Abstract
Purpose
The clinic efficiency and cost savings achieved by eliminating formal visual acuity (VA) and dilated fundus examinations (DFEs) were assessed for established patients receiving optical coherence tomography (OCT)–guided intravitreal injections.
Design
Comparative cost analysis.
Methods
Two different treatment models were evaluated. The first model included patients undergoing routine VA assessment, DFEs, OCT imaging, and intravitreal injections. The second model eliminated the routine VA assessment and DFE while using OCT imaging through an undilated pupil followed by the intravitreal injection. The 2 models incorporated both bevacizumab and aflibercept. The number of patients per clinic day, the cost per visit, and the daily revenues were compared between the 2 models.
Results
Optimized schedules with and without VA assessments and DFEs allowed for 48 and 96 patients to be injected per day, respectively. Excluding drug costs, the cost per encounter for the visits with and without a DFE were $39.33 and $22.63, respectively. Including the drug costs, the costs per encounter for the visits with and without a DFE were $85.55 and $68.85 for bevacizumab and $1787.58 and $17770.88 for aflibercept, respectively. Once the reimbursements for each visit type were included, the clinics that eliminated the VA and DFEs were more cost efficient.
Conclusion
Eliminating both VA assessments and DFEs for patients undergoing OCT-guided retreatment with intravitreal injections resulted in decreased exposure times between patients and clinic staff, decreased cost per encounter, and increased patient volumes per clinic day, resulting in improved clinic efficiency and safety while seeing more patients in a clinic day.
Intravitreal injections of vascular endothelial growth factor (VEGF) inhibitors have become the standard of care for the treatment of exudative age-related macular degeneration (eAMD), diabetic macular edema, and macular edema caused by retinal vein occlusions.1, 2, 3, 4 In clinical trials and clinical practice using anti-VEGF therapies for eAMD, patients routinely undergo visual acuity (VA) assessment, a dilated fundus examination (DFE), and optical coherence tomography (OCT) imaging before the intravitreal injection.5, 6, 7, 8, 9, 10, 11, 12 While formal clinical trials have used fixed-interval injection schedules to obtain regulatory approval, OCT-guided therapy has become the most widely adopted strategy for routine clinical care of established patients undergoing retreatment with anti-VEGF therapy.13, 14, 15, 16, 17 OCT-guided therapy usually refers to 2 different strategies that use the results of OCT imaging to determine retreatment interval. In the pro re nata (PRN) or treat-and-observe dosing strategy, patients receive fixed interval dosing until the macula is fluid-free and then the patient returns at a fixed interval, usually monthly, and retreatment is withheld until macular fluid recurs.9 , 10 , 18 In another popular strategy known as treat-and-extend (TAE) dosing,19, 20, 21 the patient receives an injection even when the macula is fluid-free and the follow-up interval is extended, usually by 2-week increments, and injections are given at all follow-up visits. If the macula remains fluid-free, then the interval between visits is extended and the process repeats itself at each follow-up visit. If fluid recurs, then a treatment is given and the follow-up interval is decreased, usually by 2 weeks. While there are many variations on the PRN and TAE treatment regimens with respect to the increase and decrease in visit intervals, most clinicians continue to perform VA assessment, a DFE, and OCT imaging at each visit although most decisions on retreatment are based solely on the OCT images.17
Whether VA assessments and DFEs are really needed to decide when to retreat was partially addressed by the HARBOR trial,10 which compared ranibizumab 0.5 mg to 2.0 mg and also compared monthly dosing with monthly PRN dosing. Of note, for the majority of PRN visits, only the OCT images and not the VA assessments or DFE were used to determine retreatment. In this study, there were no significant differences in the treatment outcomes when the doses and the treatment regimens were compared over 2 years.10
The major argument for using the DFE in deciding when to retreat rests on the belief that the presence of a small macular hemorrhage may be missed even if there was no evidence of macular fluid on OCT imaging, and if not treated or the interval extended after treatment then the patient may lose significant vision because of an enlarging hemorrhage by the next visit. To test this possibility, Patel and associates22 performed a post hoc analysis of the HARBOR trial to assess the outcomes in patients when retreatment was not offered because of the absence of fluid detected on OCT imaging even when small hemorrhages were present on color fundus images after the initial 3 monthly injections. Of note, they found no evidence that the missed hemorrhages impacted VA outcomes after 2 years, which suggests that OCT imaging could serve as the sole basis for deciding when to retreat.
Currently, the number of patients that require intravitreal anti-VEGF injections for routine care are overwhelming clinical practices.23, 24, 25, 26 In an effort to streamline office encounters and decrease the treatment burden for both patients and clinicians, injection-only encounters have been adopted for certain patients who have established a defined retreatment interval.27 , 28 In the injection-only model, the patients bypass the standard VA assessment, DFE, and OCT imaging and only receive an intravitreal injection. The goal of such an injection-only clinic is to provide treatment, adequately maintain a dry macula, decrease the visit duration, and allow the clinician to see a higher number of patients per clinic day. However, this strategy is only possible once the fixed treatment interval has been established for the individual patient or the clinician elects to manage patients using a fixed-interval dosing schedule as in the clinical trials and as approved by regulatory agencies.
Another strategy would be to eliminate the formal VA assessment and DFE and to simply perform OCT imaging through an undilated pupil. The OCT findings would allow the clinician using either the PRN or TAE regimen to shorten, maintain, or extend the retreatment interval. By using such a clinic strategy, clinicians might then perform a VA assessment and DFE just once or twice a year or when requested by the patient. When determining management solely on the OCT images obtained through an undilated pupil, the duration of the clinic visit for each patient could be shortened and more patients could be injected during a given clinic day. Given the pandemic involving the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the added benefit of eliminating a formal VA test and DFE and shortening the duration of the clinic visit would be to reduce the risk of potential virus transmission and enhance the safety for patients, office staff, and clinicians.
To assess the clinic efficiency and cost savings by eliminating a formal VA assessment and DFE, we compared 2 different clinic schedules: 1) the standard encounter in which patients undergo VA assessment, a DFE, and OCT imaging before an intravitreal injection and 2) an encounter that eliminated both the formal VA assessment and DFE with OCT imaging performed through an undilated pupil before receiving an intravitreal injection.
Methods
The models used for this analysis are based on treating patients with eAMD. An optimized schedule was created for an entire clinic day using a VA and DFE model and an OCT-only model. The models only included patients being treated with bevacizumab or aflibercept injections and managed using a TAE strategy. Each schedule was based on clinic operations at a nonhospital, provider-based practice in South Florida, although the models are generally applicable to any clinical practice regardless of the time intervals needed to perform each step in the clinic visit. The schedules were optimized by maximizing the number of vision examiners, OCT technicians, and nurses or injection technicians needed so that 1 retinal specialist could treat the maximum number of patients per day without introducing any wait times for the clinician or the staff. Start times for staff were staggered to ensure a defined beginning and end to the clinic day. The clinician and staff are made available from 7:00 AM to 4:30 PM for 8 working hours with a 1-hour break for lunch.
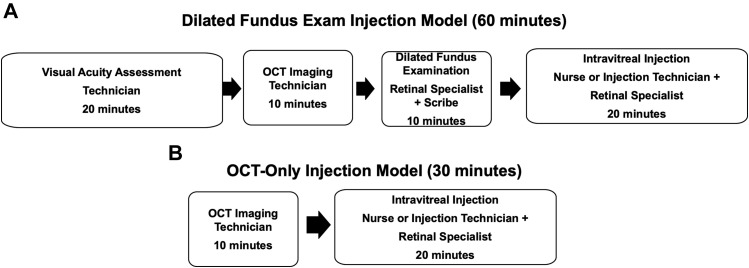
In the typical encounter with a DFE (Figure 1 ), a follow-up patient would be evaluated by a vision examiner responsible for documenting subjective complaints, medical history, intraocular pressure (IOP) measurements, and VA, followed by the instillation of dilating drops. This vision examiner, who will also be cross-trained as an OCT technician, would next perform OCT imaging. The retinal specialist then performs an intermediate evaluation that includes a dilated fundus examination, interpretation of the OCT images, and discusses follow-up plans with the patient (eye code 92012). A scribe is also present during the retinal specialist's examination. After the physician encounter, a nurse or injection technician prepares the patient for injection. The clinician performs an intravitreal injection of an anti-VEGF drug and then moves on to the next patient while the nurse cares for the patient postinjection. As shown in Figure 1, the time allocated is 20 minutes for the history documentation and VA examination, 10 minutes for OCT imaging, 10 minutes for the retinal specialist's examination and OCT interpretation, and 20 minutes for entire intravitreal injection procedure. The time allocation for retinal specialist to deliver the injection is included in the time allocated for injection. For a typical DFE encounter, a total of 60 minutes is allocated.
Figure 1.
Intravitreal injection visits with optical coherence tomography (OCT) imaging with or without a visual acuity (VA) assessment and a dilated fundus examination (DFE). (A) Components and times associated with an injection visit model with a VA assessment, OCT scan, and a DFE. (B) Components and times associated with an injection visit model with an OCT scan but without a VA assessment or a DFE.
In an OCT-only encounter (Figure 1), patients are asked if they have experienced any vision changes or patients might self-administer a VA test while in the waiting room. The OCT technician then performs OCT imaging through an undilated pupil. The retinal specialist then remotely reviews the OCT images during the time available between injections. The clinician does not interact with the patient until the injection is performed. No scribe is needed. A nurse or technician then performs an IOP measurement and prepares the patient's eye for injection. The clinician performs an intravitreal injection of an anti-VEGF drug and informs the patient when the next visit and injection would be scheduled based on the results from the OCT images. As seen in Figure 1, the time allocated for both OCT imaging and injection remain at 10 minutes and 20 minutes, respectively. For a typical OCT-only encounter, a total of 30 minutes is allocated.
The cost for a single patient encounter was calculated based on the personnel costs and the cost of medication in South Florida. The personnel costs include the vision examiner, OCT technician, nurse, and scribe, and these costs were derived from the average salary plus 40% fringe benefits from an outpatient medical clinic. The cost per patient encounter for each staff member was calculated based on this hourly wage, the time each staff member spends per patient, and the cost of the anti-VEGF drugs. The costs for the retinal specialist, front desk staff, equipment, and facility fees were not included because they remain constant regardless of the number of patients seen or the clinic model used. The reimbursements for each procedure were based on the Centers for Medicaid and Medicare Services reimbursement rates for South Florida and the economic outcomes from the 2 models were compared.
Results
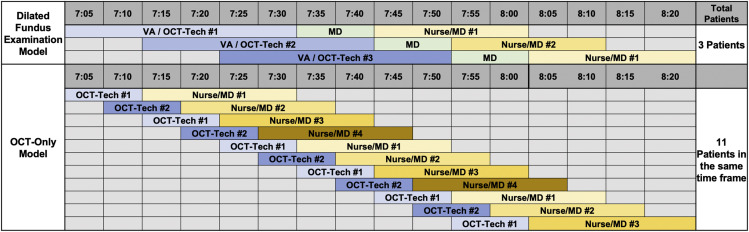
Figure 2 shows a portion of the optimized clinical schedule comparing the 2 models for the first 80 minutes of the clinic day. It can be seen that in the full DFE model, fewer patients (n = 3) can be injected compared with the OCT-only model. In both models, the staff and retinal specialist experience no wait times between patients. The optimal model with a DFE was found to require 3 vision examiners and 2 nurses or injection technicians (Table 1 ). Such a staff size would require 3 OCT instruments, 2 examination rooms, and 3 injection rooms. This model yields a maximum of 48 patients injected per day. The optimized OCT-only model would require 2 OCT technicians and 4 nurses or injection technicians (Table 1). Such a staff size would require 2 OCT instruments and 4 injection rooms. This would yield a maximum of 96 patients injected per day (Table 1).
Figure 2.
Number of patients injected using 2 different clinic models over 80 minutes. Over the first 80 minutes of clinic, a model in which visual acuity (VA) assessments and dilated fundus examinations (DFEs) were performed would result in 3 patients being injected while a clinic model that eliminated the VA assessment and DFE would result in 11 patients being injected.
Table 1.
Optimized Clinic Schedules With and Without Visual Acuity Assessments and Dilated Fundus Examinations
| Optimized Clinic Schedule | Vision Examiners/OCT Technicians, n | Nurses, n | Scribes, n | Patients Seen Per Day, n |
|---|---|---|---|---|
| Model with VA assessment and DFE | 3 | 2 | 1 | 48 |
| OCT-only model | 2 | 4 | 0 | 96 |
DFE = dilated fundus examination; OCT = optical coherence tomography; VA = visual acuity.
The cost analysis was based on the hourly salary plus fringe benefits for each staff member (Table 2 ). In the clinic models, the cost per team member per encounter was $11.33 for the vision examiner, $5.83 for the OCT technician, $5.37 for the scribe, and $16.80 for the nurse. Each dose of bevacizumab or aflibercept incurs a cost of $46.22 and $1748.25, respectively, and these amounts were added to the total encounter cost (Table 3 ).
Table 2.
Cost Per Patient Based on Utilization of Clinic Staff
| Staff Member | Hourly Salary | 40% Fringe Benefits | Minutes Per Patient Encounter | Staff Member Cost Per Patient Encounter |
|---|---|---|---|---|
| VA examiner | $25.00 | $8.98 | 20 | $11.33 |
| OCT technician | $25.00 | $10.00 | 10 | $5.83 |
| Scribe | $23.00 | $9.20 | 10 | $5.37 |
| Nurse | $36.00 | $14.40 | 20 | $16.80 |
OCT = optical coherence tomography; VA = visual acuity.
Table 3.
Total Costs Per Encounter Including Drug Cost With and Without Visual Acuity Assessment and Dilated Fundus Examination
| Component | Model with VA and DFE | OCT-Only Model |
|---|---|---|
| VA examiner (20-minute VA assessment) | $11.33 | — |
| OCT technician (10-minute OCT imaging) | $5.83 | $5.83 |
| Scribe (10-minute examination) | $5.37 | $0.00 |
| Nurse/injection technician (20-minute injection) | $16.80 | $16.80 |
| Drug cost | Bevacizumab |
Aflibercept |
Bevacizumab |
Aflibercept |
|---|---|---|---|---|
| $46.22 | $1748.25 | $46.22 | $1748.25 | |
| Total | $85.55 | $1787.58 | $68.85 | $1770.88 |
DFE = dilated fundus examination; OCT = optical coherence tomography; VA = visual acuity.
The total reimbursement from each patient encounter was calculated based on Medicare reimbursements in South Florida (Table 4 ). The OCT with interpretation code 92133 was reimbursed at $38.20. The physician examination under the established intermediate eye code 92012 was reimbursed at $90.39. The reimbursement for injection procedure under the CPT code 67028 was $106.53. Reimbursement for each dose of bevacizumab or aflibercept were $49.20 and $1889.66, respectively.
Table 4.
Total Reimbursements Per Encounter Including the Drug With and Without Visual Acuity Assessment and Dilated Fundus Examination
| Event | Model With VA and DFE | OCT-Only Model |
|---|---|---|
| Physician examination (eye code 92012) | $90.39 | — |
| OCT interpretation (CPT code 92133) | $38.20 | $38.20 |
| Injection procedure (CPT code 67028) | $106.53 | $106.53 |
| Drug reimbursement | Bevacizumab |
Aflibercept |
Bevacizumab |
Aflibercept |
|---|---|---|---|---|
| $49.20 | $1889.66 | $49.20 | $1889.66 | |
| Total | $284.32 | $2124.78 | $193.93 | $2034.39 |
CPT = Current Procedural Terminology; DFE = dilated fundus examination; OCT = optical coherence tomography; VA = visual acuity.
The total cost of the encounter in the DFE model with bevacizumab or aflibercept was $85.55 and $1787.58, respectively. The total costs of the encounter in the OCT-only encounter with bevacizumab or aflibercept were $68.85 and $1770.88, respectively (Table 3). The reimbursement per patient in the DFE model encounter with bevacizumab or aflibercept was $284.32 and $2124.78, respectively. The reimbursement per patient in the OCT-only model for bevacizumab or aflibercept was $193.93 and $2034.39, respectively (Table 4). The net difference between cost and reimbursement for each patient encounter was calculated by subtracting the total cost of each encounter from the total reimbursement for each encounter (Table 5 ). The net differences per patient encounter in the DFE model with bevacizumab and aflibercept were found to be $198.77 and $337.20, respectively, and for the OCT-only model $125.08 and $263.51, respectively.
Table 5.
Profits Generated Based on Drug Use in the Different Clinic Models
| Model | Cost Per Encounter |
Reimbursement Per Encounter |
Net Profit Per Encounter |
|||
|---|---|---|---|---|---|---|
| Bevacizumab | Aflibercept | Bevacizumab | Aflibercept | Bevacizumab | Aflibercept | |
| With VA and DFE encounter | $85.55 | $1787.58 | $284.32 | $2124.78 | $198.77 | $337.20 |
| OCT-only encounter | $68.85 | $1779.88 | $193.93 | $2034.39 | $125.08 | $263.51 |
DFE = dilated fundus examination; OCT = optical coherence tomography; VA = visual acuity.
Table 6 shows the total revenue per clinic based on maximum number of patients seen per day for both models using both drugs. For both drugs, once the reimbursements for each visit type were analyzed, the total net difference per day showed an overall financial advantage for the DFE model with bevacizumab and aflibercept of $9541.12 and $16185.76, respectively. For the OCT-only model, the overall advantage for using bevacizumb and aflibercept were $12,007.68 and $25,296.96, respectively. The OCT-only model reduced the patient visit time by 50% and improved clinic efficacy by allowing a greater number of patients to be seen in a given clinic day with less wait time compared with the DFE-model.
Table 6.
Daily Profits Generated Based on Drug Use in the Different Clinic Models
| Model | Patients Seen Per Day, n | Total Cost Per Encounter | Total Cost Per Day | Revenue Per Encounter | Total Revenue Per Day | Total Profit Per Day |
|---|---|---|---|---|---|---|
| With VA assessment and DFE | ||||||
| Bevacizumab | 48 | $85.55 | $4106.24 | $284.32 | $13,647.36 | $9540.96 |
| Aflibercept | $1787.58 | $85,803.68 | $2124.78 | $101,989.44 | $16,181.76 | |
| OCT-only model | ||||||
| Bevacizumab | 96 | $68.85 | $6049.92 | $193.93 | $18,617.28 | $12,007.68 |
| Aflibercept | $1770.88 | $70004.48 | $2034.39 | $195,301.44 | $25,296.96 | |
DFE = dilated fundus examination; OCT = optical coherence tomography; VA = visual acuity.
Discussion
By eliminating the VA assessment and the DFE, we achieved a 50% decrease in encounter time per patient, decreased per-patient cost, and increased overall clinic efficiency. The elimination of a VA assessment and DFE doubled the patient throughput and generated greater revenue for a given clinic day despite decreased billing per patient because no examination is performed. Over a full clinic day, the practice generated an additional $2466.56 by using bevacizumab and $9111.20 by using aflibercept compared with the typical visit with VA assessment and a DFE. There is, however, a difference in the number of staff, OCT instruments, and injection rooms needed to accommodate these 2 clinic paradigms. In the traditional model using VA assessments and DFEs, the staff included 3 VA/OCT technicians, 2 nurses or injection technicians, and a scribe. In the OCT-only model, the requirements included 2 OCT technicians, 4 nurses or injection technicians, and no scribe. Even with this increased staff size associated with the OCT-only model, the daily financial return was greater because more patients are injected. Furthermore, eliminating the VA assessment and DFE has additional cost-saving potential because the examination rooms normally occupied by technicians for assessing VA will no longer be needed so the overhead costs should be less and these rooms can be made available to other retina specialists or ancillary staff.
Both models were optimized by manipulating a variety of parameters so that there would not be any unproductive time for the VA examiners, OCT technicians, nurses or injection technicians, and clinicians. For example, in 1 schedule that we rejected, the OCT-only model used a staff size that was optimized for the DFE model, namely 3 OCT technicians and 2 nurses. This model resulted in no increase in patient throughput and yielded a lower revenue. In addition, OCT technicians experienced wait times of 20 minutes between patients. A second rejected schedule was a model that used a staff size optimized for the OCT-only model, namely 2 vision examiners/OCT technicians and 4 nurses. This model yielded only 32 patients per day compared with the optimized schedule of 96 patients. In addition, the retinal specialist waited 5 minutes between each patient, nurses waited 40 minutes between patients, and the 4 nurses were given a 100-minute break in order to provide a 60-minute break to the vision examiners/OCT technicians. A practice that seeks to incorporate an OCT-only model must adjust its staff size to ensure its optimal use of personnel with reduced waiting time.
Overall, with the proper staff size, the patient throughput and financial return are significantly increased by removing the time needed to complete the initial VA assessment and perform the DFE. The most efficient method of managing eAMD patients without a fixed established injection interval is to use an OCT-guided retreatment regimen under an optimized model that removes the DFE. This OCT-only model could be modified to include a patient self-administered VA test while the patient is in the waiting room before OCT imaging is performed. This self-administered VA test could be uploaded into the practice's electronic medical record, and the physician could evaluate the VA and OCT images without a formal patient encounter.
These results provide a framework to demonstrate the time savings and the increased efficiency that can be achieved by eliminating a VA assessment and DFE for any clinical practice treating follow-up patients undergoing anti-VEGF therapy. While the times allotted to each of the steps in our models may vary from practice to practice, the overall conclusions would be the same. In our experience using the OCT-only model, we have received positive feedback from our patients because of their expedited care and the decreased exposure time to other patients and staff.
We made several assumptions when designing the different clinic models. The salary of the clinician was not considered because we assumed that the clinician would be present regardless of the clinic model used. We did not consider the cost of clinic space, instrument costs, or the cost associated with the electronic medical records because these are fixed costs that the practice would incur regardless of the model. Given these fixed costs to operate the clinic and perform the injection procedures, our goal was to adjust patient flow and intravitreal injections to maximize the number of patients seen and minimize wait times for patients, staff, and clinicians. In addition, we did not specify the amount of time spent by the clinician interpreting the OCT or administering the injection. The clinician would interpret the OCT images during the allotted time when an injection is performed because we assumed that the nurse or injection technician would spend most of the allocated time preparing the patient for injection, washing out the betadine, and providing postinjection instructions after the procedure. Therefore, these specific clinic models should be adjusted to practices where the clinician is responsible for most of the procedure. We expect that the OCT-only model would still be more cost efficient.
An obvious concern when considering an OCT-only model is that elimination of a formal VA examination and DFE may put the patient at risk of inadequate treatment and worse outcomes. This risk appears to be minimal based on the HARBOR trial result that showed no difference in visual acuity outcomes in patients who were retreated in the PRN arm using OCT imaging alone.22 Moreover, clinicians can use their own experience and ask themselves how often a VA or DFE outcome influenced their plans for injection that were independent of the OCT findings. There is a concern that by eliminating formal VA assessments and DFEs the clinician increases their medicolegal risks. While it is possible that macular hemorrhages, if undetected by changes on the OCT examination, could lead to irreversible vision loss, we propose that such risk for vision loss and subsequent legal consequences can be minimized if: 1) the TAE retreatment regimen is followed and patients always receive an injection and 2) follow-up intervals are shortened or extended by only 2 weeks at a time. Other known injection associated complications, such as retinal tears or early retinal detachments, could be missed, but these events are rare, and patients typically report symptoms that necessitate a DFE.29 Moreover, in the HARBOR trial, no retinal detachments or tears were diagnosed on routine DFE.22 Another indicator of disease progression that may be overlooked by an OCT-only model is worsening VA, which may not be associated with a worsening OCT scan. While such a scenario may occur, the loss of vision is likely caused by the progression of the underlying nonexudative component of dry AMD rather than undiagnosed exudation.
If such an OCT guided injection-only model were instituted, we recommend the possible use of a patient self-administered VA test in the waiting room before the OCT scan. Smartphone applications have been shown to produce accurate and repeatable VA measurements when compared with retroilluminated clinic VA charts.30 For example, the DigiSight Checkup smartphone app (Paxos, San Francisco, California, USA) was shown to be more accurate than a standard Snellen chart in the emergency department when performed by nonophthalmic emergency department staff.31 While a number of smartphone apps have been developed to perform self-assessments of VA, few of them have been tested for accuracy and repeatability,32 so their adoption should be considered once standardization, reproducibility, and compatibility are performed with the clinician's electronic medical record system. Nonetheless, a reliable app may provide the VA examination desired in an optimal OCT-only model without compromising clinic efficiency. Currently, we are not using such VA self-assessment tests because of our reluctance to introduce any touchpad devices during the COVID-19 pandemic and our desire to move patients quickly through the visit without any delay in the waiting room.33 However, in the current paradigm, if a patient does self-report a significant decline in vision, especially in the untreated eye, then we would convert their visit to include a full DFE with VA assessment, and this conversion to a DFE could even be performed after OCT imaging if the decreased vision could not be explained by the OCT images alone.
With personal safety issues becoming a priority given the worldwide pandemic of SARS-CoV-2, particularly in elderly patients, another major advantage of the OCT-only model is the decreased time spent in clinic and the decreased exposure between patients and health care providers. Another important consideration is the close proximity between the patient and both the technician and the physician during the VA assessment and DFE, respectively. It has been noted that droplets from a cough or sneeze can be propelled for up to 6 feet, a range that includes the spacing between the patient and physician, which makes appropriate social distancing impractical.34 The safety of this OCT-only model could be improved even further if the patients could self-administer the OCT without the need of a technician. A formal prospective clinical trial is needed that compares these 2 practice models to provide greater clarity to these different approaches, but this will be delayed until the current health pandemic is resolved.
Another practice that could decrease the physician–patient contact time while increasing the number of patients injected with oversight by a clinician would be to use physician-extenders such as nurse practitioners, physician assistants, or orthoptists to deliver anti-VEGF injections. Studies from England,35 , 36 New Zealand,37 Switzerland, and Denmark38 discuss medical practices that have already adopted such methodology and have found success. Some clinics have delegated >80% of their anti-VEGF injections to nurse practitioners or orthoptists. A literature review examined 310,303 injections performed by 16 nurse practitioners and revealed short-term capacity improvement in the number of patients seen, which liberated physicians for other clinical activities. At the same time, the rate of endophthalmitis was found to be 0%-0.04%, comparable to that among physicians.39 Assuming proper training is completed, incorporating this practice with appropriate clinician oversight may serve to increase patient throughput. By using these physician-extenders to perform injections in this OCT-only model, clinic efficiency would improve even further by allowing the clinician to oversee a large number of patients.
Looking to the future with the availability of home OCT monitoring and artificial intelligence algorithms that can identify the onset of new macular fluid, we can expect to improve our ability to track and treat patients before their next scheduled appointment.40 This same technology that allows for home OCT monitoring could be adapted to accelerate the development of instruments capable of OCT imaging in the clinic without the need of a technician as described above. This innovation would improve clinic efficacy even further.
The financial results of this study are limited by the Centers for Medicaid and Medicare Services reimbursement rates and would not be replicated to non-US countries. Moreover, the times required for each encounter during the clinic visit may vary depending on different practices; however, the overall conclusions drawn from this report should remain the same. The OCT-only model should still be the most efficient strategy for treating established patients using an OCT-guided retreatment strategy. Our claim that the DFE is not necessarily required is based upon a post hoc analysis of the HARBOR study, a retrospective subgroup analysis, and this claim should be interpreted cautiously. While high-resolution infrared images that are obtained during the same session when OCT imaging is performed on certain devices may increase the detection rate of small hemorrhages, the added benefit of using infrared images in the post hoc analysis from the HARBOR study was not assessed; however, this remains an area of future investigation. However, given the positive results from using OCT alone,22 it is unlikely that the use of infrared imaging will significantly change the outcomes. In addition, we do not anticipate that most practices would use the OCT model every day, but rather, they could select certain days to schedule patients undergoing expedited OCT-only guided retreatment, and reserve other days when a full examination is warranted. Moreover, we would anticipate that even if patients are managed using the OCT-only model of TAE injections, then these patients would still undergo a DFE every 6 months or once a year.
In summary, an optimal OCT-only model for treating patients on a TAE regimen is a potential strategy to increase the volume of patient requiring intravitreal injections while minimizing visit times. Employing 2 OCT technicians and 4 nurses for the retinal specialist results in maximal patient throughput and maintains an efficient use of practice personnel. This strategy improves the practice's ability to provide intravitreal injections to the growing population of patients with exudative eye diseases while shortening visit duration and increasing patient safety during the current pandemic. In our experience using this OCT-only protocol during the current pandemic, patients have embraced this new clinic paradigm because of their expedited care and decreased exposure to other patients in the waiting room and to clinic personnel. The time periods for the visit components that have been shown are accurate for our clinical operation but may vary slightly from practice to practice. Whether an OCT-only model can be used without sacrificing patient outcomes needs to be tested prospectively; however, our anecdotal experience and the results of the HARBOR trial strongly suggest that the clinic strategy is effective in improving clinic efficiency.
Acknowledgments
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. Funding/Support: Supported by grants from the Salah Foundation (Ft. Lauderdale, Florida, USA), the National Eye Institute Center Core Grant (P30EY014801) and Research to Prevent Blindness (unrestricted grant) to the Department of Ophthalmology, University of Miami Miller School of Medicine. The funding organizations had no role in the design or conduct of the present research. Financial Disclosures: Dr Rosenfeld receives research support from Carl Zeiss Meditec, Inc (Dublin, California, USA) and Stealth Biotherapeutics, is a consultant for Apellis, Biogen, Boehringer-Ingelheim, Carl Zeiss Meditec, Chengdu Kanghong Biotech, EyePoint, Ocugenix, Ocunexus, Ocudyne, and Unity Biotechnology, and has equity interest in Apellis, Valitor, Verana Health, and Ocudyne. Dr Gregori receives research support from Carl Zeiss Meditec, Inc and the University of Miami co-owns a patent that is licensed to Carl Zeiss Meditec, Inc. Drs Trivizki, Karp, Chawla, and Yamanuha indicate no financial conflict of interest. All authors attest that they meet the current ICMJE criteria for authorship.
Other Acknowledgments: None.
Footnotes
Supplemental Material available at AJO.com.
Supplemental Data
Omer Trivizki, MD, MBA, is a medical retina fellow in Bascom Palmer Eye Institute. He finished his ophthalmology residency at Tel Aviv Medical Center and appointed as a lecturer at the Sackler Faculty of Medicine at Tel Aviv University. Omer served in the Israeli Air Intelligence as an officer. Studied medicine at the Sackler Faculty of Medicine in Tel Aviv University and completed an MBA at the Collar School of Management at Tel Aviv University.
References
- 1.Sarwar S., Clearfield E., Soliman M.K., et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2:CD011346. doi: 10.1002/14651858.CD011346.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gale R.P., Mahmood S., Devonport H., et al. Action on neovascular age-related macular degeneration (nAMD): recommendations for management and service provision in the UK hospital eye service. Eye (Lond) 2019;33:1–21. doi: 10.1038/s41433-018-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon S.D., Lindsley K., Vedula S.S., Krzystolik M.G., Hawkins B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:CD005139. doi: 10.1002/14651858.CD005139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rofagha S., Bhisitkul R.B., Boyer D.S., Sadda S.R., Zhang K., Group S.-U.S. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld P.J., Brown D.M., Heier J.S., et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Brown D.M., Kaiser P.K., Michels M., et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 7.Regillo C.D., Brown D.M., Abraham P., et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Martin D.F., Maguire M.G., Ying G.S., Grunwald J.E., Fine S.L., Jaffe G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarthy U., Harding S.P., Rogers C.A., et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Ho A.C., Busbee B.G., Regillo C.D., et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121:2181–2192. doi: 10.1016/j.ophtha.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A., Kumar N., Bandello F. Re: Dugel et al: HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration (Ophthalmology. 2019 Apr 12 [Epub ahead of print]) Ophthalmology. 2019;126:e95–e96. doi: 10.1016/j.ophtha.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Dugel P.U., Koh A., Ogura Y., et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Erfurth U., Waldstein S.M. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1–24. doi: 10.1016/j.preteyeres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 14.El-Mollayess G.M., Mahfoud Z., Schakal A.R., Salti H.I., Jaafar D., Bashshur Z.F. Fixed-interval versus OCT-guided variable dosing of intravitreal bevacizumab in the management of neovascular age-related macular degeneration: a 12-month randomized prospective study. Am J Ophthalmol. 2012;153:481–489.e1. doi: 10.1016/j.ajo.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Bogunovic H., Montuoro A., Baratsits M., et al. Machine learning of the progression of intermediate age-related macular degeneration based on OCT imaging. Invest Ophthalmol Vis Sci. 2017;58:Bio141–Bio150. doi: 10.1167/iovs.17-21789. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sheikh M., Iafe N.A., Phasukkijwatana N., Sadda S.R., Sarraf D. Biomarkers of neovascular activity in age-related macular degeneration using optical coherence tomography angiography. Retina. 2018;38:220–230. doi: 10.1097/IAE.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 17.American Society of retinal specialists preferences and trends survey. Presented at the ASRS 2018 Annual Meeting, Vancouver, British Columbia, Canada, July 20-25, 2018.
- 18.Haller J.A. Current anti-vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology. 2013;120(5 suppl):S3–S7. doi: 10.1016/j.ophtha.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 19.Berg K., Pedersen T.R., Sandvik L., Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–152. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Wykoff C.C., Croft D.E., Brown D.M., et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology. 2015;122:2514–2522. doi: 10.1016/j.ophtha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Gupta O.P., Shienbaum G., Patel A.H., Fecarotta C., Kaiser R.S., Regillo C.D. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117:2134–2140. doi: 10.1016/j.ophtha.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Patel Y., Miller D.M., Fung A.E., Hill L.F., Rosenfeld P.J. Are dilated fundus examinations needed for OCT-guided retreatment of exudative age-related macular degeneration? Ophthalmol Retina. 2020;4:141–147. doi: 10.1016/j.oret.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Iida T., Ishii K. Physician, patient, and caregiver experience of different wet age-related macular degeneration anti-VEGF treatment regimens in Japan: a qualitative assessment. Clin Ophthalmol. 2016;10:2505–2513. doi: 10.2147/OPTH.S120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prenner J.L., Halperin L.S., Rycroft C., Hogue S., Williams Liu Z., Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160:725–731.e1. doi: 10.1016/j.ajo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Layana A., Figueroa M.S., Arias L., et al. Individualized therapy with ranibizumab in wet age-related macular degeneration. J Ophthalmol. 2015;2015:412903. doi: 10.1155/2015/412903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rein D.B., Wittenborn J.S., Zhang X., Honeycutt A.A., Lesesne S.B., Saaddine J. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 27.Engman S.J., Edwards A.O., Bakri S.J. Administration of repeat intravitreal anti-VEGF drugs by retina specialists in an injection-only clinic for patients with exudative AMD: patient acceptance and safety. Semin Ophthalmol. 2011;26:380–386. doi: 10.3109/08820538.2011.622337. [DOI] [PubMed] [Google Scholar]
- 28.Thorell M.R., Nunes R.P., Chen G.W., et al. Response to aflibercept after frequent re-treatment with bevacizumab or ranibizumab in eyes with neovascular AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45:526–533. doi: 10.3928/23258160-20141118-07. [DOI] [PubMed] [Google Scholar]
- 29.Mammo D.A., Ringeisen A.L., Parke D.W., 3rd Frequency of rhegmatogenous retinal detachment after intravitreal therapy in neovascular age-related macular degeneration. Ophthalmol Retina. 2020 doi: 10.1016/j.oret.2020.03.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Brady C.J., Eghrari A.O., Labrique A.B. Smartphone-based visual acuity measurement for screening and clinical assessment. JAMA. 2015;314:2682–2683. doi: 10.1001/jama.2015.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathipati A.S., Wood E.H., Lam C.K., Sales C.S., Moshfeghi D.M. Visual acuity measured with a smartphone app is more accurate than Snellen testing by emergency department providers. Graefes Arch Clin Exp Ophthalmol. 2016;254:1175–1180. doi: 10.1007/s00417-016-3291-4. [DOI] [PubMed] [Google Scholar]
- 32.Tofigh S., Shortridge E., Elkeeb A., Godley B.F. Effectiveness of a smartphone application for testing near visual acuity. Eye (Lond) 2015;29:1464–1468. doi: 10.1038/eye.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeung W.K., Dawes P., Pye A., et al. eHealth tools for the self-testing of visual acuity: a scoping review. NPJ Digit Med. 2019;2:82. doi: 10.1038/s41746-019-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie X., Li Y., Chwang A.T., Ho P.L., Seto W.H. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 35.DaCosta J., Hamilton R., Nago J., et al. Implementation of a nurse-delivered intravitreal injection service. Eye (Lond) 2014;28:734–740. doi: 10.1038/eye.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simcock P., Kingett B., Mann N., Reddy V., Park J. A safety audit of the first 10 000 intravitreal ranibizumab injections performed by nurse practitioners. Eye (Lond) 2014;28:1161–1164. doi: 10.1038/eye.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samalia P., Garland D., Squirrell D. Nurse specialists for the administration of anti-vascular endothelial growth factor intravitreal injections. N Z Med J. 2016;129:32–38. [PubMed] [Google Scholar]
- 38.Hasler P.W., Bloch S.B., Villumsen J., Fuchs J., Lund-Andersen H., Larsen M. Safety study of 38,503 intravitreal ranibizumab injections performed mainly by physicians in training and nurses in a hospital setting. Acta Ophthalmol. 2015;93:122–125. doi: 10.1111/aos.12589. [DOI] [PubMed] [Google Scholar]
- 39.Rasul A., Subhi Y., Sørensen T.L., Munch I.C. Non-physician delivered intravitreal injection service is feasible and safe - a systematic review. Dan Med J. 2016;63:A5229. [PubMed] [Google Scholar]
- 40.Notal Vision. AI-based fluid quantification on serial OCT images from a patient self-operated home OCT device. Presented at Angiogenesis, Exudation, and Degeneration, Miami, February 8, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Omer Trivizki, MD, MBA, is a medical retina fellow in Bascom Palmer Eye Institute. He finished his ophthalmology residency at Tel Aviv Medical Center and appointed as a lecturer at the Sackler Faculty of Medicine at Tel Aviv University. Omer served in the Israeli Air Intelligence as an officer. Studied medicine at the Sackler Faculty of Medicine in Tel Aviv University and completed an MBA at the Collar School of Management at Tel Aviv University.