C-C, Hsiai TK, Hokugo A, Gkouveris I, Tetradis S, Nishimura I, Demer LL, Tintut Y, Effects of teriparatide on morphology of aortic calcification in aged hyperlipidemic mice, Am. J. Physiol. Heart Circ. Physiol 314 (2018) H1203–H1213. 10.1152/ajpheart.00718.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JLE, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP, Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography, Nat Commun. 6 (2015) 7495. 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nerlekar N, Ha FJ, Cheshire C, Rashid H, Cameron JD, Wong DT, Seneviratne S, Brown AJ, Computed Tomographic Coronary Angiography-Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis, Circ Cardiovasc Imaging. 11 (2018) e006973. 10.1161/CIRCIMAGING.117.006973. [DOI] [PubMed] [Google Scholar]
- [20].Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, Shah ASV, Pawade T, Weir-McCall JR, Roditi G, van Beek EJR, Newby DE, Nicol ED, Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study, J. Am. Coll. Cardiol 73 (2019) 291–301. 10.1016/j.jacc.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJR, Flapan AD, Uren NG, Behan MWH, Cruden NLM, Mills NL, Fox KAA, Rudd JHF, Dweck MR, Newby DE, 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial, Lancet. 383 (2014) 705–713. 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- [22].Hsu JJ, Lim J, Tintut Y, Demer LL, Cell-matrix mechanics and pattern formation in inflammatory cardiovascular calcification, Heart. 102 (2016) 1710–1715. 10.1136/heartjnl-2016-309667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bouassida A, Latin I, Bouassida S, Zalleg D, Zaouali M, Feki Y, Gharbi N, Zbidi A, Tabka Z, Parathyroid hormone and physical exercise: a brief review, J Sports Sci Med. 5 (2006)367–374. [PMC free article] [PubMed] [Google Scholar]
- [24].Gardinier JD, Mohamed F, Kohn DH, PTH Signaling During Exercise Contributes to Bone Adaptation, J. Bone Miner. Res 30 (2015) 1053–1063. 10.1002/jbmr.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee S, Prisby RD, Short-term intermittent PTH 1-34 administration and bone marrow blood vessel ossification in Mature and Middle-Aged C57BL/6 mice, Bone Rep. 10 (2019) 100193. 10.1016/j.bonr.2018.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.