INTRODUCTION
People with urothelial carcinoma of the bladder are at risk for recurrence and progression following transurethral resection of a bladder tumour. Mitomycin C (MMC) and Bacillus Calmette-Guérin (BCG) are commonly used, competing forms of intravesical therapy for intermediate- or high-risk non-muscle invasive (Ta and T1) urothelial bladder cancer but their relative merits are somewhat uncertain. Although several systematic reviews and meta-analyses have been conducted on this topic, it still remains unclear what the optimal treatment dose and schedule might be, as well as the question of which people benefit most from one or the other agent [1,2].
Objectives
We assessed the effects of MMC compared to BCG for treating intermediate- or high-risk non-muscle invasive urothelial bladder cancer.
MATERIALS AND METHODS
We updated a previously published Cochrane Review to assess the effects of MMC compared to BCG by searching systematically and comprehensively the biomedical literature in multiple databases (CENTRAL, MEDLINE, EMBASE, Web of Science, Scopus, LILACS, ClinicalTrials. gov, World Health Organization International Clinical Trials Registry Platform) up to 23th September 2019 [3]. Supplementary material 1 shows the search strategies. We also hand searched the reference lists of included articles as well as conference proceedings. We did not restrict by publication language or publication status.
We included randomised or quasi-randomised controlled trials comparing MMC to BCG for the treatment of non-muscle invasive (Ta and T1) urothelial bladder cancer in adults. Neither sequential administration of BCG and MMC nor electromotive or hyperthermic drug stimulation were the focus of this review. Two independent reviewers screened identified references, extracted data, and assessed the risk of bias according to Cochrane's methodological recommendations [4].
We performed meta-analyses using the random effects model and assessed the heterogeneity between studies with the I2 statistic. All analyses were conducted with Review Manager 5 software [5]. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to rate the certainty of the evidence for each predefined outcome [4].
RESULTS
The literature search identified 1,125 records, of which 12 studies fulfilled our inclusion criteria (based on 29 publications, including 2,932 patients, published between 1995 and 2013). Eleven were included in the meta-analyses [6,7,8,9,10,11,12,13,14,15,16]. The one study that was not included in the meta-analysis was only available as a conference proceeding, which did not provide sufficient data for inclusion in the analysis [17].
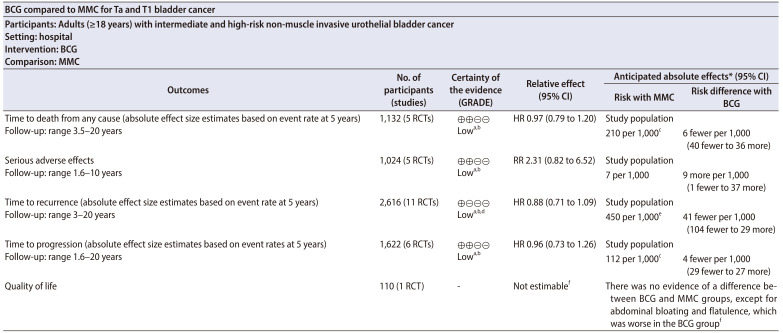
Table 1 presents the summary of findings of the main outcomes [8,13]. Supplementary Table 1 and Supplementary Fig. 1 summarises the characteristics and the risk of bias of the included studies. Supplementary material 2 lists the excluded studies and the rationale for their exclusion.
Table 1. Summary of findings table.
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
BCG, Bacillus Calmette-Guérin; MMC, mitomycin C; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; CI, confidence interval; RCT, randomised controlled trial; HR, hazard ratio; RR, risk ratio.
a:Downgraded one level for study limitations: concerns with performance or detection bias (or both), as well as with regard to allocation concealment and selective outcome reporting.
b:Downgraded one level for imprecision: 95% CI was consistent with the possibility for important benefit and large harm.
c:The assumed risk was based on five-year mortality rate from Gårdmark et al. [8]
d:Downgraded one level for inconsistency: variation in point estimates or substantial heterogeneity among studies (or both).
e:The assumed risk is based on five-year mortality rate based on Ojea et al. [13].
f:More detailed results on quality of life were not available (conference abstract only).
1. Primary outcomes
We found low-certainty evidence that BCG may make little or no difference on time to death from any cause compared to MMC (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.79 to 1.20; participants=1,132; studies=5; 567 participants in the BCG arm and 565 in the MMC arm; I2=0%). We also found low-certainty evidence that BCG may increase the risk for serious adverse effects compared to MMC (risk ratio, 2.31; 95% CI, 0.82 to 6.52; participants=1,024; studies=5; 577 participants in the BCG arm and 447 in the MMC arm; I2=0%).
2. Secondary outcomes
We found low-certainty evidence that BCG may reduce the time to recurrence compared to MMC (HR, 0.88; 95% CI, 0.71 to 1.09; participants=2,616; studies=11; 1,273 participants in the BCG arm and 1,343 in the MMC arm; I2=61%). Certainty of the evidence was also rated as low for time to progression, where BCG may make little or no difference compared to MMC (HR, 0.96; 95% CI, 0.73 to 1.26; participants=1,622; studies=6; 804 participants in the BCG arm and 818 in the MMC arm; I2=0%). There were no data on quality of life.
The visual test for publication bias did not indicate any important asymmetry. The subgroup analysis showed that higher versus lower doses of BCG resulted in higher rates of serious adverse effects when compared to MMC. We were unable to assess treatment effects between intermediate and high-risk groups due to lack of data. A sensitivity analysis based on studies with low risk of bias could not be performed due to lack of low risk studies.
DISCUSSION
The first Cochrane Review on this topic was published in 2003, and included seven trials based on 1,901 participants [3]. This review update includes further five trials, which were published meanwhile. It now reflects also the current Cochrane methodology, which includes the certainty of the evidence assessment according to the GRADE approach.
BCG may reduce the risk of recurrence over time, while it may have no effect on either the risk of progression or risk of death from any cause over time. Instead, BCG may increase the risk of serious adverse effects. All findings are based on low certainty of the evidence.
The judgement of low certainty of the evidence for all outcomes in this review means that further research is very likely to have an important impact on the confidence in the estimates of effects and is likely to change the estimates.
Of the 12 identified studies, six were planned and conducted in the 1990s and do not meet 2019s methodological quality standards. Only one trial was conducted after 2010 but results of this trial have not been published yet. One trial (recruitment 2009 to 2012) was closed prior to finalisation due to a lack of accrual. Blinding of participants did not take place in any of the 12 trials. General concerns, which led to downgrading, were study limitations (performance bias and allocation concealment), wide CIs resulting in imprecision (possibility for either important benefit or large harm) and study heterogeneity.
BCG usage must be further studied to predict patients who respond most to BCG therapy, and to determine the optimal schedule and amount of BCG delivery per patient. High-quality randomised controlled trials in people with intermediate- and high-risk bladder cancer with adequate randomisation and blinding are warranted. They should address quality of life, adverse effects and time to progression to provide more reliable results for this patient population.
CONCLUSIONS
BCG may reduce the risk of recurrence over time, while it may have little or no effect on either the risk of progression or risk of death from any cause over time. However, BCG may increase the risk of serious adverse effects. All findings are based on low certainty of the evidence.
ACKNOWLEDGMENTS
We wish to thank the Cochrane Urology Group for their support and patience during this review process.
Footnotes
This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews (CDSR) 2020, Issue 1, DOI: 10.1002/14651858.CD011935.pub2. Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the CDSR should be consulted for the most recent version of the review.
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Stefanie Schmidt and Philipp Dahm.
- Data acquisition: Stefanie Schmidt, Rick Dersch, Desiree Louise Draeger, Laura-Maria Krabbe, and Bernadette Coles.
- Statistical analysis: Samuel Kilian and Katrin Jensen.
- Data analysis and interpretation: Stefanie Schmidt, Frank Kunath, and Joerg J Meerpohl.
- Drafting of the manuscript: Stefanie Schmidt and Joerg J Meerpohl.
- Critical revision of the manuscript: Frank Kunath and Philipp Dahm.
- Administrative, technical, or material support: Stefanie Schmidt and Bernadette Coles.
- Supervision: Philipp Dahm and Joerg J Meerpohl.
- Approval of the final manuscript: Stefanie Schmidt, Frank Kunath, Bernadette Coles, Desiree Louise Draeger, Laura-Maria Krabbe, Rick Dersch, Samuel Kilian, Katrin Jensen, Philipp Dahm, and Joerg J Meerpohl.
SUPPLEMENTARY MATERIALS
Scan this QR code to see the supplementary materials, or visit https://www.icurology.org/src/sm/icurology-61-349-s001.pdf.

Search strategies
Characteristics of the included studies
Risk of bias of the included studies.
Summary of the excluded studies
References
- 1.Shelley MD, Mason MD, Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treat Rev. 2010;36:195–205. doi: 10.1016/j.ctrv.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 3.Shelley MD, Court JB, Kynaston H, Wilt TJ, Coles B, Mason M. Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2003;(3):CD003231. doi: 10.1002/14651858.CD003231. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Internet] The Cochrane Collaboration; 2011. [updated 2011 Mar]. [cited 2019 Jul 19]. Available from: http://handbook.cochrane.org. [Google Scholar]
- 5.The Nordic Cochrane Centre. Review manager (RevMan). version 5.3 [Internet] Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [cited 2019 Jul 19]. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download. [Google Scholar]
- 6.Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Navarra P, Massoud R, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol. 2003;170:777–782. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich MG, Pichlmeier U, Schwaibold H, Conrad S, Huland H. Long-term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short-term intravesical chemotherapy and short-term therapy with Bacillus Calmette-Guérin (BCG) in patients with non-muscle-invasive bladder carcinoma. Eur Urol. 2007;52:1123–1129. doi: 10.1016/j.eururo.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Gårdmark T, Jahnson S, Wahlquist R, Wijkström H, Malmström PU. Analysis of progression and survival after 10 years of a randomized prospective study comparing mitomycin-C and bacillus Calmette-Guérin in patients with high-risk bladder cancer. BJU Int. 2007;99:817–820. doi: 10.1111/j.1464-410X.2006.06706.x. [DOI] [PubMed] [Google Scholar]
- 9.Järvinen R, Kaasinen E, Sankila A, Rintala E. Long-term efficacy of maintenance bacillus Calmette-Guérin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up. Eur Urol. 2009;56:260–265. doi: 10.1016/j.eururo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Krege S, Giani G, Meyer R, Otto T, Rübben H. A randomized multicenter trial of adjuvant therapy in superficial bladder cancer: transurethral resection only versus transurethral resection plus mitomycin C versus transurethral resection plus bacillus Calmette-Guerin. Participating Clinics. J Urol. 1996;156:962–966. doi: 10.1016/s0022-5347(01)65673-8. [DOI] [PubMed] [Google Scholar]
- 11.Lamm DL, Blumenstein BA, David Crawford E, Crissman JD, Lowe BA, Smith JA, Jr, et al. Randomized intergroup comparison of bacillus calmette-guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a southwest oncology group study. Urol Oncol. 1995;1:119–126. doi: 10.1016/1078-1439(95)00041-f. [DOI] [PubMed] [Google Scholar]
- 12.Mangiarotti B, Trinchieri A, Del Nero A, Montanari E. A randomized prospective study of intravesical prophylaxis in non-musle invasive bladder cancer at intermediate risk of recurrence: mitomycin chemotherapy vs BCG immunotherapy. Arch Ital Urol Androl. 2008;80:167–171. [PubMed] [Google Scholar]
- 13.Ojea A, Nogueira JL, Solsona E, Flores N, Gómez JM, Molina JR, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol. 2007;52:1398–1406. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 14.Witjes JA, v d Meijden AP, Collette L, Sylvester R, Debruyne FM, van Aubel A, et al. Long-term follow-up of an EORTC randomized prospective trial comparing intravesical bacille Calmette-Guérin-RIVM and mitomycin C in superficial bladder cancer EORTC GU Group and the Dutch South East Cooperative Urological Group. European Organisation for Research and Treatment of Cancer. Genito-Urinary Tract Cancer Collaborative Group. Urology. 1998;52:403–410. doi: 10.1016/s0090-4295(98)00212-x. [DOI] [PubMed] [Google Scholar]
- 15.Witjes WP, Witjes JA, Oosterhof GO, Debruyne MJ. Update on the Dutch Cooperative Trial: mitomycin versus bacillus Calmette-Guérin-Tice versus bacillus Calmette-Guérin RIVM in the treatment of patients with pTA-pT1 papillary carcinoma and carcinoma in situ of the urinary bladder. Dutch South East Cooperative Urological Group. Semin Urol Oncol. 1996;14(1 Suppl 1):10–16. [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov. Mitomycin C Versus Bacillus Calmette-Guerin in the intravesical treatment of non-muscle-invasive bladder cancer patients: a randomized phase III non-inferiority trial [Internet] Bethesda: National Library of Medicine; 2009. [cited 2019 Jul 19]. Available from: www.clinicaltrials.gov/ct2/show/NCT00974818. [Google Scholar]
- 17.Michielsen D, Coomans D. Intravesical chemotherapy or immunotherapy for intermediate-risk non-muscle invasive bladder cancer: does the patient mention a different quality of life? Urology. 2013;82:S130–S131. [Google Scholar]