Abstract
COVID-19 pandemic turned the entire health-care system organization upside-down, suspending elective activities and outpatient services. In Italy, we are entering a second phase of the pandemic and several strategies have been developed to “re-open” the country, some businesses, and also health care outpatient activities. This manuscript describes the experience of a Southern Italy Respiratory Unit for safely resuming outpatient respiratory services and preventing COVID-19 transmission.
Key Words: Novel coronavirus 2019, Pulmonary function test, Outpatient clinic, Safety
Keywords: Abbreviations: COVID-19, coronavirus disease 2019; WHO, World Health Organization; HCWs, health-care workers; PFTs, pulmonary function tests; PPE, personal protective equipment
Coronavirus disease 2019 (COVID-19) has quickly spread nationwide and has officially designated as a pandemic by World Health Organization.1 At the beginning of the outbreak, the need to contain transmission caused not only the lock-down of entire nations but also the shut-down of hospitals’ outpatient care.2
The Sars-CoV-2 infection is mainly transmitted by respiratory droplets3 and close contact, and both respiratory clinicians and patients are at increased risk for transmission during the outpatient visit and the pulmonary function testing procedures.
Patients scheduled for an outpatient visit may also suffer from common respiratory symptoms that can mimic or represent undiagnosed cases of COVID-19.
Planned pulmonary visit and pulmonary function tests were suspended and postponed unless when deemed clinically essential, as recommended by The American Thoracic Society, The Thoracic Society of Australia and New Zealand and The Irish Thoracic Society,4, 5, 6, 7 at the beginning of the pandemic.
In some realities, COVID-19 has rapidly changed the way of providing medical care, with hospitals providing “virtual visits” with remote consultations through video calls using commercially available software, to fill the gap with patients.8
Nevertheless, the ongoing medical needs of patients with pre-existing chronic respiratory diseases do not disappear during pandemics. Therefore, health care organizations should implement strategies to respond to patients’ needs proactively, while maintaining physical distancing policies, preventing infection transmission, and ensuring the safety of health care workers at the same time.
In Italy, we are now experiencing a second phase of the pandemic where several efforts to cautiously “re-open” the country has been made, and despite restrictions start to ease, physical distancing is not to an untimely end and we cannot lower the guard. Therefore, a plan to manage the health issues of people with pre-existing chronic lung conditions is essential to prevent an inevitably indirect effect of COVID-19 on these frail patients that could be devastating, increasing deaths and disabilities. Moreover, a respiratory follow-up on patients who have recovered from COVID-19 pneumonia is pivotal in monitoring the possible fibrotic sequela of the disease.9
Many national/international professional organizations4, 5, 6, 7 , 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 have released operational indications on how to plan the re-opening of pulmonary function test laboratories proponing different approaches, but the practical organization still relies on local hospital policies, when available or on practicing physicians.
We share our experience in planning for a resumption of the outpatient activity at the Respiratory Unit, Policlinico-Vittorio Emanuele, Catania, Italy. We believe that our model takes into account several practical operative problems and propose constructive and easy-to-adopt solutions. Table 1 summarizes the main challenges and identified solutions for restart respiratory outpatient clinics.
Table 1.
Problems and possible solutions for resuming respiratory outpatient clinics
| Challenges | Identified solutions |
|---|---|
| Physical distancing | One patient at a specific date/time |
| COVID-19 infection control | COVID-19 risk assessment questionnaire Body temperature detection |
| HCW protection | PPE + Hand washing |
| Airborne/Droplet transmission | HCW sitting in the same direction as the patient during PFTs Plexiglas wall between HCW and the patient |
| Poorly ventilated room | Natural ventilation + Window exhaust fan |
| Contact transmission | Cleaning of the equipment and surfaces |
COVID-19 = Coronavirus 2019; HCW = health-care worker; PPE = personal protective equipment; PFTs = pulmonary function tests.
Patients will be informed telephonically and instructed to come on specific dates and times. At the entrance, an ad hoc trained “outpatient triage nurse” with adequate personal protective equipment (PPE)19 will evaluate the patient providing a questionnaire for COVID-19 risk assessment, detecting temperature, and assigning a level of risk (high or low risk).
High-risk individuals will be referred to the emergency room department for nasopharyngeal swab testing and their outpatient visit rescheduled. This screening is an important aspect to consider since it might also trace under-recognized COVID-19 patients. Patients deemed at low risk will be provided with a surgical mask and an alcohol-based hand rub for hand hygiene and proceed to registration.19
Pulmonologist evaluation will be carried out in a room with minimal furnishes, with physicians equipped with PPE, a stethoscope, a portable wireless echography probe for lung ultrasound evaluation, and an oximeter. Equipment will be disinfected with alcohol solutions between use for each patient.19
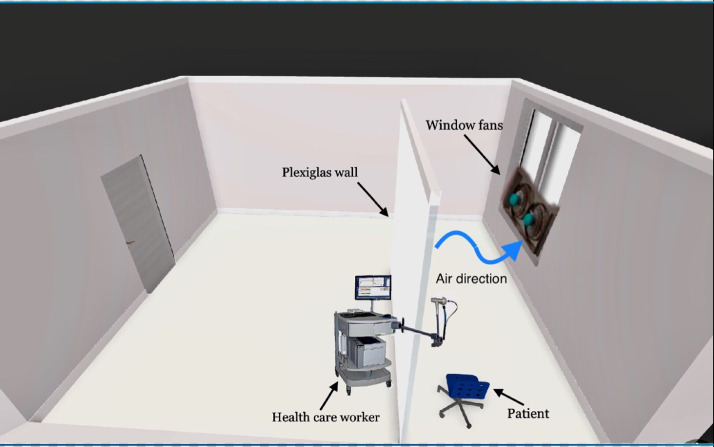
Pulmonary Function Tests will be performed in a room with a minimum presence of furnishings; the patient will be placed near the window with a plexiglass wall between he/she and the operator.9 The technician will wear PPE19 and sit in the same direction as the patient for protection in case of coughing or sneezing in the test area.9
Pulmonary Function Tests will be performed using disposable nose-clips and in-line antibacterial and viral filters10 that will also serve as disposable mouthpieces, to prevent the transmission of infection from within the circuit. The bronchodilator testing will be performed using patients’ salbutamol inhaler or a disposable aerochamber.20
Adequate ventilation of each room will be ensured using natural ventilation and an exhaust fan at the window to boost ventilation and direct airflow.21 A 3D model of room design is shown in Figure 1 .
Fig 1.
3D image of spirometry room design.
After each patient, adequate house-keeping and cleaning of the equipment and surfaces (eg, exam bed, countertop, chair, equipment) with alcoholic solutions will be performed,19 as well as safe waste management.
Pulmonary Function Tests will be performed only in essential cases for diagnosis of current illness, with immunocompromised patients tested first.7 , 12 , 14 Early afternoon appointments will be scheduled for the most severe chronic respiratory patients. COVID-19 pneumonia recovered patients will be scheduled after at least 30 days of 2 consecutive negative swabs.13
We need to change the way we provided care to patients so far and co-habit with the “silent enemy,” since chronic severe medical conditions still exist despite COVID-19, and we need to take care of them taking urgent and effective actions in continuing to assist chronic respiratory diseases while preventing infection dissemination.
The proposed approach represents a prudent and easy-to-reproduce plan to restart respiratory outpatient services while dealing with this prolonged health-care emergency. We hope that our experience might provide a practical and useful guide for other respiratory units while resuming their respiratory outpatient activity.
Acknowledgments
None.
Footnotes
Conflicts of interest: None to report.
References
- 1.WHO. Coronavirus Disease (COVID-2019) Situation Report 2020. Available at:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed May 17, 2020
- 2.WHO. Critical Preparedness, Readiness and Response Actions for COVID-19: Interim Guidance. Available at: https://apps.who.int/iris/handle/10665/331511. Accessed May 17, 2020
- 3.Anfinrud P., Stadnytskyi V., Bax C.E., Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med. 2020;382:2061–2063. doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Thoracic Society. Pulmonary Function Laboratories: Advice Regarding COVID-19. Available at: https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/pulmonary-function-laboratories.php. Accessed May 17, 2020
- 5.Irish Thoracic Society Guidance on Lung Function Testing: SARS COVID-19. Available at: https://irishthoracicsociety.com/wp-content/uploads/2020/03/ITS-Guideline-on-lung-function-testing-24.03-FINAL.pdf. Accessed May 17, 2020
- 6.TSANZ/ANZSRS. Peak Respiratory Bodies Recommend Suspension of Lung Function Testing. Available at: https://www.anzsrs.org.au/images/anzsrs/peak_respiratory_bodies_recommend_suspension_of_lung_function_testing_002.pdf
- 7.ARTP COVID19 Guidelines. Available at: https://www.artp.org.uk/News/artp-covid19-update-18th-march-2020. Accessed May 17, 2020
- 8.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for covid-19. N Engl J Med. 2020;18:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 9.Task Force of Pulmonary Function Testing. Clinical Respiratory Physiology. Chinese Association of Chest Physicians. Pulmonary Function Testing Group and R. T. G. C. T. Society Expert consensus on pulmonary function testing during the epidemic of Corona Virus Disease 2019. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E032. doi: 10.3760/cma.j.cn112147-20200225-00175. [DOI] [PubMed] [Google Scholar]
- 10.TSANZ/ANZSRS. Update on Suspension Of Lung Function Testing. Available at: https://www.anzsrs.org.au/images/Updated_Lung_Function_Testing_Guidelines_-_30th_April_2020.pdf. Accessed May 17, 2020
- 11.AIPO-ITS Le prove di funzionalità respiratoria nell'era della pandemia da COVID-19 - Position Paper. Available at: http://www.aiponet.it/news/speciale-covid-19/2471-le-prove-di-funzionalita-respiratoria-nell-era-della-pandemia-da-covid-19-position-paper-aipo-its.html. Accessed May 17, 2020
- 12.Italian Respiratory Society. SIP-IRS. Esami di funzionalità respiratoria nel contesto COVID-19 Position Paper. Available at: https://irn.sipirs.it/storage/61/Documento-EsamiFunzionalitàRes-Covid_Vers.1_12.05.2020.pdf. Accessed May 17, 2020
- 13.Recommendation from ERS Group 9.1 (Respiratory function technologists/scientists) lung function testing during COVID-19 pandemic and beyond. Available at: https://ers.app.box.com/s/zs1uu88wy51monr0ewd990itoz4tsn2h. Accessed May 17, 2020
- 14.Sociedad Uruguaya de Neumología. Recomendaciones para realizar Estudios Funcionales Respiratorios durante la epidemia causada por el coronavirus SARS–CoV–2 (COVID19). Available at: https://suneumo.org/descargar/adjunto/32-grl8hr-coronavirus-2020-15mar-reco1.pdf. Accessed May 17, 2020
- 15.Sociedade Portuguesa de Pneumologia (SPP). Reabertura de Laboratórios de função Pulmonar. Available at: https://www.sppneumologia.pt/uploads/subcanais_conteudos_ficheiros/adenda-pfr-covid19.pdf. Accessed May 17, 2020
- 16.Sociedade Portuguesa de Pneumologia. Recomendações da SPP para a realização de provas funcionais respiratórias durante o surto COVID-19. Available at:https://www.sppneumologia.pt/uploads/subcanais_conteudos_ficheiros/adenda-pfr-covid19.pdf. Accessed May 17, 2020
- 17.UNMC Nebraska Medicine. Recommendations on performing Pulmonary Function Test (PFT) in PFT lab. Available at: https://www.nebraskamed.com/sites/default/files/documents/covid-19/Guidance-regarding-pulmonary-function-testing-(PFT).pdf?date=05122020. Accessed May 17, 2020
- 18.UNMC Nebraska Medicine. SBAR for performing pulmonary function test in PFT lab. Available at:https://www.nebraskamed.com/sites/default/files/documents/covid-19/Guidance-regarding-pulmonary-function-testing-(PFT).pdf?date=04012020. Accessed May 17, 2020
- 19.WHO. Infection prevention and control during health care when COVID-19 is suspected. Interim Guidance. Available at: https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125. Accessed May 7, 2020
- 20.Saeed H., Abdelrahim M.E., Rabea H., Salem H.F. Evaluation of disposable and traditional accessory devices for use with a pressurized metered-dose inhaler. Respir Care. 2020;3:320–325. doi: 10.4187/respcare.06835. [DOI] [PubMed] [Google Scholar]
- 21.Qian H., Li Y., Seto W.H., Ching P., Ching W.H., Sun H.Q. Natural ventilation for reducing airborne infection in hospitals. Build Environ. 2010;3:559–565. doi: 10.1016/j.buildenv.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]