Abstract
Radiation exposure is among the few factors known to be associated with risk of central nervous system (CNS) tumors. However, the patterns of radiation risk by histological type, sex or age are unclear. We evaluated radiation risks of first primary glioma, meningioma, schwannoma, and other or not otherwise specified (other/NOS) tumors in the Life Span Study (LSS) cohort of atomic bomb survivors. Cases diagnosed between 1958 and 2009 were ascertained through population-based cancer registries in Hiroshima and Nagasaki. To estimate excess relative risk per Gy (ERR/Gy), we fit rate models using Poisson regression methods. There were 285 CNS tumors (67 gliomas, 107 meningiomas, 49 schwannomas, and 64 other/NOS tumors) among 105,444 individuals with radiation dose estimates to the brain contributing 3.1 million person-years of observation. Based on a simple linear model without effect modification, ERR/Gy was 1.67 (95% confidence interval, CI: 0.12 to 5.26) for glioma, 1.82 (95% CI: 0.51 to 4.30) for meningioma, 1.45 (95% CI: −0.01 to 4.97) for schwannoma, and 1.40 (95% CI: 0.61 to 2.57) for all CNS tumors as a group. For each tumor type, the dose-response was consistent with linearity and appeared to be stronger among males than among females, particularly for meningioma (P = 0.045). There was also evidence that the ERR/Gy for schwannoma decreased with attained age (P = 0.002). More than 60 years after the bombings, radiation risks for CNS tumors continue to be elevated. Further follow-up is necessary to characterize the lifetime risks of specific CNS tumors following radiation exposure.
Keywords: CNS tumor, etiology, cohort studies, atomic bombs, risk assessment
Tumors of the central nervous system (CNS) are a heterogeneous group, including tumors of different histological origin, mutational profile, etiology, and clinical behavior [1, 2]. The most common CNS tumors are gliomas, meningiomas, and schwannomas [1]. Gliomas are tumors that arise from diverse populations of neuroepithelial progenitor cells and themselves are a heterogeneous group [3]. Glioblastoma multiforme, the most common type of glioma in adults, is one of the deadliest cancers [1]. Meningiomas are neoplasms developing from arachnoidal cap cells in the meningeal coverings of the spinal cord and brain [4, 5]. These are usually encapsulated and benign tumors, although intracranial location often leads to serious morbidity [5]. Virtually all schwannomas (or neurilemmomas) are benign and slow-growing nerve sheath tumors originating from Schwann cells [6]. The most common type is vestibular schwannoma (or acoustic neuroma) [1]. Collectively, brain and other CNS tumors (both malignant and non-malignant) are relatively uncommon neoplasms with an annual age-standardized incidence rate varying between 10 to 20 per 100,000 population [7, 1, 8].
Radiation exposure is among the few factors known to be associated with risk of CNS tumors [7]. Moderate-to-high doses of radiation received during childhood for treatment of tinea capitis [9], enlarged tonsils [10], and cancer [11–14] have been associated with increased risk of major histological types of CNS tumors. Increased risks for CNS tumors were also observed following childhood exposure to low radiation doses due to treatment of hemangioma [15] and, more recently, diagnostic computerized tomography (CT) scans [16–19]. Previous studies of Japanese atomic bomb survivors [20, 21], exposed to a wide range of radiation doses at different ages, similarly found radiation-associated increased risks for glioma, meningioma, schwannoma, and for all CNS tumors combined. For CNS tumors, other than schwannoma, there was a suggestion of greater risk following radiation exposure at earlier ages [21]. Overall, data concerning histology-specific patterns of radiation risks by sex, age at exposure, and attained age are limited and inconsistent, largely due to a small number of radiation excess cases in individual studies [22]. The recent findings from CT studies added more controversy, as radiation risks in these studies did not exhibit a decreasing pattern with increasing age at exposure [23–26, 17, 27, 28] and appeared higher than those from studies of therapeutically irradiated populations, raising concerns about confounding by indication.
As part of a series of updated solid cancer incidence reports in the Life Span Study (LSS) of atomic bomb survivors [29], we evaluated the incidence of CNS tumors, adding 11 years of follow-up since the last report [21] and using improved radiation dose estimates [30]. Our objective was to clarify CNS tumor-specific radiation risk estimates and modification patterns by sex and age.
Materials and Methods
Study population and case ascertainment
Study methods have been described previously [29, 31]. The LSS cohort consists of 120,321 individuals, including 93,741 atomic bomb survivors from Hiroshima and Nagasaki and 26,580 city residents who were not in either city at the time of the bombings (NIC). Vital status was ascertained through the national family registration system (koseki) followed by ascertainment of causes of death from death certificates, while cancer incidence was ascertained through population-based cancer registries in Hiroshima and Nagasaki (since 1958). All malignant CNS tumors and benign or uncertain behavior CNS tumors at the following locations (International Classification of Diseases for Oncology, ICD-O-3T): C70.0, C70.1, C70.9 (cerebral and spinal meninges), C71.0-C71.9 (brain), C72.0-C72.5 (spinal cord, cauda equqina, cranial nerves), C72.8-C72.9 (nervous system not otherwise specified, overlapping lesions of brain and CNS), and C75.1-C75.3 (pituitary, craniopharyngeal duct, pineal gland) are reportable to the cancer registries. The ascertainment of tumors is also supplemented by data from Hiroshima and Nagasaki tumor tissue registries and surgical and autopsy programs at the Radiation Effect Research Foundation (RERF) active between 1948 and 1988. As in Grant et al. [29], analyses are based on 105,444 individuals who were not known to have cancer as of 1958 and had known radiation dose estimates.
Cases were first primary CNS tumors diagnosed in the cancer registry catchment areas between 1958 and 2009. To classify tumors, we used the ICD-O-3 topography, morphology, and behavior codes. In the analysis, we categorized tumors as “glioma” (tumors of neuroepithelial and embryonal origin), “meningioma” (tumors of meningothelial cells), “schwannoma” (tumors of cranial and paraspinal nerves), “other” (mesenchymal or other rare tumors), or “not otherwise specified” (NOS). The latter two groups were further combined into one “other/NOS” group. Details of topography and morphology codes used to define specific CNS tumor types are shown in Supplemental Table S1 and Table S2, respectively. To minimize dose-related ascertainment bias [29], 56 tumors diagnosed at autopsy and not suspected clinically (“autopsy only”) were excluded from the analyses except for comparison with the results of previous LSS studies [20, 21].
Radiation doses
Dosimetry System 2002 (DS02) provided individual DS02 revision 1 (DS02R1) brain dose estimates for survivors exposed to radiation from the bombings [30, 32]. Estimated doses were adjusted to account for implausibly large estimates (total shielded kerma > 4 Gy) and random errors in dose assignments [33]. Analyses used weighted absorbed brain doses calculated using a neutron weighting factor of 10.
Data organization and statistical analyses
Person-years (PY) of observation were computed from January 1, 1958 until the earliest of the following: date of first primary CNS tumor or other cancer diagnosis, date of death, 110th birthday, or December 31, 2009. Because tumors diagnosed outside of the catchment areas were not ascertainable, PYs were adjusted for migration into and out of the catchment areas by applying sex-, age- and time-dependent residence probabilities estimated from the Adult Health Study clinical contact data [29, 34].
The analyses were based on a highly stratified table of PYs and number of cases by city, sex, age at exposure, attained age, calendar year, distance from the hypocenter (including a separate category for NIC), brain dose, and shielded kerma dose >4 Gy [29]. Further time-dependent stratification was made for smoking. This information was collected in several mailed surveys or clinic-based questionnaires and was available for 60% of the cohort [35, 29]. For more details see Supplemental Material and Data. We applied Poisson regression methods to model incidence rates of all CNS tumors as a group and specific histological types as functions of radiation dose (d), city (c), sex (s), attained age (a), age at exposure (e), year of birth (b), or calendar year (y). In describing background rates, we distinguished between in-city survivors and NIC residents (n) as well as between proximal (within 3 km of the hypocenter) and distal survivors (dis) within each city so that radiation effects were quantified relative to proximal in-city survivors with 0 dose [36]. The effects of attained age in the background were described using linear-quadratic (meningioma and schwannoma) or quadratic spline functions (glioma and all CNS tumors), while the birth cohort effect was described using linear (schwannoma) or linear-quadratic functions (glioma, meningioma, and all CNS tumors). Both attained age and birth cohort effects were allowed to vary by sex, if needed. To control for a residual period effect, we applied a method suggested by Carstensen [37]. In brief, we first fit the best-fitting tumor-specific model including attained age and year of birth. We than fixed estimated parameters and evaluated the calendar period-related “drift” by adding a linear-quadratic function in calendar year. The final model was fit by fixing the calendar-year “drift” parameters and re-estimating all other parameters freely.
Background rate, radiation and smoking effects were modeled simultaneously using an excess relative risk (ERR) model that can be represented as:
where λ0 is the background rate for unexposed (radiation dose = 0) individuals, ERR(d,s,a,e,h) describes change in rates due to radiation relative to the background rates after allowing for modification of radiation risk by sex, attained age, or age at exposure, if needed, and shielded kerma dose >4 Gy (h); and ERRsmk describes change in rates due to smoking. Smoking effect was modeled as a function of pack years allowing for modification by smoking intensity and duration (when data permitted). See details in the Supplement. Radiation effect modification was evaluated using multiplicative log-linear models and testing each factor individually, unless stated otherwise.
We evaluated dose-response shape (overall and by sex) using several dose-response models:
The primary test for departure from linearity involved testing the hypothesis that γ=0 in the linear-quadratic model. In the dose-response plot showing categorical dose-response estimates, the ERR estimates and their confidence limits were smoothed using running weighted-average smoothers [29].
To compare our results with those of a previous study [21], we repeated analyses for all CNS tumors as a group including “autopsy only” cases and applying statistical models from Preston et al. to the current data with a follow-up through 1998 and 2009.
The difference in radiation effects for CNS tumors of different histological origin was tested using a joint endpoint analysis analogous to the analysis of competing risks [38]. This approach allowed us to fit separate background parameters for each tumor type (as in tumor-specific analyses) while simultaneously testing for a difference in ERRs or effect modifiers across different tumors.
Estimated parameters, likelihood ratio tests (LRT), and likelihood-based 95% confidence intervals (CI) were computed with the AMFIT module of the Epicure software [39]. Relative quality of model fit was evaluated using Akaike information criterion (AIC). All statistical tests were two-sided and P-values less than 0.05 was considered statistically significant.
Ethical considerations
This study was approved by the RERF Human Investigation Committee via approval of Research Plans 1–75 (Study of Lifespan of A-bomb survivors, Hiroshima and Nagasaki) and 18–61 (Tumor registry study in Hiroshima and Nagasaki). The Hiroshima and Nagasaki Prefectures approved the linkages between LSS cohort and data from the Cancer Registries.
Results
Between 1958 and 2009, we identified 285 CNS tumors among 105,444 LSS subjects with 3.1 million PYs of follow-up. These included 67 gliomas (24%; 64 brain and 3 spinal), 107 meningiomas (38%; 102 cranial, 3 spinal, 2 unknown CNS location), 49 schwannomas (17%; 37 cranial nerves and 12 spinal nerves), 16 tumors of other histologies (6%; 12 cranial, 2 spinal, 2 unknown CNS location), and 46 NOS tumors (16%; 42 cranial, 4 spinal). Histologic confirmation was available for 87% of gliomas, 88% of meningiomas and tumors of other histologies, and 92% of schwannomas. More than half of NOS tumors (52%) were ascertained from death certificates and 28% from radiologic or clinical records. With the exception of two cases (1 benign choroid plexus tumor and 1 uncertain behavior neuroepithelioma), all gliomas were malignant, while all except 6 meningiomas (1 malignant and 5 uncertain behavior) and all schwannomas were benign tumors.
Distribution of CNS cases, person years at risk, and crude incidence rates are summarized in Table 1. The overall incidence rate was 0.93 per 10,000 PY for all CNS tumors combined, 0.22 per 10,000 PY for glioma, 0.35 per 10,000 PY for meningioma, and 0.16 per 10,000 PY for schwannoma. The schwannoma and other/NOS tumor rates were somewhat higher in Hiroshima than Nagasaki survivors. Males had higher rates of glioma and other/NOS tumors than females, while females had higher rates of meningioma. The crude rates tended to increase monotonically with attained age for glioma and other/NOS tumors and to decrease after 70 years for meningioma and schwannoma. The decreasing trend with increasing age at exposure or decreasing year of birth (which are perfectly correlated in the LSS) was observed for schwannoma, while the opposite trend was observed for other/NOS tumors. For each tumor type, distal survivors with zero dose had higher incidence rates than NIC residents or proximal survivors exposed to < 5 mGy. This pattern was observed in both Hiroshima and Nagasaki (data not shown). Also, for each tumor type, there was a clear increasing trend in crude rates with increasing radiation dose among survivors exposed to ≥ 5 mGy.
Table 1.
Number of person years, CNS tumor cases, and crude incidence rates according to selected characteristics in the LSS cohort, 1958–2009
| Glioma | Meningioma | Schwannoma | Other/NOS | All CNSa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PY | N | Rateb | N | Rate | N | Rate | N | Rate | N | Rate | |
| Cityc | |||||||||||
| Hiroshima | 2,193,250 | 46 | 0.21 | 78 | 0.36 | 40 | 0.18 | 50 | 0.23 | 214 | 0.98 |
| Nagasaki | 886,204 | 21 | 0.24 | 29 | 0.33 | 9 | 0.10 | 12 | 0.14 | 71 | 0.80 |
| Sex | |||||||||||
| Male | 1,142,207 | 33 | 0.29 | 21 | 0.18 | 17 | 0.15 | 28 | 0.25 | 99 | 0.87 |
| Female | 1,937,246 | 34 | 0.18 | 86 | 0.44 | 32 | 0.17 | 34 | 0.18 | 186 | 0.96 |
| Age at exposure, yr | |||||||||||
| 0 – <15 | 1,197,560 | 28 | 0.23 | 44 | 0.37 | 25 | 0.21 | 15 | 0.13 | 112 | 0.94 |
| 15 –<25 | 705,871 | 15 | 0.21 | 39 | 0.55 | 11 | 0.16 | 16 | 0.23 | 81 | 1.15 |
| 25+ | 1,176,020 | 24 | 0.20 | 24 | 0.20 | 13 | 0.11 | 31 | 0.26 | 92 | 0.78 |
| Attained age, yr | |||||||||||
| <50 | 1,132,390 | 17 | 0.15 | 9 | 0.08 | 11 | 0.10 | 13 | 0.11 | 50 | 0.44 |
| 50 – <70 | 1,265,870 | 31 | 0.24 | 72 | 0.57 | 29 | 0.23 | 22 | 0.17 | 154 | 1.22 |
| 70+ | 681,186 | 19 | 0.28 | 26 | 0.38 | 9 | 0.13 | 27 | 0.40 | 81 | 1.19 |
| DS02R1 brain dose, Gy | |||||||||||
| NIC | 761,554 | 16 | 0.21 | 12 | 0.16 | 6 | 0.08 | 14 | 0.18 | 48 | 0.63 |
| 0 (distal) | 667,734 | 17 | 0.25 | 34 | 0.51 | 12 | 0.18 | 15 | 0.22 | 78 | 1.17 |
| 0 – <0.005 | 333,480 | 5 | 0.15 | 9 | 0.27 | 3 | 0.09 | 5 | 0.15 | 22 | 0.66 |
| 0.005 – <0.100 | 800,918 | 15 | 0.19 | 24 | 0.30 | 15 | 0.19 | 16 | 0.20 | 70 | 0.87 |
| 0.100 – <0.500 | 340,902 | 7 | 0.21 | 14 | 0.41 | 6 | 0.18 | 9 | 0.26 | 36 | 1.06 |
| 0.500 – <1.000 | 100,265 | 3 | 0.30 | 5 | 0.50 | 2 | 0.20 | 0 | 10 | 1.00 | |
| 1.00+ | 74,597 | 4 | 0.54 | 9 | 1.21 | 5 | 0.67 | 3 | 0.40 | 21 | 2.82 |
| Total | 3,079,454 | 67 | 0.22 | 107 | 0.35 | 49 | 0.16 | 62 | 0.20 | 285 | 0.93 |
Note. NOS, not otherwise specified. CNS, central nervous system. PY, person-years. NIC, not in Hiroshima or Nagasaki city at the time of the bombings.
Including glioma, meningioma, schwannoma, and other/NOS tumors.
Per 10,000 PY.
City of exposure or residence at the time of the 1950 National Census (for NIC residents).
Details of the background models for each tumor type and all CNS tumors combined are shown in the Supplement (Table S3–Table S7). Smoking had no significant effect on rates of any CNS tumor and, therefore, was not retained in the final models.
Based on a simple linear model without effect modification, we found a significant dose-response for all CNS tumors combined (P < 0.001, Table 2) and, by histological type, for glioma (P = 0.029) and meningioma (P < 0.001). The estimated ERRs per Gy were 1.40 (95% CI: 0.61 to 2.57), 1.67 (95% CI: 0.12 to 5.26) and 1.82 (95% CI: 0.51 to 4.30), respectively. The ERR per Gy for schwannoma was 1.45 (95% CI: −0.01 to 4.97) but not statistically significant at the nominal 0.05 level (P = 0.053). There was little evidence for a radiation effect on risk of other/NOS CNS tumors (P = 0.493) with estimated ERR per Gy of 0.40 (95% CI: <−0.58 to 2.59). Both tests of heterogeneity of tumor-specific ERRs, a 3 degree-of-freedom test (glioma, meningioma, schwannoma, other/NOS tumors) and a 2 degree-of-freedom, test (glioma, meningioma, and schwannoma) were not statistically significant (P = 0.672 and P = 0.967, respectively).
Table 2.
Excess relative risks per 1 Gy and 95% confidence intervals for CNS tumors of different histologic origin in the LSS, 1958–2009
| ERR per 1 Gya | 95% CI b | Pc | P d | |
|---|---|---|---|---|
| Glioma | 1.67 | 0.12 to 5.26 | 0.029 | 0.409 |
| Meningioma | 1.82 | 0.51 to 4.30 | <0.001 | 0.424 |
| Schwannoma | 1.45 | −0.01 to 4.97 | 0.053 | 0.682 |
| Other/NOS | 0.40 | <−0.58 to 2.59 | 0.493 | 0.572 |
| All CNS e | 1.40 | 0.61 to 2.57 | <0.001 | 0.980 |
Note. ERR, excess relative risk. NOS, not otherwise specified. CNS, central nervous system.
ERR per 1 Gy derived from simple linear model without effect modification.
95% confidence interval.
P value for linear dose trend.
P value for quadratic departure from linearity.
Including glioma, meningioma, schwannoma, and other/NOS tumors.
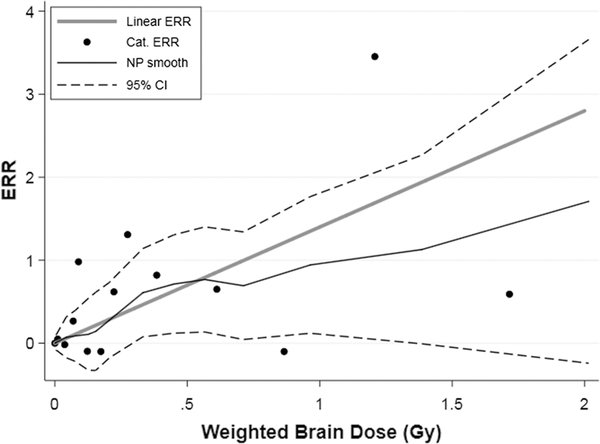
We found little evidence for lack of fit for the linear dose-response for any tumor type (all P values > 0.4, Table 2). Evaluation of the dose-response shape by sex for individual histologies was not possible but, for all CNS tumors as a group, a test of quadratic departure from linearity was not significant either among males (P = 0.562) or females (P = 0.466). Figure 1 presents categorical dose-response estimates, fitted linear model, and smoothed non-parametric estimates with uncertainty bounds (+/− one standard error) for all CNS tumors as a group. This figure illustrates that while the linear model provides an adequate fit to the data, there is substantial uncertainty in dose response.
Figure 1.
CNS tumor excess relative risk (ERR) in relation to weighted absorbed DS02R1 brain dose: fitted linear ERR dose response function (thick gray line), the ERR estimates for 14 dose categories (Cat., black points), and a non-parametric smoothed estimate (NP, thin black line) with pointwise 95% confidence intervals (95% CI, dashed black curves)
The effect modification results are summarized in Table 3 for glioma, meningioma, schwannoma, and all CNS tumors combined. For each tumor type, males had higher ERRs than females, with a statistically significant sex difference for meningioma (P = 0.045). While schwannoma exhibited a significant decreasing trend in ERR with increasing age at exposure (P = 0.015), there was no clear pattern by age at exposure for other tumor types. Also for schwannoma, we found a significant decreasing trend in the ERR with attained age (P = 0.002). When both attained age and age at exposure effect modifiers were included in the risk model for schwannoma, the effect of attained age on ERR persisted (P = 0.015), while the effect of age at exposure weakened (P = 0.148). There was little evidence for attained age effect modification for either glioma (P = 0.348) or meningioma (P = 0.389). When all CNS tumors were considered together, we found a suggestion of ERR effect modification by sex (P = 0.053) but not by age at exposure (P = 0.299) or attained age (P = 0.192).
Table 3.
Excess relative risks and 95% confidence intervals for major types of CNS tumors according to sex, age at exposure, and attained age
| Glioma (N=67) | Meningioma (N=107) | Schwannoma (N=49) | All CNSa (N=285) | |||||
|---|---|---|---|---|---|---|---|---|
| ERRb | 95% CIc | ERR | 95% CI | ERR | 95% CI | ERR | 95% CI | |
| Sex d | ||||||||
| Male | 3.12 | 0.48 to 10.08 | 5.54 | 1.32 to 17.09 | 2.99 | <−0.62 to 12.20 | 2.46 | 1.00 to 4.89 |
| Female | −0.04 | <−0.85 to 3.42 | 0.99 | <−0.15 to 3.13 | 0.17 | <−0.09 to 3.31 | 0.77 | 0.05 to 1.95 |
| P heterogeneity | 0.105 | 0.045 | 0.116 | 0.053 | ||||
| Age at exposure,yr d | ||||||||
| 0 – <10 e | 1.90 | <−0.73 to 8.11 | 2.05 | 0.14 to 6.60 | 3.26 | <2.09 to 11.91 | 2.20 | 0.73 to 4.74 |
| 10 – <20 | 1.45 | <−0.69 to 6.67 | 1.41 | 0.05 to 4.53 | 0.28 | <−0.86 to3.80 | 1.02 | 0.15 to 2.47 |
| 20+ | 1.70 | <−0.73 to 7.83 | 2.24 | <−0.17 to 7.11 | −0.06 | <−1.70 to 3.55 | 1.10 | −0.02 to 2.97 |
| P heterogeneity | 0.979 | 0.851 | 0.122 | 0.475 | ||||
| % change per 10 yr | 45.2 0 | −54.01 to 277.1 | 0.90 | −99.38 to 105.1 | −90.84 | −100.0 to −29.0 | −32.71 | −94.83 to 29.4 |
| P trend | 0.395 | 0.979 | 0.015 | 0.299 | ||||
| Attained age, yrd | ||||||||
| <50 | 0.70 | 0.44 to 5.92 | 4.48 | <−0.80 to23.42 | 4.96 | <−0.52 to 18.01 | 1.97 | 0.44 to 4.76 |
| 50 – < 70 | 1.33 | <−0.64 to 5.72 | 1.40 | 0.08 to 4.06 | 0.40f | −0.82 to 3.08 | 1.25 | 0.31 to 2.71 |
| 70+ | 4.26 | 0.30 to 15.98 | 2.20 | 0.22 to 6.87 | 1.24 | 0.17 to 3.13 | ||
| P heterogeneity | 0.446 | 0.621 | 0.044 | 0.773 | ||||
| Age power | 1.94 | −1.73 to 25.53 | −2.32 | −7.40 to 5.01 | −6.88 | −14.67 to − 2.18 | −1.31 | −3.23 to 0.82 |
| P trend | 0.348 | 0.389 | 0.002 | 0.192 | ||||
Note. CNS, central nervous system. ERR, excess relative risk.
Including glioma, meningioma, schwannoma, and other/NOS tumors.
Excess relative risk at 1 Gy.
95% confidence interval.
Effect of each modifier was tested individually.
Age at exposure categories for glioma 0–<15, 15–<20, 20+ yr.
Two attained age categories were combined as the ERR at 1 Gy could not be estimated for subjects ≥70 yr.
To compare our findings with the results of previous LSS reports, we repeated analyses for all CNS tumors as a group including 56 “autopsy only” cases and evaluated effects of the extended follow-up (1958–2009 vs 1958–1998) and alternative models (current vs Preston et al. [21]) (Table S8). We found that with longer follow-up, the estimate of ERR per Gy increased by 20% under the Preston et al. model and evidence for effect modification, particularly by attained age, became weaker (column 2 vs column 1). The ERR per Gy estimated under the current model was, in turn, 45% higher than that under the model of Preston et al. for the same follow-up period, while the magnitude of the effect modification, particularly by age at exposure, was attenuated (column 4 vs column 2). Based on additional analyses, we found that the single most influential factor contributing to differences in radiation effect-estimates from the two models was adjustment for location at the time of the bombings (i.e., city-specific distal vs proximal, column 4 vs column 3). The primary effect of excluding “autopsy only” cases was a 15% increase in the ERR per Gy with little effect on effect modification parameters (column 5 vs column 4).
To facilitate comparison of LSS derived radiation risk estimates with those from studies of CT exposed populations, we estimated linear ERR per Gy for individuals exposed under 20 years of age over entire follow-up period and within 20 years after the bombings allowing for effect modification by age at exposure and time since exposure (Supplemental Table S9). The linear ERR per Gy estimates obtained over the entire range of doses for both scenarios were only slightly higher (1.46, 95% CI: 0.60 to 2.79 and 1.51, 95% CI: 0.63 to 2.85, respectively) than the ERR per Gy from simple linear model without effect modification (1.40, 95% CI: 0.61 to 2.57). In the restricted dose range (0–150 mGy), the respective linear ERR per Gy estimates were larger, but more uncertain than those obtained over entire range of doses (2.67, 95% CI: <−7.22 to 11.57 and 2.14, 95% CI:<−4.72 to 11.51, respectively).
Discussion
In this updated study of CNS tumor incidence among atomic bomb survivors, we found a statistically significant linear dose response for glioma and meningioma and a nearly significant linear dose-response for schwannoma. For each tumor type, males had a numerically higher ERR per Gy estimate than females; the sex difference was statistically significant for meningioma. While schwannoma exhibited a significant decreasing trend in ERR with both increasing age at exposure and attained age, the modifying age at exposure effect was accounted for by the effect of attained age. For other CNS tumors there was no clear pattern of variation in the ERR with either attained age or age at exposure.
The objective of the current study was to clarify CNS tumor-specific radiation risks and modification patterns extending previous LSS follow-up [21] by 11 years. We focused on analyses of glioma, meningioma, and schwannoma, three common types of CNS tumors because these have different histological origin and sex-age-specific patterns of baseline risk potentially leading to heterogeneity in radiation risk estimates and effect modification patterns. As the total number of CNS tumors increased by 21%, elevated radiation risks for glioma and meningioma became statistically significant and there was evidence for a radiation effect on schwannoma in which the ERR decreased with increasing attained age. Unlike before [20, 21], we modeled the background rates for each CNS tumor type separately, but the ERR per Gy estimates from simple linear models without effect modification were remarkably similar and there was no evidence of non-linearity for any tumor type. Our findings are generally consistent with previous LSS reports with each tumor-specific 95% CI including the previously reported ERR per Gy estimate [20, 21]. The suggestion of a downturn in the schwannoma dose-response above 1 Gy noted in earlier analyses [20] was not supported by the current data.
Compared to other studies of radiation-exposed populations (Table 4), the ERR per Gy for glioma in the LSS appears to be larger than the ERR per Gy estimated in studies of childhood cancer survivors [12, 14] and substantially lower than that seen in studies of diagnostic CT imaging [16, 17, 19]. However, given the uncertainties as indicated by the wide and overlapping 95% confidence intervals, these studies all provide compatible risk estimates. The ERR per Gy for meningioma in the LSS similarly falls in the middle of the ERR spectrum reported in studies of medically exposed populations [16]. Beyond the LSS, only one study of patients treated with radiation for benign head and neck conditions estimated a radiation risk for schwannoma [10]. The reported OR per Gy in that study seems lower than the simple RR per Gy estimate (i.e., ERR+1) in the current study. Given that there is a wide variation in mean doses, degree of fractionation, age at exposure and length of follow-up across radiation studies, comparison of simple risk estimates should be treated cautiously and could be misleading if the true CNS tumor-specific radiation risks vary by sex, age at exposure, or attained age.
Table 4.
CNS tumor-type specific excess relative risks per 1 Gy and 95% confidence intervals reported by radioepidemiological studies
| Glioma | Meningioma | Schwannoma | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | Mean dose, Gy (range) | Mean age at exposure, yr (range) | ERR per 1 Gy | 95% CIa | ERR per 1 Gy | 95% CI | ERR per 1 Gy | 95% CI |
| Present | 0.13b (0 to 3.8) | 22b (0 to 81) | 1.67 | 0.12 to 5.26 | 1.82 | 0.51 to 4.30 | 1.45 | −0.01 to 4.97 |
| Neglia et al. [12] | 9c (<1 to >45) | 6c (0 to 20) | 0.33 | 0.07 to 1.71 | 1.06 | 0.21 to 8.15 | ||
| Taylor et al. [14] | 9d (0 to >40) | 6e (0 to 14) | 0.079 | 0.021 to 0.229 | 5.1 | 0.7 to 107.7 | ||
| Pearce et al. [16] | 0.045f (<0.005 to >0.200) | 11f (0 to 21) | 19g | 3 to 70 | ||||
| Meulepas et al. [19] | 0.039 (<0.005 to >0.120) | (0 to < 18) | 11.7h | 0.4 to 51.3 | 0.6c | −0.35 to 64.4 | ||
| Sadetzki et al. [9] | 1.5 (0.98 to 6.0) | 7 (<1 to 15) | 5.01 | 2.66 to 9.80 | ||||
| Schneider et al. [10] | 4.6i (0.01 to 18.2) | 3.6i (0 to <16)) | RR/Gy=1.14 | 1.0 to 1.3 | ||||
Note. ERR, excess relative risk. RR, relative risk.
95% confidence interval.
Among proximal and distal survivors.
Estimated weighted mean among all controls.
Estimated weighted mean in glioma and meningioma controls combined.
Estimated weighted mean in the entire cohort.
Estimated person-years weighted mean.
For comparison with LSS and other studies, ERR is expressed per 1 Gy based on the original ERR estimate expressed per 1 mGy.
For comparison with LSS and other studies, ERR is expressed per 1 Gy based on the original ERR estimate expressed per 100 mGy.
Median.
Due to the small number of radiation-associated excess cases in our study, evaluation of effect modification is underpowered. However, we noted several interesting patterns in radiation risk by sex and age. For each tumor type, the ERR per Gy estimates among males were higher than among females, a pattern suggested in previous LSS reports [20, 21]. Because the background rates of meningioma in the LSS were substantially lower among males than females, the ERR difference by sex for meningioma could partially reflect the difference in the background rates. In contrast, suggestively higher ERRs for glioma and scwhannoma among males compared to females coupled with higher background rates of these tumors among males may reflect differences in radiosensitivity. Non-significantly higher radiation risks for glioma and meningioma among males were also reported in a case-control study of CNS neoplasms in childhood cancer survivors [12]. However, radiation risks for benign meningioma among males and females were similar in a study of brain tumors following radiation treatment for tinea capitis [9]; and radiation risks for all CNS tumors combined were non-significantly higher among females than among males in two studies of diagnostic CT imaging [16, 19].
Differences in radiation risks according to age at exposure are thought to be related to age-dependent tissue-specific growth patterns and stem cell dynamics [40, 41]. During early childhood, brain and nervous system undergo profound changes reaching 90% of adult size by age 5 with CNS maturation continuing until adulthood [23]. More precise understanding of the dynamic of cells that give rise to specific types of CNS tumors is currently lacking. Studies of CNS tumors after therapeutic radiation during childhood and adolescence generally suggest that radiation exposure appears to be more effective in tumor induction than adult exposure, particularly for malignant tumors [12, 9, 15]. However, the data on adult exposures are limited and not all studies of childhood exposure observed significant decrease in radiation risks with increasing age at exposure [42, 11]. An opposite trend, significant increase in ERR per Gy with increasing age at exposure, found in one study of brain tumors after diagnostic CT radiation [16], remains currently unexplained. Previous studies of atomic bomb survivors noted suggestive evidence for age at exposure effect on radiation risk of CNS tumors other than schwannoma [20]. In the present study, this evidence weakened, partly due to extended follow-up and adjustment for location at the time of the bombings (see below). The ERRs for glioma and meningioma did not vary by age at exposure and there was no evidence of a decreasing trend for schwannoma once both attained age and age at exposure modifiers were included in the risk model.
Statistically significant modification of radiation risk by attained-age or time-since exposure has not been reported before for any type of CNS tumors. A strong, decreasing trend with increasing attained age for schwannoma ERR in the LSS is a new finding. In one other study of radiation-related schwannoma [10], investigators found no correlation between radiation exposure and tumor latency, but association with attained age was not evaluated. An inverse attained-age effect for schwannoma and meningioma was suggested in the LSS previously [20] with schwannoma trend strengthening and meningioma trend weakening in the current study. As the LSS cohort ages, and more individuals exposed in early childhood enter cancer-prone years, age-at-exposure and attained age patterns need to be reevaluated.
Direct comparison of current and previously reported radiation risk estimates for CNS tumors in the LSS is complicated due to differences in study methods [20, 21, 29]. Therefore, we emphasize consistency of radiation risk patterns rather than radiation risk estimates. The extended follow-up and exclusion of “autopsy only” cases each somewhat increased the ERR per Gy for all CNS tumors combined, however, the largest increase in ERR per Gy and improvement in fit (as evidenced by AIC change) was seen after adjustment for city-specific location at the time of the bombings and estimating radiation risks relative to proximal survivors with zero doses. Difference in rates of all solid cancer incidence and mortality between distal and proximal survivors, and its potential impact on radiation risk estimates, was noted before and thought to be due to city-dependent urban/rural differences, i.e., distal survivors from Hiroshima being more likely rural residents than proximal survivors and distal survivors from Nagasaki being more likely urban residents than proximal survivors [36, 43]. The reasons for higher rates of CNS tumors among distal compared with proximal survivors in both cities are not entirely clear, but we believe that including distal survivors with zero doses in the reference group should be avoided as it would bias the CNS tumor ERR per Gy downward.
The observations in the LSS offer useful insights for other studies of radiation-related CNS tumors. It suggests that, for rare outcomes, a long follow-up might be needed to detect the radiation-related increase and characterize the effect-modification patterns. The updated LSS risks estimated for individuals comparable in characteristics to the CT-exposed cohorts remain substantially lower, although statistically compatible with the risks reported for CNS tumors by the CT studies [16, 17]. Whenever numbers permit, studies should emphasize histology-specific radiation risk estimates allowing for effect modification. Combining histologically and etiologically distinct tumors, often performed to increase statistical power, may obscure the true patterns of risks (e.g., attained age effect for schwannoma). To overcome insufficient data in individual studies, pooling of data from several cohorts of radiation-exposed populations using the same case definitions and modeling methods might allow us to draw more precise inferences about excess CNS tumor-specific risks by sex, age, and time, an approach proven successful for radiation-related thyroid and breast cancers, and leukemia [44, 20, 45].
The strengths of our study include a well-defined cohort with long follow-up, availability of individual radiation doses for nearly all cohort members, and ascertainment of both malignant and benign CNS tumors from population-based cancer registries. In the analysis, we reconsidered the appropriateness of including “autopsy only” cases to avoid potential dose-age-period related bias [46, 29]. Nearly 90% of gliomas, meningiomas, schwannomas, and tumors of other histologies were confirmed microscopically, while most of NOS tumors were ascertained from death certificates, radiological or clinical records. For 153 cases ascertained between 1958 and 1995 and included in both the current and previous LSS study [20], that relied on expert review of available slides, pathology reports, and medical records, diagnostic agreement was 92% at the level of major CNS tumor types (data not shown). Thus, reliability of cancer registry-based diagnoses appears high and appreciable misclassification of CNS tumors is unlikely. Atomic bomb survivors were exposed to a wide range of doses at all ages and did not have an underlying disease that may have affected development and/or ascertainment of CNS tumors. While being one of the largest studies in the field, the LSS lacks power for precise characterization of the radiation effects for CNS tumor types accurately due to the small number of radiation excess cases. Consequently, potential heterogeneity in ERR estimates for neuroepithelial tumors (astrocytoma, glioblastoma, oligodendroglioma, etc.) was not evaluated. Radiation risk estimates were adjusted for city-specific location at the time of the bombings as a surrogate for urban/rural lifestyle. However, additional information on potential confounders preceding the bombings is unavailable and could not be controlled.
In conclusion, our findings demonstrate that radiation exposure from the atomic bombs is associated with elevated risks of major types of CNS tumors that appear to persist throughout life, except possibly for schwannoma. The radiation ERRs appear to be higher among males than females. The data also suggest that radiation exposure at any age can increase the CNS tumor risks. Further follow-up is necessary to characterize the lifetime risks for specific CNS tumor types among the atomic bomb survivors.
Supplementary Material
Acknowledgments
Financial support: The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the U.S. Department of Energy (DOE). The research was also funded in part through DOE award DE-HS0000031 to the National Academy of Sciences and contract HHSN261201400009C through the U.S. National Cancer Institute (NCI), with additional support from the Division of Cancer Epidemiology and Genetics in the NCI Intramural Research Program. This publication was supported by RERF Research Protocol 1–75 and 18–61.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report:Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-oncology. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villa C, Miquel C, Mosses D, Bernier M, Di Stefano AL. The 2016 World Health Organization classification of tumours of the central nervous system. Presse Med. 2018;47(11–12 Pt 2):e187–e200. [DOI] [PubMed] [Google Scholar]
- 3.Alcantara Llaguno SR, Parada LF. Cell of origin of glioma: biological and clinical implications. British journal of cancer. 2016;115(12):1445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalamarides M, Stemmer-Rachamimov AO, Niwa-Kawakita M, Chareyre F, Taranchon E, Han ZY et al. Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes. Oncogene. 2011;30:2333. [DOI] [PubMed] [Google Scholar]
- 5.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. Journal of neuro-oncology. 2010;99(3):307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonig A, Gandhi V, Nanda A. From the cell of Schwann to schwannoma--a century’s fruition. World neurosurgery. 2014;82(5):906–11. [DOI] [PubMed] [Google Scholar]
- 7.Amirian ES, Ostrom QT, Liu Y, Barnholtz-Sloan J, Bondy ML. Nervous System In: Thun MJ, Linet MS, Cerhan JR, Haiman C, Schottenfeld D, editors. Cancer epidemiology and prevention. Fourth edition ed. New York, NY: Oxford University Press; 2018. p. 1039–60. [Google Scholar]
- 8.Kaneko S, Nomura K, Yoshimura T, Yamaguchi N. Trend of brain tumor incidence by histological subtypes in Japan: estimation from the Brain Tumor Registry of Japan, 1973–1993. Journal of neuro-oncology. 2002;60(1):61–9. [DOI] [PubMed] [Google Scholar]
- 9.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiation research. 2005;163(4):424–32. [DOI] [PubMed] [Google Scholar]
- 10.Schneider AB, Ron E, Lubin J, Stovall M, Shore-Freedman E, Tolentino J et al. Acoustic neuromas following childhood radiation treatment for benign conditions of the head and neck. Neuro-oncology. 2008;10(1):73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little MP, de Vathaire F, Shamsaldin A, Oberlin O, Campbell S, Grimaud E et al. Risks of brain tumour following treatment for cancer in childhood: modification by genetic factors, radiotherapy and chemotherapy. Int J Cancer. 1998;78(3):269–75. [DOI] [PubMed] [Google Scholar]
- 12.Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528–37. [DOI] [PubMed] [Google Scholar]
- 13.Svahn-Tapper G, Garwicz S, Anderson H, Shamsaldin A, De Vathaire F, Olsen JH et al. Radiation dose and relapse are predictors for development of second malignant solid tumors after cancer in childhood and adolescence: a population-based case-control study in the five Nordic countries. Acta Oncol. 2006;45(4):438–48. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AJ, Little MP, Winter DL, Sugden E, Ellison DW, Stiller CA et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(36):5287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson P, Holmberg E, Lundell M, Mattsson A, Holm LE, Wallgren A. Intracranial tumors after exposure to ionizing radiation during infancy: a pooled analysis of two Swedish cohorts of 28,008 infants with skin hemangioma. Radiation research. 1998;150(3):357–64. [PubMed] [Google Scholar]
- 16.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrington de Gonzalez A, Salotti JA, McHugh K, Little MP, Harbron RW, Lee C et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. British journal of cancer. 2016;114(4):388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. Bmj. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meulepas JM, Ronckers CM, Smets A, Nievelstein RAJ, Gradowska P, Lee C et al. Radiation Exposure From Pediatric CT Scans and Subsequent Cancer Risk in the Netherlands. J Natl Cancer Inst. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, Kishikawa M et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94(20):1555–63. [DOI] [PubMed] [Google Scholar]
- 21.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiation research. 2007;168(1):1–64. [DOI] [PubMed] [Google Scholar]
- 22.Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro-oncology. 2012;14(11):1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boice JD Jr. Radiation epidemiology and recent paediatric computed tomography studies. Ann ICRP.2015;44(1 Suppl):236–48. [DOI] [PubMed] [Google Scholar]
- 24.Hauptmann M, Meulepas JM. CT scans in childhood and risk of leukaemia and brain tumours. Lancet.2012;380(9855):1736; author reply −7. [DOI] [PubMed] [Google Scholar]
- 25.Gardavaud F, Luciani A, Rahmouni A. CT scans in childhood and risk of leukaemia and brain tumours. Lancet. 2012;380(9855):1735; author reply 6–7. [DOI] [PubMed] [Google Scholar]
- 26.Zopf DA, Green GE. CT scans in childhood and risk of leukaemia and brain tumours. Lancet.2012;380(9855):1735–6; author reply 6–7. [DOI] [PubMed] [Google Scholar]
- 27.Meulepas JM, Ronckers CM, Merks J, Weijerman ME, Lubin JH, Hauptmann M. Confounding of the association between radiation exposure from CT scans and risk of leukemia and brain tumors by cancer susceptibility syndromes. J Radiol Prot. 2016;36(4):953–74. [DOI] [PubMed] [Google Scholar]
- 28.Meulepas JM, Hauptmann M, Lubin JH, Shuryak I, Brenner DJ. Is there Unmeasured Indication Bias in Radiation-Related Cancer Risk Estimates from Studies of Computed Tomography? Radiation research. 2018;189(2):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M et al. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiation research. 2017;187(5):513–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullings HM, Grant EJ, Egbert SD, Watanabe T, Oda T, Nakamura F et al. DS02R1: Improvements to Atomic Bomb Survivors’ Input Data and Implementation of Dosimetry System 2002 (DS02) and Resulting Changes in Estimated Doses. Health physics. 2017;112(1):56–97. [DOI] [PubMed] [Google Scholar]
- 31.Ozasa K, Cullings HM, Ohishi W, Hida A, Grant EJ. Epidemiological studies of atomic bomb radiation at the Radiation Effects Research Foundation. Int J Radiat Biol. 2019:1–13. [DOI] [PubMed] [Google Scholar]
- 32.Cullings HM, Fujita S, Funamoto S, Grant EJ, Kerr GD, Preston DL. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiation research. 2006;166(1 Pt 2):219–54. [DOI] [PubMed] [Google Scholar]
- 33.Pierce DA, Stram DO, Vaeth M. Allowing for random errors in radiation dose estimates for the atomic bomb survivor data. Radiation research. 1990;123(3):275–84. [PubMed] [Google Scholar]
- 34.Sposto R, Preston DL. Correcting for catchment area non-residency in studies based on tumor registry data Hiroshima, Japan: Radiation Effects Research Foundation1992; Report No.: CR 1–92. [Google Scholar]
- 35.Furukawa K, Preston DL, Lonn S, Funamoto S, Yonehara S, Matsuo T et al. Radiation and smoking effects on lung cancer incidence among atomic bomb survivors. Radiation research. 2010;174(1):72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.French B, Cologne J, Sakata R, Utada M, Preston DL. Selection of reference groups in the Life Span Study of atomic bomb survivors. Eur J Epidemiol. 2017;32(12):1055–63. [DOI] [PubMed] [Google Scholar]
- 37.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med. 2007;26(15):3018–45. [DOI] [PubMed] [Google Scholar]
- 38.Pierce DA, Preston DL. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiation research. 1993;134(2):134–42. [PubMed] [Google Scholar]
- 39.Preston DL, Lubin JH, Pierce DA, McConney ME, Shilnikova NS. Epicure Risk Regression and Person-Year Computation Software: Command Summary and User Guide. Ottawa, ON, Canada: Risk Sciences International; 2015. [Google Scholar]
- 40.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation. Effects of radiation exposure of children New York: United Nations; 2013. [Google Scholar]
- 41.ICRP Publication 131: Stem Cell Biology with Respect to Carcinogenesis Aspects of Radiological Protection. SAGE Publications; 2016. [DOI] [PubMed] [Google Scholar]
- 42.Shore RE, Moseson M, Harley N, Pasternack BS. Tumors and other diseases following childhood x-ray treatment for ringworm of the scalp (Tinea capitis). Health physics. 2003;85(4):404–8. [DOI] [PubMed] [Google Scholar]
- 43.Cologne JB, Preston DL. Impact of comparison group on cohort dose response regression: an example using risk estimation in atomic-bomb survivors. Health physics. 2001;80(5):491–6. [DOI] [PubMed] [Google Scholar]
- 44.Veiga LH, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ et al. Thyroid Cancer after Childhood Exposure to External Radiation: An Updated Pooled Analysis of 12 Studies. Radiation research. 2016;185(5):473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little MP, Wakeford R, Borrego D, French B, Zablotska LB, Adams MJ et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: a pooled analysis of nine historical cohort studies. The Lancet Haematology. 2018;5(8):e346–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yonehara S, Brenner AV, Kishikawa M, Inskip PD, Preston DL, Ron E et al. Clinical and epidemiologic characteristics of first primary tumors of the central nervous system and related organs among atomic bomb survivors in Hiroshima and Nagasaki, 1958–1995. Cancer. 2004;101(7):1644–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.