Abstract
In the first two decades of the 21st century, there have been three outbreaks of severe respiratory infections caused by highly pathogenic coronaviruses (CoVs) around the world: the severe acute respiratory syndrome (SARS) by the SARS-CoV in 2002–2003, the Middle East respiratory syndrome (MERS) by the MERS-CoV in June 2012, and Coronavirus Disease 2019 (COVID-19) by the SARS-CoV-2 presently affecting most countries In all of these, fatalities are a consequence of a multiorgan dysregulation caused by pulmonary, renal, cardiac, and circulatory damage; however, COVID patients may show significant neurological signs and symptoms such as headache, nausea, vomiting, and sensory disturbances, the most prominent being anosmia and ageusia. The neuroinvasive potential of CoVs might be responsible for at least part of these symptoms and may contribute to the respiratory failure observed in affected patients. Therefore, in the present manuscript, we have reviewed the available preclinical evidence on the mechanisms and consequences of CoVs-induced CNS damage, and highlighted the potential role of CoVs in determining or aggravating acute and long-term neurological diseases in infected individuals. We consider that a widespread awareness of the significant neurotropism of CoVs might contribute to an earlier recognition of the signs and symptoms of viral-induced CNS damage. Moreover, a better understanding of the cellular and molecular mechanisms by which CoVs affect CNS function and cause CNS damage could help in planning new strategies for prognostic evaluation and targeted therapeutic intervention.
Keywords: Neuroinflammation, Coronaviruses, Viral encephalitis, COVID-19, SARS
1. Introduction
Coronaviruses (CoVs), a subfamily of RNA enveloped plus-strand viruses belonging to the order Nidovirales, family Coronaviridae, have recently gained worldwide notoriety as the agents causing the ongoing Coronavirus Disease 2019 (COVID-19) pandemic, already responsible for hundreds of thousands of deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). More than 50 different CoVs, classified into four genera (α-, β-, γ-, and δ-CoVs) on the basis of phylogenetic clustering (de Groot et al., 2012), are currently known. While most CoVs cause animal diseases, until recently only four of them were known to be responsible for human diseases, usually self-limiting respiratory infections (van der Hoek, 2007): two α-CoVs (HCoV-229E and -NL63) and two β-CoVs (HCoV-OC43 and -HKU1). At the beginning of the new millennium, three new β-CoVs highly pathogenic for humans, have been proven responsible for the severe acute respiratory syndrome (SARS; SARS-CoV) and the Middle East respiratory syndrome (MERS; MERS-CoV) epidemics, and more recently for the COVID-19 pandemic (SARS-CoV-2). All three viruses are strictly related to CoVs infecting lower animal species from which they are believed to have originally spread (Sheahan et al., 2008; Han et al., 2016; Ahmad et al., 2020).
Recent studies have shown that significant neurological signs and symptoms such as headache, nausea, vomiting, and sensory disturbances, the most prominent being anosmia and ageusia, may occur in COVID-19 patients. Furthermore, it has been hypothesized that the neuroinvasive potential of CoVs might be responsible for at least part of these symptoms and may contribute to the respiratory failure observed in affected patients. Therefore, in the present manuscript, we have reviewed the available preclinical evidence on the mechanisms and consequences of the CoVs-induced CNS damage, and highlighted the potential role of CoVs in determining or aggravating acute and long-term neurological diseases in infected individuals. To this aim, we searched repositories of articles (PubMed; https://pubmed.ncbi.nlm.nih.gov/) for studies (no date limits) published in English in peer-reviewed International journals using the following research terms: Coronavirus, SARS, MERS, COVID-19, MERS-CoV, SARS-CoV, SARS-CoV-2, neurovirulence and neuroinfection mechanisms, neurological diseases, immune-mediated neurological damage, and animal models. In addition, clinical trial databases (https://clinicaltrials.gov/) were searched for ongoing clinical studies investigating potential therapies for COVID-19 involving some of the pathophysiological mechanisms herein described. The screening of titles and abstracts was performed by all authors, and those considered relevant for the purpose of the present review were retrieved, analyzed, and their main findings reported in the present manuscript.
2. Neurovirulence of coronaviruses: evidence from animal models
The high neurotropism of β-CoVs in experimental animals has been known for decades. In fact, the first evidence that CoVs may infect the CNS was gathered at the end of 1940s when a neurotropic strain of the β-CoVs mouse hepatitis virus-4 (MHV-4), the JHM virus (JHMV) named in honor of the microbiologist John Howard Mueller (Pappenheimer, 1958), was isolated from mice developing spontaneous hind leg paralysis, and was shown to induce a diffuse encephalomyelitis with patchy areas of demyelination when inoculated in mice (Cheever et al., 1949; Bailey et al., 1949). In newborn and weanling rats, JHMV induces either an acute panencephalomyelitis, which is usually fatal in a few days, or a subacute paralytic disease which progresses over several months, or a chronic progressive paralysis appearing 6–8 month after virus inoculation (Nagashima et al., 1978a). These conditions differ significantly in their gross and microscopic pathology with widespread neuronal death, necrosis and polymorphonucleate infiltration in the acute form, perivascular cuffing consisting of plasma cells and macrophages and large demyelinating lesions in which axons are largely preserved in the subacute form, and diffuse myelomalacia, cerebral cortex thinning and hydrocephalus in the chronic form (Nagashima et al., 1978a; Nagashima et al., 1978b), suggesting multiple pathogenic mechanisms. The demyelinating lesions found in the subacute paralytic disease displayed considerable histopathological resemblance to those of multiple sclerosis (MS); therefore, JHMV infection has become a widely used experimental model for this disease (Sorensen and Dales, 1985). JHMV is not the only neurovirulent CoV in rodents. In fact, a few years after its identification, Dick et al. (Dick et al., 1956) isolated a mutant neurotropic strain of the mouse hepatitis virus-3 (MHV-3), that did not cause hepatitis but induced a fatal meningo-encephalitis when injected intracerebrally in mice. It was later reported that, in a few susceptible mice strains such as CH3 and AG2 mice, also the wild type MHV-3 variant induced neurological manifestations with lack of coordination and limb paresis associated to histological evidence of brain vasculitis and meningoependymitis (Virelizier et al., 1975). Interestingly, CoVs may induce neurological diseases also in species different from rodents, as seen in the β-CoV porcine hemagglutinating encephalomyelitis virus (PHEV) of the VW572 strain that causes encephalomyelitis in piglets (Desforges et al., 2013), and the feline α-CoV (FCoV) that produces significant CNS damage in cats (Desforges et al., 2013).
As previously mentioned, CoVs responsible for dramatic outbreaks in humans in the last 20 years probably originated from animal species and later spread to humans. It is therefore crucial to understand whether the neurovirulence of CoVs is species-specific. Even though available evidence is very limited, CoVs can easily adapt to infect brains from species different from their original hosts. In fact, neurotropic MHV may also induce encephalitis in rats (Nagashima et al., 1978a; Nagashima et al., 1978b) and monkeys (Cabirac et al., 1995; Murray et al., 1992a; Murray et al., 1997), and, remarkably, HCoV-OC43 can induce acute encephalitis in mice with clinical signs and brain histopathological alterations very similar to those described for the JHMV (Butler et al., 2006; McIntosh et al., 1967). These findings confirm that CoVs can cause brain damage in species different from their natural host, and suggest that human pathogenic CoVs are also endowed with significant neurovirulence potential.
Different animal species including mice, ferrets, hamsters and monkeys have been infected with SARS-CoV; however, unlike the devastating human infection, these animals only developed a mild form of the disease (Subbarao and Roberts, 2006). In particular, intranasal instillation of SARS-CoV in C57BL/6 mice only causes a transient replication of the virus in the lungs and very few symptoms, with failure to thrive yet no increase in mortality (Glass et al., 2004a). Intriguingly, in these mice, SARS-CoV spreads to the brain and mainly targets the hippocampus (Glass et al., 2004b). The low virulence of SARS-CoV for C57BL/6 mice is believed to be a consequence of the low affinity of the virus (whose receptors are represented by human ACE2, see below) for murine ACE. In fact, a lethal infection was successfully induced when SARS-CoV was inoculated intranasally in transgenic mice overexpressing the human ACE2 enzyme (K18-hACE2 mice), with high viral titres in neurons of the cerebrum, thalamus, and brainstem, and relative sparing of the olfactory bulb and cerebellum (McCray Jr. et al., 2007). However, intranasal infection with SARS-CoV-2 of transgenic mice overexpressing human ACE2 (ACE2-HB-01 mice) only caused a mild disease course with pulmonary inflammation but no evidence of brain involvement (Bao et al., 2020).
A strategy using transgenic mice overexpressing the MERS-CoV receptor hCD26/DPP4 was also needed to replicate the severity of human MERS (Agrawal et al., 2015). When hCD26/DPP4 mice were infected with moderate loads of MERS-CoV, significant brain damage occurred, mainly localized in the brainstem, where perivascular cuffing, apoptotic bodies, and activated microglia were observed. Conversely, only a mild meningitis occurred in the cortex (Tao et al., 2016).
3. Mechanisms for CoVs neuroinvasion
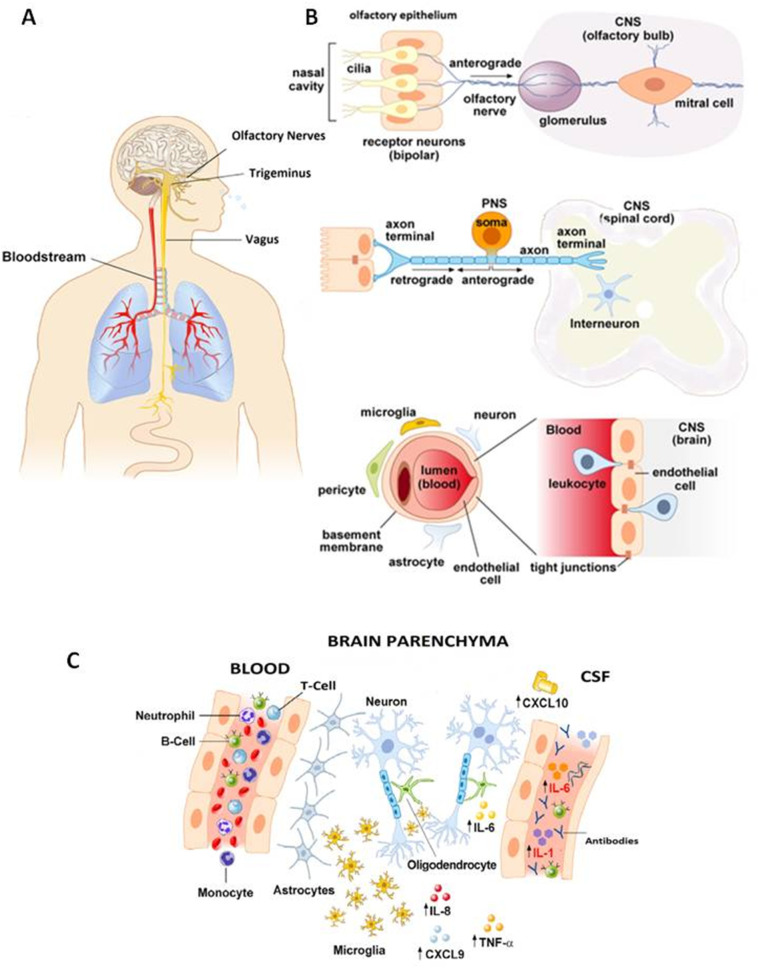
CoVs can access the CNS in two ways: the hematogenous and the neurogenic routes (Fig. 1 ). Hematogenous invasion occurs when a neurovirulent agent enters the brain after a transient viremic episode and crosses the blood brain barrier (BBB). Hematogenous neuroinvasion by CoVs, as with the human respiratory syncytial neurotropic virus (hRSV), may occur through two non-mutually excluding mechanisms: by direct penetration of the free viral particle across the endothelial cells of the BBB, or by hijacking peripheral blood cells (mainly monocytes/macrophages) which are infected in the airways during the acute phase and then transmigrate from the blood to the brain via the paracellular route between endothelial cells of the BBB (Bohmwald et al., 2018). Indeed, neutrophils, macrophages, and natural-killer (NK) lymphocytes may support MHV replication in the early stages of the infection, and can be recruited in MHV-infected brain tissue (Bergmann et al., 2006). Moreover, different strains of CoVs, including SARS-CoV, infect peripheral myeloid cells during respiratory infections, and infected leukocytes can serve as reservoirs for CNS invasion (Collins, 2002; Desforges et al., 2007; Mesel-Lemoine et al., 2012; Desforges et al., 2019). On the other hand, CoVs can also directly penetrate the brain causing meningeal inflammation and damage to the integrity of the BBB through the release of inflammatory mediators (Tohidpour et al., 2017). Lymph nodes, which are often colonized in the early phases of CoVs infection, may represent additional intermediate sites for hematogenous invasion (Barthold and Smith, 1992).
Fig. 1.
Schematic representation of the potential anatomical (A), cellular (B), and molecular (C) mechanisms for CoVs neuroinvasion. See text for explanation of each panel.
During neurogenic invasion on the other hand, viruses enter the CNS through retrograde axonal transport along specific peripheral nerves depending on the main site of infection. For instance, respiratory viruses which colonize the olfactory epithelium may use olfactory nerve fibers to enter the CNS (Swanson 2nd and McGavern, 2015). This invasion mechanism is used, among others, by Herpes Simplex virus-1, vesicular stomatitis virus, Borna disease virus, rabiae virus, influenza A virus (IAV), hRVS, and CoVs (Koyuncu et al., 2013). Other nerves such as the trigeminus and the vagus serving the nasal cavities and upper airways represent additional major invasion routes for respiratory viruses such as neurotropic IAVs (Matsuda et al., 2004), but seem to play only a minor role in CoV neuroinvasion (Dube et al., 2018). The vagus nerve may serve as a gateway to the CNS for CoVs that infect the gastrointestinal mucosa, as seen for PHEV-67 N, which, after oronasal inoculation, infects the epithelial cells of the small intestinal mucosa in pigs and is thereafter transported retrogradely via peripheral nerves to central neurons in charge of peristaltic function of the digestive tract, resulting in the so-called vomiting disease (Andries and Pensaert, 1980).
3.1. CoV receptors
The ability of CoVs to enter the primary infection site and to later spread to distant sites including the CNS, relies on their interaction with specific receptors located on the host cells. A large body of experimental work firmly points to the spike (S) protein as the main determinant of cell tropism and pathogenicity of CoVs (Walls et al., 2020). The S protein, which forms heavily glycosylated transmembrane trimers in the viral envelope, mediates CoVs entry into susceptible host cells via the interaction with specific cellular receptors represented by aminopeptidase N (APN) for the α-CoV HCoV-229E (Yeager et al., 1992), angiotensin-converting enzyme 2 (ACE2) for SARS-CoV (Li et al., 2003) and SARS-CoV-2 (Hoffmann et al., 2020), and dipeptidyl-peptidase 4 (DPP4) in the case of MERS-CoV (Raj et al., 2013). Instead, the immunoglobulin-like CEACAM1a protein is the receptor for MHVs (Bergmann et al., 2006; Williams et al., 1990; Williams et al., 1991). It is important to note that these receptors are also expressed in CNS neurons, glial and endothelial cells, where they could mediate cell-specific targeting (Baig et al., 2020; Bender et al., 2010). The S protein has the structure of class I fusion proteins, consisting of two functional subunits contained in the same protein, the N-terminal surface subunit (S1) responsible for receptor binding, and the C-terminal transmembrane subunit (S2) that mediates viral internalization (Bosch et al., 2003). Upon binding to its receptor, the S protein is cleaved by specific proteases, thereby allowing the release of the S2 subunit, initiating membrane fusion and virus internalization (Bosch et al., 2003). Cleavage of SARS-CoV (Glowacka et al., 2011) and SARS-CoV-2 (Hoffmann et al., 2020) S protein is mediated by the Transmembrane Serine Protease 2 (TMPRSS2); pharmacological inhibition of TMPRSS2 significantly reduces infection with SARS-CoV-2, representing therefore a potential strategy for the treatment of COVID-19 (Yamaya et al., 2020).
3.2. Neuroinvasion by neurotropic MHVs
Neurotropic MHVs use different neuroinvasion mechanisms depending on the administration pathways. Systemic administration of MHV through intravenous or intraperitoneal routes is followed by hematogenous propagation in the brain, a process facilitated by the ability of CoVs to infect brain microvascular endothelial cells (BMECs) and to loosen the BBB (Cabirac et al., 1995; Bleau et al., 2015).The severity of BMECs infection induced by different MHVs correlates well with their neuroinvasiveness (Joseph et al., 1995; Godfraind et al., 1997). The hepatotropic MHV-3 virus invades the brain by impairing tight junctions and loosening the BBB without triggering the release of inflammatory cytokines, yet decreasing IFNβ production by infected BMECs (Bohmwald et al., 2018). Conversely, the MHV-A59 virus directly loosens the BBB by reducing the expression and the plasmamembrane localization of connexin 43 (Cx43), a critical determinant of the glial-pial gap junctions (Bose et al., 2018). Finally, JHMV-induced BBB damage depends on cytokine release and metalloproteinase activation (Zhou et al., 2005).
The ability of MHVs to invade the CNS by the neurogenic route was demonstrated by sequential immunochemistry analysis showing that, after intranasal inoculation in mice, JHMV first infects the nasal mucosa and then progresses into the brain through the olfactory fibers to reach the anterior part of the brain and, after at least four days, invade the posterior regions (Barthold, 1988). In this model, the trigeminal nerve may also be infected by JHMV (Perlman et al., 1989); however, since no brain infection occurs in mice upon removal of the olfactory bulbs, it is clear that the trigeminal route is not a major invasion pathway (Barnett and Perlman, 1993). Brain invasion through the olfactory fibers has also been demonstrated for the MHV-A59 virus (Lavi et al., 1988).
3.3. Neuroinvasion by HCoV-OC43
HCoV-OC43 can invade the CNS through the neurogenic route. After intranasal inoculation in mice, HCoV-OC43 first appears in the cell bodies and dendrites of olfactory neurons 3 days after infection, and then propagates to other brain regions including the hippocampus, the brain stem where it can damage the respiratory nuclei, and, finally, the cortex and the spinal cord (Dube et al., 2018; Niu et al., 2020). Destruction of the olfactory epithelium with zinc sulphate abrogated brain invasion, further supporting the critical role of olfactory fibers for neuroinvasion; by contrast, hematogenous invasion was ruled out because viral particles were not found in the general circulation after intranasal viral inoculation (Dube et al., 2018). Finally, although HCoV-OC43 may cause gastroenteritis in humans, no evidence for its propagation to the CNS though the vagal route has been reported so far.
3.4. Neuroinvasion by SARS-CoV and MERS-CoV
The mechanism of neuroinvasion by SARS-CoV has been investigated in the above mentioned K18-hACE2 transgenic mice overexpressing the human ACE2, a valuable experimental model of human SARS (Netland et al., 2008). The analysis of brains collected at serial times after virus inoculation in these transgenic mice showed that the virus enters the brain through the olfactory bulb to be rapidly cleared from these neurons, followed by spreading to more distal regions.
Remarkably, not only regions directly connected with the olfactory bulb (including the piriform cortex, the basal ganglia and the midbrain), but also regions with no direct synaptic connection are rapidly invaded, suggesting that alternative routes of invasion besides the trans-synaptic propagation could be involved, possibly including the Virchow-Robin spaces. The dorsal vagal complex is among the brain structures not directly connected with the olfactory bulb which are invaded early during the disease. Its damage could thus contribute to the death of the animal by cardiorespiratory failure. The route used by SARS-CoV to invade the dorsal vagal nuclei is still unclear, but it does not likely involve the propagation from the gut through the vagal fibers since the virus has not been found in the gut after intranasal instillation. Importantly, after intranasal administration, SARS-CoV-2 can be found only in neurons, with no glial colonization or inflammatory cell infiltration, ruling out the spread to the CNS through the hematogenous route (Netland et al., 2008). Notably, the pattern of neuroinvasion by SARS-CoV emerging from studies in K18-hACE2 transgenic mice is different from that observed by Glass et al. (Glass et al., 2004b) in wild type C57 mice. This study found no evidence of olfactory bulb involvement and the location of the virus was predominantly in the hippocampus, an area frequently spared or only slightly and lately infected in transgenic mice. It is presently unknown whether this different neuroinvasion pattern occurs because SARS-CoV penetrates the brain through alternative routes in the absence of high affinity hACE2 receptors, or because it is simply a consequence of the slow progression of brain infection in wild type mice.
The mechanism of neuroinvasion by MERS-CoV has not been investigated so far. However, the occurrence of perivascular infiltrates in the brainstem and meningitis in the cortex of hCD26/DPP4 transgenic mice is hardly compatible with a neurogenic pattern of infection, and better fits with hematogenous invasion (Tao et al., 2016).
4. Cellular and molecular mechanisms for CoV-induced CNS damage
In vitro experiments using murine or human cell cultures exposed to JHMV, HCoV-OC43, or SARS-CoV have shown that CoVs can infect neurons, glial cells and BMECs (Arbour et al., 1999a; Bonavia et al., 1997; Houtman and Fleming, 1996; Yamashita et al., 2005). Importantly, neuronal and glial cells infected in vitro or in vivo may harbor CoVs for a very long time (Arbour et al., 1999b; Sun et al., 1995; Stohlman and Weiner, 1978; Perlman et al., 1988; Lavi et al., 1984; Fleming et al., 1993), possibly accounting for their long persistence in the brain after the initial infection and for their potential involvement in chronic neurodegenerative diseases (Sorensen and Dales, 1985) (see below). Non-neuronal cells have a role in the neurobiology of CoV infection of the CNS since they may be instrumental for tissue damage or healing. In addition, their dysfunction contributes to the pathogenesis of relevant neurological disorders such as stroke (Verkhratsky et al., 2019; Liu and Chopp, 2016; Ma et al., 2017) or seizures (Devinsky et al., 2013; Ravizza et al., 2013; Vezzani et al., 2008) that can complicate severe brain infection by CoVs.
Evidence has been reported that JHMV may kill cultured oligodendrocytes by promoting apoptosis (Liu et al., 2006; Liu and Zhang, 2007), and that HCoV-OC43 may induce an unfolded protein response causing necroptosis in neuronal cells (Favreau et al., 2009; Meessen-Pinard et al., 2016). Direct virus-induced cell demise may have a role in acute or hyperacute forms of CoV-induced CNS damage which occur with limited tissue inflammation in mice (Netland et al., 2008) or humans (Morfopoulou et al., 2016). In the majority of cases, however, CoV neuroinvasion triggers a major immune response with tissue inflammation that can either clear the virus and help resolving the infection, or cause immune-mediated CNS injury. Importantly, even when CoVs are no longer detectable, sterile immunity is rarely achieved since the virus persists in the brain in a latent form.
4.1. CoV-induced CNS damage during acute infection
Whether a specific CoV-induced neuroinfection will evolve to resolution, progression, or chronicization, depends on a fine balance between factors that promote or restrain inflammatory responses (Savarin and Bergmann, 2018). While the activation of both innate and adaptive immune responses is crucial for CoVs clearance and infection healing, the release of inflammatory mediators may seriously damage the CNS. Studies on JHMV-induced neuroinfection in mice unveiled a specific temporal sequence of the immune response: once inside the CNS, the virus triggers innate immunity with significant polymorphonucleate, macrophage and NK cell infiltration. The release of cytokines and chemokines causes tissue damage, and eventually long lasting adaptive immunity is established, thus eradicating the infection or initiating CNS autoimmune aggression (Bergmann et al., 2006). Early activation of innate immunity is associated with the release of INFα/β by microglial cells. A strong INF response predicts the resolution of the infection, whereas a weak response is associated to a higher risk of virus persistence and chronic CNS infection (Bergmann et al., 2006; Savarin and Bergmann, 2018). Wheeler et al. (Wheeler et al., 2018) recently reported that microglial activation is essential for the survival of mice with a severe JHMV variant-induced encephalitis. Proinflammatory cytokines and chemokines, including IL-1α, IL-1β, IL-6, IFN-γ, TNF-α, and CXCL10 are released by CNS resident cells (macrophages, glia and endothelium) as well as by infiltrating cells as part of the innate immunity response, and play a crucial role both in damaging the nervous tissue and in the subsequent activation of the adaptive immunity (Joseph et al., 1993). As a matter of fact, it has been demonstrated that there is a correlation between the levels of the mRNAs for some of the aforementioned mediators of the innate immune response (such as TNF-α, IL-12 p40, IL-6, IL-15, and IL-1) and the neurovirulence of different MHV strains, further strengthening the role of these cytokines as master regulators of CoV neurovirulence (Li et al., 2004). In fact, IL-1 and IL-6 mRNA transcription is robustly activated in mice brains following infection with the neurovirulent JHMV, but not with the more attenuated MHV-A59 strain (Rempel et al., 2004). Moreover, high levels of IL-1β were found in brain-derived CD11+ cells, microglia, and macrophages from MHV intracranially-infected mice. Survival of these mice was increased by treatment with the IL-1 receptor antagonist (IL-Ra) anakinra (Allan et al., 2005; Vijay et al., 2017).
The pathophysiological relevance of some of these mechanisms also receives support from studies in humans. The levels of proinflammatory cytokines such as IL-1β, IL-6, IL-8 and TNFα and their ratio with antinflammatory cytokines such as IL-Ra and IL-10 correlate with damage progression in many CNS inflammatory diseases, including neuromyelitis optica, transverse myelitis, acute disseminated encephalomyelitis (ADEM), amyotrophic lateral sclerosis, herpes simplex encephalitis, Parkinson's disease, traumatic brain injury, epilepsy, and stroke (Beridze et al., 2011; de Vries et al., 2016; Rodney et al., 2018; Vezzani et al., 2002; Waje-Andreassen et al., 2005; Welsh et al., 2009; Zaremba and Losy, 2001; Vezzani et al., 2019; West et al., 2019). As a matter of fact, high levels of IL-6, IL-8, MCP-1, and GM-CSF were found in both serum and cerebrospinal fluid (CSF) samples from hospitalized children with acute encephalitis-like syndrome and serological evidence of recent CoV-induced respiratory tract infections (Li et al., 2016). Among the chemokines released in the early stages of CoV neuroinfection, CXCL9 and CXCL10, both induced by IFN-γ, are among the earliest inflammatory markers in the peripheral blood of patients affected with SARS (Jiang et al., 2005; Xu et al., 2005). The levels of both CXCL9 and CXCL10 were abnormally elevated in brain samples from a SARS-affected patient (Xu et al., 2005). A potential link between direct cytotoxic and indirect immune-mediated neurovirulence mechanisms triggered by HCoV-OC43 in mice has been hypothesized upon demonstration that IL-6 promotes glutamate-dependent exocitoxicity (Brison et al., 2014), and that the NMDA glutamate receptor antagonist memantine produces significant neuroprotection in HCoV-OC43-infected mice (Brison et al., 2011). Finally, a hyperactive immune response leading to the massive release of cytokines in the general circulation (cytokine storm) and to intravascular disseminated coagulation and multiorgan failure, is a major mechanism of death in COVID-19 patients. This further highlights the crucial role of the immune system in the progression of CoV-induced diseases (Jose and Manuel, 2020).
4.2. CoV-induced CNS damage during latent infection
As mentioned before, immune response to CoVs does not always result in sterile immunity and CoVs remain latent in neuronal or glial cells. This has been shown in mice which develop a subacute demyelinating disease after JHMV infection (Knobler et al., 1982). Likewise, the persistence of MHV-A59 has been demonstrated in the CNS of C57BL/6 mice, showing demyelinating lesions of the brain and the spinal cord (Lavi et al., 1984). In addition, viral particles and RNA were found in the brain of owl monkeys up to 215 days after intravenous injection of the JHM OMP1 virus, a JHM strain neurovirulent for primates (Cabirac et al., 1993). Moreover, in BALB/c mice injected intracerebrally with HCoV-OC43 at 8 postnatal days, viral RNA persisted in the CNS for at least 5 months after infection (Jacomy and Talbot, 2001).
Virus persistence in the CNS is critical for the development of the subacute and chronic forms of MHV-, and possibly HCoV-OC43-induced demyelinating diseases. In these infections, histopathological damage appears to be mainly immune-mediated, with little or no direct cytopathic effects. In fact, while no demyelination occurs after JHMV infection in mice with defective B- and T-cell maturation, demyelinating lesions are instead observed when virus-infected splenocytes from normal mice are transferred (Wu and Perlman, 1999). Although JHMV viral infection may trigger the development of autoreactive T cell clones, this does not seem the major mechanism for demyelination (Savarin et al., 2015). Instead, persistent activation of inflammatory cells that may damage oligodendrocytes seems to play a major role. In fact, JHMV latent infection favors CNS maintenance of CD4+ and CD8+ T lymphocytes (Marten et al., 2000) which both attract macrophages through the release of the chemokine CCL5 and of IFN-γ, respectively (Lane et al., 2000). An important role in recruiting macrophages and lymphocytes in the lesions is also played by the release of CXCL10 by resident glial cells (Dufour et al., 2002). As a matter of fact, immunoneutralization of either CCL5 or CXCL10 significantly reduces the severity of demyelination in mice chronically infected with JHMV (Glass et al., 2004a; Liu et al., 2001). Time course analysis of gene expression in different cell types infiltrating the demyelinating lesions showed that long-lasting damage is probably due to microglial cells, whereas macrophages are probably more important at earlier stages (Savarin et al., 2018). Glial cells may prompt tissue damage not only by releasing chemoattractant molecules, but also nitric oxide and cytokines such as TNF-α, IL-1β, IL-6 (Sun et al., 1995; Edwards et al., 2000; Grzybicki et al., 1997). An additional mechanism of damage during latent infection is the impairment of differentiation of oligodendrocyte precursors caused either directly by JHMV (Liu and Zhang, 2006) or indirectly through IFNγ release under CXCL10 stimulation (Weinger et al., 2013) (Chew et al., 2005; Tirotta et al., 2011).
Although responsible for demyelination, immune response is also essential to prevent the reactivation of a latent CNS CoV infection. In fact, reactivation of JHMV infection has been shown to occur in mouse models in which humoral immunity is impaired. For example, in μMT mice that lack mature B cells JHMV are not persistently cleared from the CNS as in wild type mice, but, after a transitory clearance by cell immunity, remerge mainly in oligodendrocytes and cause encephalitis and animal death (Lin et al., 1999; Ramakrishna et al., 2003) .
5. CoVs neurovirulence may be responsible for acute CNS diseases in humans
More than thirty years ago, seizures and signs of meningitis or radiculitis in patients with serologically proven HCoV-OC43 infection were first reported (Riski and Hovi, 1980). Nonetheless, the etiological involvement of CoVs in acute encephalitis in humans has only been demonstrated in immunosuppressed patients. The presence of HCoV-OC43 in a brain biopsy sample from a 11-month old boy with severe combined immunodeficiency and signs and symptoms of encephalitis was demonstrated using RNA sequencing and confirmed by real-time PCR and immunocytochemistry (Morfopoulou et al., 2016). The radiological findings in this child did not show demyelination but an extensive gray matter damage with loss of brain volume. Very recently, the presence of the HCoV-OC43 genome has been documented in a brain biopsy sample from a 19 month-old child affected with pre-B acute lymphoblastic leukaemia who developed a persistent airway disease during chemotherapy followed by a fatal encephalitis (Nilsson et al., 2020).
CoVs may also play a role in the genesis of acute disseminated encephalomyelitis (ADEM), a monophasic autoimmune demyelinating disease usually occurring in children a few days after an infection or a vaccination (Pohl et al., 2016; Cole et al., 2019). The histopathological features of ADEM are similar to those observed in the subacute form of JHMV-induced encephalitis in rats, with perivascular sleeves of demyelination abundantly infiltrated by T cells but not by polymorphonucleates (Pohl et al., 2016; Cole et al., 2019). Moreover, the case of a 15 year-old boy who developed ADEM with numbness in the lower limbs a week after a common cold and whose CSF was positive for HCoV-OC43 at RT-PCR analysis (Yeh et al., 2004), further supports a potential link between CoVs infection and subacute demyelinating diseases in humans. Intriguingly, a common laboratory finding in ADEM patients is the significant increase in CSF levels of several cytokines and chemokines, also including those induced upon CoV infection in experimental animals, such as IL-6, IFN-γ, TNF-α, CXCL9, and CXCL10 (Kothur et al., 2016).
However, it is presently unknown whether highly pathogenic SARS-CoV and MERS-CoV can also cause encephalitis in humans, possibly because of the difficulties in identifying symptoms and signs of brain involvement in patients who are deeply sedated during mechanical ventilation, and due to the limited availability of autoptic material. Nonetheless, genome sequences of SARS-CoV have been found in brain samples from all eight SARS-affected patients reported by Gu et al. (Gu et al., 2005) who underwent a thorough autoptic examination, with neuronal death and necrosis at histopathology in 6 of them. Moreover, the SARS-CoV genome was identified by RT-PCR in the CSF of a 32 year-old woman affected with SARS who developed generalized tonic-clonic seizure (Lau et al., 2004), and in brain autoptic samples from a 39 year-old patient who died from SARS and showed neurological symptoms suggestive of encephalitis (Xu et al., 2005). In addition, three patients with MERS-CoV infection displayed severe neurological impairment, which variably included altered mental status from confusion to coma, ataxia and focal motor deficits, and MRI findings suggestive of ongoing encephalitis (Arabi et al., 2015). As far as the involvement of the CNS during infection with SARS-CoV-2 is concerned, it has been suggested that neurological symptoms frequently occurring at the onset of COVID-19 (Mao et al., 2020; Nath, 2020) may represent early warning signs of the disease (Lechien et al., 2020). In a retrospective analysis of 214 COVID-19 patients admitted to three hospitals in Wuhan, China, neurological signs were observed in more than 36% of the cases (Mao et al., 2020). Although in the majority of cases these neurological symptoms were mild and consisted in headache, dizziness augeusia and hyposmia, altered consciousness or stroke also occurred (Mao et al., 2020). Additional reports documenting serious neurological complications in COVID-19 patients have been subsequently published. For instance, a severe encephalopathy with diffuse EEG slowing was described in a 74-year-old man presenting with altered mental status and later shown to be affected with COVID-19 (Filatov et al., 2020). Altered mental status at presentation was also documented in a middle-aged female whose CT scan was diagnostic for acute hemorrhagic necrotizing encephalopathy (ANE), a rare complication of influenza and other viral infections causing BBB breakdown but without direct viral invasion or parainfectious demyelination (intracranial cytokine storm) (Poyiadji et al., 2020). Seizures may occur at the time of COVID-19 onset as reported in a 24-year-old man with meningo-encephalitis found positive for SARS-CoV-2 in the CSF but not in nasopharyngeal swabs, or may complicate the course of the disease (Sohal and Mossammat, 2020; Moriguchi et al., 2020). Altered mental status and seizures have been also observed in COVID-19 patients showing demyelinating lesions of the brain and spinal cord resembling those observed in acute MHV infection (Zanin et al., 2020). It has also been hypothesized (but not formally proven) that SARS-CoV-2-induced acute encephalopathic damage of the brainstem respiratory centers may contribute to the respiratory failure observed in seriously affected COVID-19 patients (Li et al., 2020).
Among the acute neurological complications of SARS-CoV-2 worth mentioning is stroke, whose prevalence is highly increased in COVID-19 just as it was in SARS (Umapathi et al., 2004; Tsai et al., 2005). In a retrospective series (Mao et al., 2020), stroke occurred in about 5.7% of severely-affected COVID-19 patients, whereas its prevalence was 2.5% among 388 consecutive COVID-19 patients admitted to a University hospital in Milan (Lodigiani et al., 2020). Remarkably, stroke in COVID-19 patients may occur at a much younger age than in the general population, often in the absence of classical risk factors (Oxley et al., 2020). Many of these COVID-19-related strokes are subcortical or distal cortical, but large vessel occlusions are also observed (Mao et al., 2020; Helms et al., 2020a; Helms et al., 2020b; Helms et al., 2020c; Helms et al., 2020d). Stroke in COVID-19 patients, which may have a subacute course and a subtle clinical presentation preceded by encephalopathy (Helms et al., 2020a; Deliwala et al., 2020) or present as an acute cerebrovascular accident (Avula et al., 2020), is likely just one of the clinical consequences of the increased thrombotic risk observed in this disease. This trombophilic status depends on multiple factors including immobilization, hypoxia, diffuse endothelial damage and severe coagulopathy, and may either occur in the form of the so called COVID-19-associated coagulopathy (CAC) with increased levels of dimer, fibrin, fibrinogen, or fibrinogen degradation product, or as a classical disseminated intravascular coagulation (DIC), with decreased levels of these proteins (Klok et al., 2020a; Klok et al., 2020b). In addition, it has also been hypothesized that direct viral infection of brain endothelial cells, where the SARS-CoV-2 receptor ACE2 is expressed, may participate in the neurovascular damage of these patients (Nath, 2020). Importantly, in COVID-19 patients, stroke occurs despite pharmacological thromboprophylaxis recommended by the American Society of Hematology (Kollias et al., 2020), suggesting that anticoagulation strategies specifically tailored for COVID-19 should be developed.
SARS-CoV-2 human infection may not only induce acute neurological complications but also subacute neurological disorders reminiscent of the supposed involvement of HCoV-OC43 in ADEM. More specifically, several cases of Guillain Barrè syndrome have been described in COVID-19 patients, with neurological symptoms emerging 5–10 days after the first respiratory symptoms (Camdessanche et al., 2020; Padroni et al., 2020; Sedaghat and Karimi, 2020; Toscano et al., 2020; Virani et al., 2020). Even though the majority of these patients were seriously ill, milder forms of the Guillain-Barrè syndrome are also observed in COVID-19 (Scheidl et al., 2020). Finally, the occurrence of Miller-Fisher syndrome, a variant of the Guillain-Barrè syndrome characterized by the tryad of ataxia, areflexia, and ophthalmoplegia, has also been reported in two COVID-19 patients (Gutierrez-Ortiz et al., 2020).
6. Do CoVs play any role in chronic human neurodegenerative diseases?
The profile of CoVs as neurovirulent agents is not only that of rapid-acting killers, but also of slow-acting saboteurs. As previously mentioned, CoVs may remain in the CNS of experimental animals for a very long time after an acute infection, possibly inducing slowly progressing tissue damage through immunological mechanisms. The histopathologic and pathogenetic similarities between experimental CoV infections in rodents and human demyelinating diseases such as MS have already been highlighted (Nagashima et al., 1978a; Nagashima et al., 1978b; Jacomy et al., 2006). One of the first evidence supporting CoVs role in the pathogenesis of MS comes from the electron microscopic identification of donut-shaped CoV-like particles in the active brain lesions of a young woman deceased with MS (Tanaka et al., 1976). A few years later, two CoVs similar to HCoV-OC43 (named SK and SD) were isolated from autoptic cerebral samples of two MS patients (Burks et al., 1980). These two viruses induced encephalitis with demyelination when intracerebrally injected in non-human primates (Murray et al., 1992a). However, the etiological role for the SK and SD viruses in MS in these patients has not been firmly established, and the possibility that they could represent a murine contaminant has never been excluded (Weiss, 1983). HCoV-OC43 and HCoV-229E RNA and/or antigens have also been detected in brain samples from MS patients (Cristallo et al., 1997; Murray et al., 1992b; Stewart et al., 1992). Moreover, T cells cross-reacting to myelin basic protein and human CoVs antigens were isolated from peripheral blood (Talbot et al., 1996), and intrathecal synthesis of antibodies to HCoV-OC43 and HCoV-229E was documented in MS patients (Salmi et al., 1982).
Among neurodegenerative diseases, it should be mentioned that CoVs could also take part in the pathogenesis of Parkinson's disease (PD), mainly based on the observation that basal ganglia are damaged during experimental CoV infection both in rodents and primates (Fishman et al., 1985; Kersting and Pette, 1956). However, only one human study has shown higher titers of antibodies against JHMV and HCoV-OC43 in the CSF of PD patients when compared to normal age-matched controls (Fazzini et al., 1992).
It is worth noticing that, by promoting the local release of cytokines such as IL-1 and IL-6, CoVs could actually trigger a more general pathogenic mechanism of CNS damage that is activated in many chronic neurodegenerative diseases including MS, Alzheimer, Parkinson's disease, and epilepsy, also in the absence of documented viral infections (Aarli, 2003; Rothaug et al., 1863; Erta et al., 2012). However, no direct finding supporting this hypothesis is currently available.
7. Conclusions and future perspectives
The evidence examined in the present review highlights the long-known but currently little-considered ability of CoVs to invade the CNS and cause neurological diseases. It also shows, however, that most of our knowledge of CoV neurovirulence derives from studies performed in rodents infected with MHVs or HCoV-OC43. On the other hand, the information on the new human CoVs is much more limited. Many basic questions concerning the neurobiology of these new viruses still need to be addressed in experimental animals, and major clinical points concerning CoVs infections in human patients remain to be urgently clarified for SARS-CoV-2 in light of the devastating health consequences of the ongoing COVID-19 pandemic. Reliable animal models are critical to successfully address these issues (Natoli et al., 2020). In fact, both MERS and SARS can hardly be replicated in rodents, and transgenic animals overexpressing the receptors for their causative viruses display a non-physiological distribution pattern of CoV receptors in the CNS which does not faithfully replicate the neurobiology of these viruses (McCray Jr. et al., 2007; Agrawal et al., 2015). Moreover, even in hACE2 overexpressing mice, no evidence of CNS invasion was detected upon experimental infection with SARS-CoV-2 (Bao et al., 2020); thus, better animal models of SARS-CoV-2 neuroinvasion and/or different experimental conditions for neuroinfection are also needed.
Many questions regarding the neurological consequences of COVID-19 in humans are still unanswered. For instance, it is currently unknown whether the neurological signs described in COVID-19 patients are a consequence of a direct SARS-CoV-2 CNS infection; however, the fact that SARS-CoV-2 genome has been isolated from the CSF of a COVID-19 patient (Zhou et al., 2020) suggests direct viral neuroinvasion. Further attempts to isolate SARS-CoV-2 from the CSF, and autopsies of COVID-19 victims may shed some light. In addition, knowing whether the encephalopathy occurring during the acute phase of the disease leads to long lasting sequelae such as chronic epilepsy or cognitive impairment would be critical for rigorous prognostic evaluation; however, we presently don't know whether SARS-CoV-2 establishes latent infections that could either reactivate or trigger chronic neurological disorders. Shared procedures for early identification of COVID-19 patients at high risk of neurological involvement are essential. In order to better define the extent and pathomechanism of CNS damage, special attention should be given to signs and symptoms that could suggest brainstem impairment. These may then prompt further diagnostic investigations with auditory brainstem responses, neuroimaging tools, or CFS analysis, as recently suggested (Ogier et al., 2020). Studies aiming to better characterize the immunological mechanisms responsible for disease pathogenesis could additionally provide solid conceptual basis for the rational use of immune modulators such as the anti-IFN-γ emapalumab, the IL-1 antagonist anakinra, and the two monoclonal antibodies targeting the IL-6 pathway sarilumab and tocilizumab. All these drugs, potentially representing targeted therapeutic interventions to prevent or dampen neuroinflammation in COVID-19 patients, are currently under active investigation (https://clinicaltrials.gov/ct2/show/NCT04330638; https://www.clinicaltrials.gov/ct2/show/NCT04339712;
Author contributions
M.C., G.P., and M.T. wrote the initial draft; they all read, amended, and approved the final manuscript.
Funding information
The present work was also supported by a grant from the Italian Ministry for University and Research (MIUR) (PRIN 2017ALCR7C) to MT.
Declaration of Competing Interest
M.C., G.P., and M.T. have nothing to disclose.
References
- Aarli J.A. Role of cytokines in neurological disorders. Curr. Med. Chem. 2003;10:1931–1937. doi: 10.2174/0929867033456918. [DOI] [PubMed] [Google Scholar]
- Agrawal A.S., Garron T., Tao X., Peng B.H., et al. Generation of a transgenic mouse model of middle east respiratory syndrome coronavirus infection and disease. J. Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad T., Khan M., Haroon, Musa T.H., et al. Covid-19: zoonotic aspects. Travel. Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101607. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan S.M., Tyrrell P.J., Rothwell N.J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Andries K., Pensaert M.B. Virus isolated and immunofluorescence in different organs of pigs infected with hemagglutinating encephalomyelitis virus. Am. J. Vet. Res. 1980;41:215–218. [PubMed] [Google Scholar]
- Arabi Y.M., Harthi A., Hussein J., Bouchama A., et al. Severe neurologic syndrome associated with middle east respiratory syndrome corona virus (mers-cov) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N., Cote G., Lachance C., Tardieu M., et al. Acute and persistent infection of human neural cell lines by human coronavirus oc43. J. Virol. 1999;73:3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N., Ekande S., Cote G., Lachance C., et al. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229e. J. Virol. 1999;73:3326–3337. doi: 10.1128/jvi.73.4.3326-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula A., Nalleballe K., Narula N., Sapozhnikov S., et al. Covid-19 presenting as stroke. Brain Behav. Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the covid-19 virus targeting the cns: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Bailey O.T., Pappenheimer A.M., Cheever F.S., Daniels J.B. A murine virus (jhm) causing disseminated encephalomyelitis with extensive destruction of myelin: II. Pathology. J. Exp. Med. 1949;90:195–212. doi: 10.1084/jem.90.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., et al. The pathogenicity of sars-cov-2 in hace2 transgenic mice. Nature. 2020 doi: 10.1038/s41586-020-2312-y. May 7. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- Barnett E.M., Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of cns entry for mouse hepatitis virus, strain jhm. Virology. 1993;194:185–191. doi: 10.1006/viro.1993.1248. [DOI] [PubMed] [Google Scholar]
- Barthold S.W. Olfactory neural pathway in mouse hepatitis virus nasoencephalitis. Acta Neuropathol. 1988;76:502–506. doi: 10.1007/BF00686390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S.W., Smith A.L. Viremic dissemination of mouse hepatitis virus-jhm following intranasal inoculation of mice. Arch. Virol. 1992;122:35–44. doi: 10.1007/BF01321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender S.J., Phillips J.M., Scott E.P., Weiss S.R. Murine coronavirus receptors are differentially expressed in the central nervous system and play virus strain-dependent roles in neuronal spread. J. Virol. 2010;84:11030–11044. doi: 10.1128/JVI.02688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C.C., Lane T.E., Stohlman S.A. Coronavirus infection of the central nervous system: host-virus stand-off. Nat. Rev. Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beridze M., Sanikidze T., Shakarishvili R., Intskirveli N., et al. Selected acute phase csf factors in ischemic stroke: findings and prognostic value. BMC Neurol. 2011;11:41. doi: 10.1186/1471-2377-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau C., Filliol A., Samson M., Lamontagne L. Brain invasion by mouse hepatitis virus depends on impairment of tight junctions and beta interferon production in brain microvascular endothelial cells. J. Virol. 2015;89:9896–9908. doi: 10.1128/JVI.01501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmwald K., Galvez N.M.S., Rios M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia A., Arbour N., Yong V.W., Talbot P.J. Infection of primary cultures of human neural cells by human coronaviruses 229e and oc43. J. Virol. 1997;71:800–806. doi: 10.1128/jvi.71.1.800-806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class i virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Basu R., Maulik M., Das Sarma J. Loss of cx43-mediated functional gap junction communication in meningeal fibroblasts following mouse hepatitis virus infection. Mol. Neurobiol. 2018;55:6558–6571. doi: 10.1007/s12035-017-0861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison E., Jacomy H., Desforges M., Talbot P.J. Glutamate excitotoxicity is involved in the induction of paralysis in mice after infection by a human coronavirus with a single point mutation in its spike protein. J. Virol. 2011;85:12464–12473. doi: 10.1128/JVI.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison E., Jacomy H., Desforges M., Talbot P.J. Novel treatment with neuroprotective and antiviral properties against a neuroinvasive human respiratory virus. J. Virol. 2014;88:1548–1563. doi: 10.1128/JVI.02972-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks J.S., DeVald B.L., Jankovsky L.D., Gerdes J.C. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980;209:933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- Butler N., Pewe L., Trandem K., Perlman S. Murine encephalitis caused by hcov-oc43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology. 2006;347:410–421. doi: 10.1016/j.virol.2005.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabirac G.F., Soike K.F., Butunoi C., Hoel K., et al. Coronavirus jhm omp1 pathogenesis in owl monkey cns and coronavirus infection of owl monkey cns via peripheral routes. Adv. Exp. Med. Biol. 1993;342:347–352. doi: 10.1007/978-1-4615-2996-5_53. [DOI] [PubMed] [Google Scholar]
- Cabirac G.F., Murray R.S., McLaughlin L.B., Skolnick D.M., et al. In vitro interaction of coronaviruses with primate and human brain microvascular endothelial cells. Adv. Exp. Med. Biol. 1995;380:79–88. doi: 10.1007/978-1-4615-1899-0_11. [DOI] [PubMed] [Google Scholar]
- Camdessanche J.P., Morel J., Pozzetto B., Paul S., et al. Covid-19 may induce guillain-barre syndrome. Rev. Neurol. (Paris) 2020;176:516–518. doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever F.S., Daniels J.B., et al. A murine virus (jhm) causing disseminated encephalomyelitis with extensive destruction of myelin. J. Exp. Med. 1949;90:181–210. doi: 10.1084/jem.90.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew L.J., King W.C., Kennedy A., Gallo V. Interferon-gamma inhibits cell cycle exit in differentiating oligodendrocyte progenitor cells. Glia. 2005;52:127–143. doi: 10.1002/glia.20232. [DOI] [PubMed] [Google Scholar]
- Cole J., Evans E., Mwangi M., Mar S. Acute disseminated encephalomyelitis in children: an updated review based on current diagnostic criteria. Pediatr. Neurol. 2019;100:26–34. doi: 10.1016/j.pediatrneurol.2019.06.017. [DOI] [PubMed] [Google Scholar]
- Collins A.R. In vitro detection of apoptosis in monocytes/macrophages infected with human coronavirus. Clin. Diagn. Lab. Immunol. 2002;9:1392–1395. doi: 10.1128/CDLI.9.6.1392-1395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristallo A., Gambaro F., Biamonti G., Ferrante P., et al. Human coronavirus polyadenylated rna sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol. 1997;20:105–114. [PubMed] [Google Scholar]
- de Groot R.J.B.S., Baric R., et al. Elsevier; 2012. Coronaviridae. [Google Scholar]
- de Vries E.E., van den Munckhof B., Braun K.P., van Royen-Kerkhof A., et al. Inflammatory mediators in human epilepsy: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016;63:177–190. doi: 10.1016/j.neubiorev.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Deliwala S., Abdulhamid S., Abusalih M.F., Al-Qasmi M.M., et al. Encephalopathy as the sentinel sign of a cortical stroke in a patient infected with coronavirus disease-19 (covid-19) Cureus. 2020;12 doi: 10.7759/cureus.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Miletti T.C., Gagnon M., Talbot P.J. Activation of human monocytes after infection by human coronavirus 229e. Virus Res. 2007;130:228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M.F.D., Brison É., Desjardins J., Meessen-Pinard M., Jacomy H., Talbot P.J. In: Neuroviral infections: Rna viruses and retroviruses. Ruzek S.S., editor. CRC Press; Boca Raton, Florida: 2013. Human coronavirus: Respiratory pathogens revisited as infectious neuroinvasive, neurotropic, and neurovirulent agents. [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Vezzani A., Najjar S., De Lanerolle N.C., et al. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Dick G.W., Niven J.S., Gledhill A.W. A virus related to that causing hepatitis in mice (mhv) Br. J. Exp. Pathol. 1956;37:90–98. [PMC free article] [PubMed] [Google Scholar]
- Dube M., Le Coupanec A., Wong A.H.M., Rini J.M., et al. Axonal transport enables neuron-to-neuron propagation of human coronavirus oc43. J. Virol. 2018;92:e00404–e00418. doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour J.H., Dziejman M., Liu M.T., Leung J.H., et al. Ifn-gamma-inducible protein 10 (ip-10; cxcl10)-deficient mice reveal a role for ip-10 in effector t cell generation and trafficking. J. Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Edwards J.A., Denis F., Talbot P.J. Activation of glial cells by human coronavirus oc43 infection. J. Neuroimmunol. 2000;108:73–81. doi: 10.1016/S0165-5728(00)00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau D.J., Desforges M., St-Jean J.R., Talbot P.J. A human coronavirus oc43 variant harboring persistence-associated mutations in the s glycoprotein differentially induces the unfolded protein response in human neurons as compared to wild-type virus. Virology. 2009;395:255–267. doi: 10.1016/j.virol.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzini E., Fleming J., Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with parkinson’s disease. Mov. Disord. 1992;7:153–158. doi: 10.1002/mds.870070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (covid-19): encephalopathy. Cureus. 2020;12 doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P.S., Gass J.S., Swoveland P.T., Lavi E., et al. Infection of the basal ganglia by a murine coronavirus. Science. 1985;229:877–879. doi: 10.1126/science.2992088. [DOI] [PubMed] [Google Scholar]
- Fleming J.O., Houtman J.J., Alaca H., Hinze H.C., et al. Persistence of viral rna in the central nervous system of mice inoculated with mhv-4. Adv. Exp. Med. Biol. 1993;342:327–332. doi: 10.1007/978-1-4615-2996-5_50. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Hickey M.J., Hardison J.L., Liu M.T., et al. Antibody targeting of the cc chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J. Immunol. 2004;172:4018–4025. doi: 10.4049/jimmunol.172.7.4018. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Subbarao K., Murphy B., Murphy P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (sars-cov) pulmonary infection of mice. J. Immunol. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Muller M.A., Allen P., et al. Evidence that tmprss2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind C., Havaux N., Holmes K.V., Coutelier J.P. Role of virus receptor-bearing endothelial cells of the blood-brain barrier in preventing the spread of mouse hepatitis virus-a59 into the central nervous system. J. Neuro-Oncol. 1997;3:428–434. doi: 10.3109/13550289709031188. [DOI] [PubMed] [Google Scholar]
- Grzybicki D.M., Kwack K.B., Perlman S., Murphy S.P. Nitric oxide synthase type ii expression by different cell types in mhv-jhm encephalitis suggests distinct roles for nitric oxide in acute versus persistent virus infection. J. Neuroimmunol. 1997;73:15–27. doi: 10.1016/S0165-5728(96)00159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., et al. Multiple organ infection and the pathogenesis of sars. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Ortiz C., Mendez A., Rodrigo-Rey S., San Pedro-Murillo E., et al. Miller fisher syndrome and polyneuritis cranialis in covid-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009619. In press. [DOI] [PubMed] [Google Scholar]
- Han H.J., Yu H., Yu X.J. Evidence for zoonotic origins of middle east respiratory syndrome coronavirus. J. Gen. Virol. 2016;97:274–280. doi: 10.1099/jgv.0.000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., et al. Neurologic features in severe sars-cov-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Meziani F. More on neurologic features in severe sars-cov-2 infection. Reply. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2015132. [DOI] [PubMed] [Google Scholar]
- Helms J., Severac F., Merdji H., Angles-Cano E., et al. Prothrombotic phenotype in covid-19 severe patients. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06082-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., et al. High risk of thrombosis in patients with severe sars-cov-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., et al. Sars-cov-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. (e278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman J.J., Fleming J.O. Pathogenesis of mouse hepatitis virus-induced demyelination. J. Neuro-Oncol. 1996;2:361–376. doi: 10.3109/13550289609146902. [DOI] [PubMed] [Google Scholar]
- Jacomy H., Talbot P.J. Susceptibility of murine cns to oc43 infection. Adv. Exp. Med. Biol. 2001;494:101–107. doi: 10.1007/978-1-4615-1325-4_16. [DOI] [PubMed] [Google Scholar]
- Jacomy H., Fragoso G., Almazan G., Mushynski W.E., et al. Human coronavirus oc43 infection induces chronic encephalitis leading to disabilities in balb/c mice. Virology. 2006;349:335–346. doi: 10.1016/j.virol.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., et al. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- Jose R.J., Manuel A. Covid-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8 doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Grun J.L., Lublin F.D., Knobler R.L. Interleukin-6 induction in vitro in mouse brain endothelial cells and astrocytes by exposure to mouse hepatitis virus (mhv-4, jhm) J. Neuroimmunol. 1993;42:47–52. doi: 10.1016/0165-5728(93)90211-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Kim R., Siebert K., Lublin F.D., et al. Organ specific endothelial cell heterogeneity influences differential replication and cytopathogenicity of mhv-3 and mhv-4. Implications in viral tropism. Adv. Exp. Med. Biol. 1995;380:43–50. doi: 10.1007/978-1-4615-1899-0_6. [DOI] [PubMed] [Google Scholar]
- Kersting G., Pette E. Histopathology and pathogenesis of experimental jhm-virus encephalomyelitis in apes. Dtsch. Z. Nervenheilkd. 1956;174:283–304. [PubMed] [Google Scholar]
- Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., et al. Incidence of thrombotic complications in critically ill icu patients with covid-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill icu patients with covid-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R.L., Lampert P.W., Oldstone M.B. Virus persistence and recurring demyelination produced by a temperature-sensitive mutant of mhv-4. Nature. 1982;298:279–280. doi: 10.1038/298279a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., et al. Thromboembolic risk and anticoagulant therapy in covid-19 patients: emerging evidence and call for action. Br. J. Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothur K., Wienholt L., Mohammad S.S., Tantsis E.M., et al. Utility of csf cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating, anti-nmdar and enteroviral encephalitis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu O.O., Hogue I.B., Enquist L.W. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T.E., Liu M.T., Chen B.P., Asensio V.C., et al. A central role for cd4(+) t cells and rantes in virus-induced central nervous system inflammation and demyelination. J. Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.K., Yu W.C., Chu C.M., Lau S.T., et al. Possible central nervous system infection by sars coronavirus. Emerg. Infect. Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Gilden D.H., Highkin M.K., Weiss S.R. Persistence of mouse hepatitis virus a59 rna in a slow virus demyelinating infection in mice as detected by in situ hybridization. J. Virol. 1984;51:563–566. doi: 10.1128/jvi.51.2.563-566.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Fishman P.S., Highkin M.K., Weiss S.R. Limbic encephalitis after inhalation of a murine coronavirus. Lab. Investig. 1988;58:31–36. [PubMed] [Google Scholar]
- Lechien J.R., Chiesa‑Estomba C.M., De Siati D.R., Horol M., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‑to‑moderate forms of the coronavirus disease (COVID‑19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., et al. Angiotensin-converting enzyme 2 is a functional receptor for the sars coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu L., Gonzales D.M., Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J. Virol. 2004;78:3398–3406. doi: 10.1128/JVI.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li H., Fan R., Wen B., et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of sars-cov2 may play a role in the respiratory failure of covid-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.T., Hinton D.R., Marten N.W., Bergmann C.C., et al. Antibody prevents virus reactivation within the central nervous system. J. Immunol. 1999;162:7358–7368. [PubMed] [Google Scholar]
- Liu Z., Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang X. Persistent coronavirus infection of progenitor oligodendrocytes. Adv. Exp. Med. Biol. 2006;581:379–384. doi: 10.1007/978-0-387-33012-9_67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang X. Murine coronavirus-induced oligodendrocyte apoptosis is mediated through the activation of the fas signaling pathway. Virology. 2007;360:364–375. doi: 10.1016/j.virol.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.T., Keirstead H.S., Lane T.E. Neutralization of the chemokine cxcl10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J. Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- Liu Y., Pu Y., Zhang X. Role of the mitochondrial signaling pathway in murine coronavirus-induced oligodendrocyte apoptosis. J. Virol. 2006;80:395–403. doi: 10.1128/JVI.80.1.395-403.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., et al. Venous and arterial thromboembolic complications in covid-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang J., Wang Y., Yang G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Mao L WM, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Li Y, Jin H, Hu B (2020). Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study. Neurological Manifestations of Hospitalized Patients With covid-19 in Wuhan, China: A Retrospective Case Series Study. 2020;Apr 10;e201127.
- Marten N.W., Stohlman S.A., Bergmann C.C. Role of viral persistence in retaining cd8(+) t cells within the central nervous system. J. Virol. 2000;74:7903–7910. doi: 10.1128/jvi.74.17.7903-7910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Park C.H., Sunden Y., Kimura T., et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet. Pathol. 2004;41:101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., et al. Lethal infection of k18-hace2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Becker W.B., Chanock R.M. Growth in suckling-mouse brain of “ibv-like” viruses from patients with upper respiratory tract disease. Proc. Natl. Acad. Sci. U. S. A. 1967;58:2268–2273. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meessen-Pinard M., Le Coupanec A., Desforges M., Talbot P.J. Pivotal role of receptor-interacting protein kinase 1 and mixed lineage kinase domain-like in neuronal cell death induced by the human neuroinvasive coronavirus oc43. J. Virol. 2016;91:e01513–e01516. doi: 10.1128/JVI.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesel-Lemoine M., Millet J., Vidalain P.O., Law H., et al. A human coronavirus responsible for the common cold massively kills dendritic cells but not monocytes. J. Virol. 2012;86:7577–7587. doi: 10.1128/JVI.00269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfopoulou S., Brown J.R., Davies E.G., Anderson G., et al. Human coronavirus oc43 associated with fatal encephalitis. N. Engl. J. Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., et al. A first case of meningitis/encephalitis associated with sars-coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.S., Cai G.Y., Hoel K., Zhang J.Y., et al. Coronavirus infects and causes demyelination in primate central nervous system. Virology. 1992;188:274–284. doi: 10.1016/0042-6822(92)90757-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.S., Brown B., Brian D., Cabirac G.F. Detection of coronavirus rna and antigen in multiple sclerosis brain. Ann. Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.S., Cai G.Y., Soike K.F., Cabirac G.F. Further observations on coronavirus infection of primate cns. J. Neuro-Oncol. 1997;3:71–75. doi: 10.3109/13550289709015795. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Wege H., ter Meulen V. Early and late cns-effects of corona virus infection in rats. Adv. Exp. Med. Biol. 1978;100:395–409. doi: 10.1007/978-1-4684-2514-7_28. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978;44:63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Neurologic complications of coronavirus infections. Neurology. 2020;94:809–810. doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- Natoli S., Oliveira V., Calabresi P., Maia L.F., et al. Does sars-cov-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020 doi: 10.1111/ene.14277. Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ace2. J. Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A., Edner N., Albert J., Ternhag A. Fatal encephalitis associated with coronavirus oc43 in an immunocompromised child. Infect. Dis. (Lond.) 2020;52:419–422. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- Niu J., Shen L., Huang B., Ye F., et al. Non-invasive bioluminescence imaging of hcov-oc43 infection and therapy in the central nervous system of live mice. Antivir. Res. 2020;173:104646. doi: 10.1016/j.antiviral.2019.104646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier M., Andeol G., Sagui E., Bo G.D. How to detect and track chronic neurologic sequelae of covid-19? Use of auditory brainstem responses and neuroimaging for long-term patient follow-up. Brain Behav. Immun. Health. 2020;100081 doi: 10.1016/j.bbih.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., et al. Guillain-barre syndrome following covid-19: New infection, old complication? J. Neurol. 2020;267:1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A.M. Pathology of infection with the jhm virus. J. Natl. Cancer Inst. 1958;20:879–891. [PubMed] [Google Scholar]
- Perlman S., Jacobsen G., Moore S. Regional localization of virus in the central nervous system of mice persistently infected with murine coronavirus jhm. Virology. 1988;166:328–338. doi: 10.1016/0042-6822(88)90503-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Jacobsen G., Afifi A. Spread of a neurotropic murine coronavirus into the cns via the trigeminal and olfactory nerves. Virology. 1989;170:556–560. doi: 10.1016/0042-6822(89)90446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl D., Alper G., Van Haren K., Kornberg A.J., et al. Acute disseminated encephalomyelitis: updates on an inflammatory cns syndrome. Neurology. 2016;87:S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., et al. Covid-19-associated acute hemorrhagic necrotizing encephalopathy: Ct and mri features. Radiology. 2020;201187 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-emc. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna C., Bergmann C.C., Atkinson R., Stohlman S.A. Control of central nervous system viral persistence by neutralizing antibody. J. Virol. 2003;77:4670–4678. doi: 10.1128/JVI.77.8.4670-4678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T., Kostoula C., Vezzani A. Immunity activation in brain cells in epilepsy: mechanistic insights and pathological consequences. Neuropediatrics. 2013;44:330–335. doi: 10.1055/s-0033-1358601. [DOI] [PubMed] [Google Scholar]
- Rempel J.D., Murray S.J., Meisner J., Buchmeier M.J. Differential regulation of innate and adaptive immune responses in viral encephalitis. Virology. 2004;318:381–392. doi: 10.1016/j.virol.2003.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riski H., Hovi T. Coronavirus infections of man associated with diseases other than the common cold. J. Med. Virol. 1980;6:259–265. doi: 10.1002/jmv.1890060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodney T., Osier N., Gill J. Pro- and anti-inflammatory biomarkers and traumatic brain injury outcomes: a review. Cytokine. 2018;110:248–256. doi: 10.1016/j.cyto.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Rothaug M., Becker-Pauly C., Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta. 1863;2016:1218–1227. doi: 10.1016/j.bbamcr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Salmi A., Ziola B., Hovi T., Reunanen M. Antibodies to coronaviruses oc43 and 229e in multiple sclerosis patients. Neurology. 1982;32:292–295. doi: 10.1212/wnl.32.3.292. [DOI] [PubMed] [Google Scholar]
- Savarin C., Bergmann C.C. Fine tuning the cytokine storm by ifn and il-10 following neurotropic coronavirus encephalomyelitis. Front. Immunol. 2018;9:3022. doi: 10.3389/fimmu.2018.03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Hinton D.R., Valentin-Torres A., Chen Z., et al. Astrocyte response to ifn-gamma limits il-6-mediated microglia activation and progressive autoimmune encephalomyelitis. J. Neuroinflammation. 2015;12:79. doi: 10.1186/s12974-015-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Dutta R., Bergmann C.C. Distinct gene profiles of bone marrow-derived macrophages and microglia during neurotropic coronavirus-induced demyelination. Front. Immunol. 2018;9:1325. doi: 10.3389/fimmu.2018.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barre syndrome during sars-cov-2 pandemic: a case report and review of recent literature. J. Peripher. Nerv. Syst. 2020;25:204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat Z., Karimi N. Guillain Barre syndrome associated with covid-19 infection: a case report. J. Clin. Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T., Rockx B., Donaldson E., Sims A., et al. Mechanisms of zoonotic severe acute respiratory syndrome coronavirus host range expansion in human airway epithelium. J. Virol. 2008;82:2274–2285. doi: 10.1128/JVI.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal S., Mossammat M. Covid-19 presenting with seizures. IDCases. 2020 doi: 10.1016/j.idcr.2020.e00782. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Dales S. In vivo and in vitro models of demyelinating disease: Jhm virus in the rat central nervous system localized by in situ cdna hybridization and immunofluorescent microscopy. J. Virol. 1985;56:434–438. doi: 10.1128/jvi.56.2.434-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.N., Mounir S., Talbot P.J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191:502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S.A., Weiner L.P. Stability of neurotropic mouse hepatitis virus (jhm strain) during chronic infection of neuroblastoma cells. Arch. Virol. 1978;57:53–61. doi: 10.1007/BF01315637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., Roberts A. Is there an ideal animal model for sars? Trends Microbiol. 2006;14:299–303. doi: 10.1016/j.tim.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Grzybicki D., Castro R.F., Murphy S., et al. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology. 1995;213:482–493. doi: 10.1006/viro.1995.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P.A., 2nd, McGavern D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P.J., Paquette J.S., Ciurli C., Antel J.P., et al. Myelin basic protein and human coronavirus 229e cross-reactive t cells in multiple sclerosis. Ann. Neurol. 1996;39:233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Iwasaki Y., Koprowski H. Intracisternal virus-like particles in brain of a multiple sclerosis patient. J. Neurol. Sci. 1976;28:121–126. doi: 10.1016/0022-510X(76)90053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Garron T., Agrawal A.S., Algaissi A., et al. Characterization and demonstration of the value of a lethal mouse model of middle east respiratory syndrome coronavirus infection and disease. J. Virol. 2016;90:57–67. doi: 10.1128/JVI.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirotta E., Ransohoff R.M., Lane T.E. Cxcr2 signaling protects oligodendrocyte progenitor cells from ifn-gamma/cxcl10-mediated apoptosis. Glia. 2011;59:1518–1528. doi: 10.1002/glia.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidpour A., Morgun A.V., Boitsova E.B., Malinovskaya N.A., et al. Neuroinflammation and infection: molecular mechanisms associated with dysfunction of neurovascular unit. Front. Cell. Infect. Microbiol. 2017;7:276. doi: 10.3389/fcimb.2017.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., et al. Guillain-barre syndrome associated with sars-cov-2. N. Engl. J. Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.K., Hsieh S.T., Chang Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwanica. 2005;14:113–119. [PubMed] [Google Scholar]
- Umapathi T., Kor A.C., Venketasubramanian N., Lim C.C., et al. Large artery ischaemic stroke in severe acute respiratory syndrome (sars) J. Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L. Human coronaviruses: what do they cause? Antivir. Ther. 2007;12:651–658. [PubMed] [Google Scholar]
- Verkhratsky A., Ho M.S., Vardjan N., Zorec R., et al. General pathophysiology of astroglia. Adv. Exp. Med. Biol. 2019;1175:149–179. doi: 10.1007/978-981-13-9913-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A., Moneta D., Richichi C., Aliprandi M., et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl. 5):30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A., Ravizza T., Balosso S., Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49(Suppl. 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A., Balosso S., Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- Vijay R., Fehr A.R., Janowski A.M., Athmer J., et al. Virus-induced inflammasome activation is suppressed by prostaglandin d2/dp1 signaling. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E5444–E5453. doi: 10.1073/pnas.1704099114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani A., Rabold E., Hanson T., Haag A., et al. Guillain-barre syndrome associated with sars-cov-2 infection. IDCases. 2020 doi: 10.1016/j.idcr.2020.e00771. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J.L., Dayan A.D., Allison A.C. Neuropathological effects of persistent infection of mice by mouse hepatitis virus. Infect. Immun. 1975;12:1127–1140. doi: 10.1128/iai.12.5.1127-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waje-Andreassen U., Krakenes J., Ulvestad E., Thomassen L., et al. Il-6: an early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., et al. Structure, function, and antigenicity of the sars-cov-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger J.G., Marro B.S., Hosking M.P., Lane T.E. The chemokine receptor CXCR2 and coronavirus-induced neurologic disease. Virology. 2013;435:110–117. doi: 10.1016/j.virol.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R. Coronaviruses sd and sk share extensive nucleotide homology with murine coronavirus mhv-a59, more than that shared between human and murine coronaviruses. Virology. 1983;126:669–677. doi: 10.1016/S0042-6822(83)80022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh P., Barber M., Langhorne P., Rumley A., et al. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc. Dis. 2009;27:247–253. doi: 10.1159/000196823. [DOI] [PubMed] [Google Scholar]
- West P.K., Viengkhou B., Campbell I.L., Hofer M.J. Microglia responses to interleukin-6 and type i interferons in neuroinflammatory disease. Glia. 2019;67:1821–1841. doi: 10.1002/glia.23634. [DOI] [PubMed] [Google Scholar]
- Wheeler D.L., Sariol A., Meyerholz D.K., Perlman S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Invest. 2018;128:931–943. doi: 10.1172/JCI97229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.K., Jiang G.S., Snyder S.W., Frana M.F., et al. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (mhv)-a59 from mouse liver and identification of a nonfunctional, homologous protein in mhv-resistant sjl/j mice. J. Virol. 1990;64:3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.K., Jiang G.S., Holmes K.V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.F., Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J. Virol. 1999;73:8771–8780. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., Li L., et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Yamate M., Li G.M., Ikuta K. Susceptibility of human and rat neural cell lines to infection by sars-coronavirus. Biochem. Biophys. Res. Commun. 2005;334:79–85. doi: 10.1016/j.bbrc.2005.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M., Nishimura H., Deng X., Kikuchi A., et al. Protease inhibitors: candidate drugs to inhibit severe acute respiratory syndrome coronavirus 2 replication. Tohoku J. Exp. Med. 2020;251:27–30. doi: 10.1620/tjem.251.27. [DOI] [PubMed] [Google Scholar]
- Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., et al. Human aminopeptidase n is a receptor for human coronavirus 229e. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E.A., Collins A., Cohen M.E., Duffner P.K., et al. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., Renisi G., et al. Sars-cov-2 can induce brain and spine demyelinating lesions. Acta Neurochir. (Wien.) 2020:1–4. doi: 10.1007/s00701-020-04374-x. May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba J., Losy J. Early tnf-alpha levels correlate with ischaemic stroke severity. Acta Neurol. Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- Zhou J., Marten N.W., Bergmann C.C., Macklin W.B., et al. Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis. J. Virol. 2005;79:4764–4773. doi: 10.1128/JVI.79.8.4764-4773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Zhang M., Wang J., Gao J. Sars-cov-2: underestimated damage to nervous system. Travel Med. Infect. Dis. 2020;101642 doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]