Abstract
Background
Women living with HIV (WLWH) are at higher risk of acquisition and progression of human papillomavirus (HPV) infection. Evidence on effect of HPV vaccination in this population is limited.
Methods
This phase IV randomized controlled observer-blind study assessed immunogenicity and safety of two HPV vaccines (AS04-HPV-16/18 vs. 4vHPV) given in WLWH (stage 1) and HIV- females aged 15–25 years. Co-primary endpoints were to demonstrate, in WLWH subjects, non-inferiority (and if demonstrated, superiority) of AS04-HPV-16/18 vs. 4vHPV for HPV-16 and HPV-18 by pseudovirion-based neutralization assay (PBNA) at month 7 and safety. Non-inferiority criteria was lower limit (LL) of the 95% confidence interval (CI) of the GMT ratio AS04-HPV-16/18/4vHPV above 0.5, in the according to protocol population. NCT01031069
Findings
Among 873 subjects recruited between 26-Oct-2010 and 14-May-2015, 546 were randomized (1:1) and received at least one vaccine dose (total vaccinated cohort, TVC): 257 were WLWH (129 AS04-HPV-16/18; 128 4vHPV) and 289 were subjects without HIV (144 AS04-HPV-16/18; 145 4vHPV). Baseline CD4 cell count in WLWH was at least 350 cells/mm3.
At month 7, AS04-HPV-16/18 showed immunological superiority to 4vHPV in WLWH. Neutralizing anti-HPV-16 and HPV-18 antibody GMTs were 2·74 (95% CI: 1·83; 4·11) and 7·44 (95% CI: 4·79; 11·54) fold higher in AS04-HPV-16/18 vs. 4vHPV (LL of the GMT ratio >1 in TVC, p<0·0001), respectively. Similar results were observed by ELISA up to month 24.
Solicited local and general symptoms were in line with product labels. The number of reported serious adverse events (SAEs) was balanced throughout the study.
Interpretation
Both vaccines showed an acceptable safety profile in all subjects. Despite the absence of an immunological correlate of protection for HPV, differences in immune responses elicited by the vaccines especially for HPV-18 may translate into longer lasting or more robust protection against cervical cancer with the AS04-HPV-16/18 vaccine in WLWH.
Funding
GlaxoSmithKline Biologicals S.A. funded this study (NCT01031069) and related publications.
Trial registration
Keywords: Immunogenicity, Safety, HPV, Vaccine, HIV, AS04-HPV-16/18 vaccine, 4-valent HPV vaccine
Research in context.
Evidence before this study
Women living with HIV (WLWH) are at greater risk of cervical cancer from higher rates of persistent HPV infection when compared to women without HIV. Limited evidence on the effect of the use of HPV vaccines in WLWH exists. Previous studies have shown that AS04-HPV-16/18 (in young women) and 4vHPV (in children) both produced an antibody response and were well tolerated in WLWH. However immunogenic responses were lower in WLWH versus women without, and in those with more advanced HIV disease.
Added value of this study
This study compared two HPV vaccines in asymptomatic WLWH and with CD4 cell counts of 350 cells/mm3 or higher. AS04-HPV-16/18 demonstrated immunological superiority in terms of antibody titers against HPV-16 and HPV-18 and overall higher immunogenicity compared to 4vHPV in WLWH. In addition, AS04-HPV-16/18 showed a similar response to HPV-16/18 in WLWH compared to 4vHPV in subjects without HIV.
Implications of all the available evidence
Despite the absence of an immunological correlate of protection for HPV, differences in immune responses elicited by the vaccines may translate into longer lasting or more robust protection against cervical cancer with the AS04-HPV-16/18 vaccine in WLWH, in particular for HPV-18.
Alt-text: Unlabelled box
1. Introduction
Cervical cancer is the fourth most common cancer among women worldwide, with around 569,000 new cases and 311,000 deaths reported in 2018 [1]. Persistent human papillomavirus (HPV) infection is recognized as the central cause of cervical cancer [2,3] with up to 71% of cases attributable to high-risk HPV types 16 and 18 [4]. Women living with HIV (WLWH) have higher rates of persistent HPV infection and are at greater risk of developing cervical cancer than women without HIV infection [5], [6], [7] HPV vaccination is therefore likely to be highly beneficial in this high-risk group.
Two HPV vaccines, AS04-adjuvanted HPV-16/18 vaccine (AS04-HPV-16/18, Cervarix, GSK) and HPV-6/11/16/18 vaccine (4vHPV, Gardasil, Merck) were licensed in 2007 and 2006, respectively. The former is formulated with AS04, which contains aluminum hydroxide salts and the TLR4 agonist MPL (3-O-desacyl-4′-monophosphoryl lipid A) while the latter only includes aluminum hydroxyphosphate sulfate. Both are indicated for the prevention of (ano)genital lesions and cervical and anal cancers caused by certain oncogenic HPV types. For women aged 15 years and older, three doses are recommended, according to a 0-, 1- and 6-month schedule for AS04-HPV-16/18 [8] or a 0-, 2- and 6-month schedule for 4vHPV [9]. A large trial comparing these two vaccines in 18- to 45-year-old women showed significantly higher HPV-16 and HPV-18 neutralizing antibody responses with AS04-HPV-16/18 vs. 4vHPV, as well as higher positivity rates in cervicovaginal secretions (CVS) and higher HPV-16 and HPV-18 specific B-cell frequencies, one month after the last dose [10]. Five years after vaccination, serum neutralizing antibody levels were still 7.8-fold higher with AS04-HPV-16/18 compared to 4vHPV for HPV-16, and 12.1-fold for HPV-18 in 18–26 year olds [11]. In a study in 9- to 14-year-old girls, AS04-HPV-16/18 was administered as a two-dose schedule (0, 6 months) and was also shown to be superior to two-dose and three-dose of 4vHPV in terms of immune responses to both HPV-16 and HPV-18 up to 36 months after vaccination [12]. The AS04 adjuvant is thought to play a key role in the difference of immunogenicity and efficacy profiles between the two vaccines [13,14].
Inactivated vaccines can generally be safely administered to people with altered immunocompetence, although safety and efficacy may differ according to immunodeficiency type and severity [15]. AS04-HPV-16/18 and 4vHPV contain no live infectious or inactivated agents. Instead, they contain recombinant proteins assembled as virus-like particles (VLP), thereby reducing the potential for harmful effects. At the time of study design, limited data were available on the use (or effects) of the two HPV vaccines in WLWH, while it was known that WLWH could mount a humoral response to HPV antigen [16].
A South African study in WLWH and women without HIV aged 18 to 25 years who received three doses of AS04-HPV-16/18 vaccine showed that all women were seropositive for both HPV-16 and HPV-18 after the second vaccine dose and until one year after the first dose, irrespective of baseline HPV status. While antibody titers against HPV-16 and HPV-18 were lower in WLWH than in women without HIV, they remained 26-fold and 16-fold higher after 12 months, respectively, than those observed in healthy women with an immune response to natural infection [17]. A study in children living with HIV aged 7 to 12 years who received either three or four doses of 4vHPV and followed- up to five years showed seropositivity rates 86–93% for types 6, 11 and 16 but 64% for HPV-18. These rates were similar to those observed one month after completing vaccination, suggesting a poor response to 4vHPV vaccination for some subjects [18,19]. Another study with 4vHPV in girls and WLWH aged 13 to 45 years showed that the vaccine had an acceptable safety profile and was immunogenic. Lower seroconversion rates for HPV-6, −11, -16 and −18 were observed however after three doses in WLWH with a viral load of >10,000 copies/mL and/or CD4 count <200 cells/µL [20]. A double-blind head to head study with the AS04-HPV-16/18 and the 4vHPV vaccines in older males and WLWH found the AS04 adjuvanted vaccine overall more immunogenic [21]. A follow-up of this study found both vaccines to induce comparable cellular immune responses, although the limited sample size sounds a note of caution when interpreting these results [22]. Last, a recent efficacy study with 4vHPV in 279 WLWH suggested a higher risk for vaccine failure compared to women without HIV. All four breakthrough infection observed were HPV-18 which was unexpected considering the low HPV-18 prevalence in the study [23].
This phase IV randomized controlled observer-blind study (NCT01031069) was conducted to assess the safety and immunogenicity of AS04-HPV-16/18 compared to 4vHPV in females aged 15 to 25 years who were living with HIV (clinical stage 1) or without HIV over 24 months.
2. Patients and methods
2.1. Study design and participants
This multicenter, randomized, controlled, observer-blind phase IV study (NCT01031069) was conducted in clinical stage 1 (asymptomatic) WLWH and in women without HIV aged 15 to 25 years. Study participants were randomized 1:1 to receive a 0·5 mL dose of either AS04-HPV-16/18 or 4vHPV at day 0, week 6 and month 6. Vaccines were administered by intramuscular injection into the deltoid of the non-dominant arm. The study duration of two years included an active phase up to month 7, and a follow-up phase until month 24. An independent data monitoring committee (IDMC) provided oversight throughout the study.
2.2. randomization
Subjects were enrolled in Brazil, Estonia, India, and Thailand. Randomization was performed using MATEX, a program developed for use in SAS (Cary, NC, USA) by GSK. A randomization blocking scheme (1:1 ratio) was used to ensure balance between treatments was maintained: a treatment number was allocated at each dose for each subject. Treatment allocation at the investigator site was performed using a central randomization system on the Internet. Subjects were stratified according to country, baseline HIV infection status, and age (15 to 17 years and 18 to 25 years). In addition, WLWH were randomized according to baseline CD4 cell count (350–500 cells/mm3 or >500 cells/mm3) and whether they were on highly-active antiretroviral therapy (HAART) or not. The randomization algorithm for WLWH used a minimization procedure accounting for CD4 cell count and HAART.
2.3. Study conduct
Data were collected in an observer-blind manner. Due to the different visual appearance of the HPV vaccines, qualified medical personnel not otherwise involved in the conduct of the study prepared and administered the vaccines. The vaccine recipient and study personnel conducting or evaluating study endpoints were unaware of which vaccine was administered. Analyses were performed by an external statistician.
The study was conducted in accordance with the Good Clinical Practice (GCP) Guidelines and the Declaration of Helsinki, and the protocol and associated documents were reviewed and approved by local ethics committees. Written informed consents were obtained from the subjects or their legally acceptable representatives, and assents were obtained from subjects below the legal age of consent who consented after reaching 18 years of age during the course of the study. The study protocol is provided in the Supplementary materials. The manuscript was developed in accordance with the CONSORT checklist.
A sample size of 120 evaluable WLWH per vaccine group was needed to demonstrate superiority for HPV-18 and HPV-16 immune response with a power of 97%. A total of 600 subjects (300 with and 300 without HIV) were to be enrolled. Due to data integrity issues at one site (protocol non-compliance, high drop-out rate), additional subjects were enrolled to maintain statistical power for analysis. Thus, approximately 700 subjects were to be enrolled to obtain 480 evaluable subjects.
Subjects with no previous vaccination against HPV or previous administration of 3-O-desacyl-4′- monophosphoryl lipid A (MPL) or AS04, a GSK proprietary-Adjuvant System containing MPL (50 µg) adsorbed on Aluminum salt (500 µg), were included. WLWH, according to the World Health Organization (WHO) case definition, were defined as having positive HIV antibody test report (rapid or laboratory-based enzyme immunoassay, confirmed by a second and different HIV antibody test relying on different antigens or of different operating characteristics and/or positive virological test for HIV or its components such as HIV-RNA, HIV-DNA or ultrasensitive HIV P24 antigen). The protocol was amended during the study to be in line with the updates of the WHO guidelines. Subjects had to be asymptomatic regardless of their prior clinical stage. If they were currently taking antiretrovirals (ARV), subjects were to be on HAART for at least one year, have undetectable viral load (i.e., viral load ≤400 copies/mL) for at least six months, and have a CD4 cell count >350 cells/mm3 at study entry. WLWH diagnosed with active tuberculosis (TB), or subjects on TB therapy were not enrolled.
2.4. Study assessments
Immunogenicity assessments were performed as previously described in Leung et al. (2018) [12], including determination of antibody titers to HPV-16 and HPV-18 in all subjects using pseudovirion-based neutralization assay (PBNA) up to month 7 and Enzyme-linked Immunosorbent Assay (ELISA) until study end; cell-mediated immune response (memory B-cells by Enzyme-linked Immunospot [ELISPOT] and, CD4 and CD8 T cells by Intracellular Cytokine Staining) in a randomized subset of 100 subjects (25 from each arm); and total immunoglobulin G and anti-HPV-16/18 antibody titers in cervicovaginal secretions from a subset of post-menarcheal subjects who volunteered, by ELISA (Table 1). The conversion factor from ELISA to international units (IU) for the serum assays were determined as 1/6.1 for HPV-16 and 1/5.7 for HPV-18 [24]. Solicited adverse events (AEs) occurring within seven days and unsolicited AEs occurring within one month of vaccination were recorded on a diary card by the subject or the legally acceptable representative. Diary cards were transcribed by investigators one month after each vaccine dose, and the intensity of AEs and relationship to vaccination (for solicited general and unsolicited symptoms) were assessed. Serious adverse events (SAEs) and medically-significant conditions (MSCs) were recorded at each visit.
Table 1.
Study timepoints for vaccination schedule and immunogenicity assessments.
| D 0 | W 6 | W 10 | M 6 | M 7 | M 12 | M 18 | M 24 | |
|---|---|---|---|---|---|---|---|---|
| Vaccination (AS04-HPV-16/18 or 4vHPV) | Dose 1 | Dose 2 | – | Dose 3 | – | – | – | – |
| HPV-16/18 humoral response | PBNA | – | – | – | PBNA | – | – | – |
| ELISA | ELISA | ELISA | – | ELISA | ELISA | ELISA | ELISA | |
| HPV-16/18 cell-mediated immunity* | ELISPOT | ELISPOT | ELISPOT | – | ELISPOT | ELISPOT | – | – |
| ICS | ICS | ICS | – | ICS | ICS | – | – | |
| cervico-vaginal secretions⁎⁎ | ELISA | ELISA | ELISA | – | ELISA | ELISA | – | ELISA |
D: day; ELISA: Enzyme-linked Immunosorbent Assay; ELISPOT: Enzyme-linked Immunospot; HPV: human papillomavirus; ICS: Intracellular Cytokine Staining; M: month; PBNA: Pseudovirion-Based Neutralization Assay; W: week.
Memory B-cells by ELISPOT and CD4/CD8 T cells by ICS.
Immunoglobulin G and anti-HPV16/18 by ELISA in cervico-vaginal secretion samples.
The co-primary immunogenicity and safety endpoints were i) to demonstrate non-inferiority of AS04-HPV-16/18 vs. 4vHPV in the according to protocol (ATP) cohort and if non-inferiority was demonstrated, to demonstrate superiority in the total vaccinated cohort (TVC) in terms of geometric mean titers (GMTs) against HPV-16 and HPV-18 measured by PBNA one month after administration of the third dose (i.e., month 7) in WLWH; ii) to assess safety and reactogenicity of both vaccines in WLWH up to month 7 in the TVC.
Non-inferiority was to be demonstrated if the lower limit of the 95% confidence interval (CI) for the ratio of GMTs (AS04-HPV-16/18 over 4vHPV) was above 0·5 for both HPV types. Superiority was to be demonstrated if the lower limit of the 95% CI for the ratio of GMTs (AS04-HPV-16/18 over 4vHPV) was above 1·0 for both HPV types, with a statistically significant p-value.
Secondary immunogenicity endpoints were to i) demonstrate superiority of AS04-HPV-16/18 vs. 4vHPV in subjects without HIV using PBNA at month 7, ii) evaluate antibody response to both vaccines using ELISA from day 0 to month 24 in all subjects, iii) evaluate antibody response using ELISA in CVS from day 0 to month 24 in post-menarcheal subjects who volunteered for this procedure, iv) evaluate memory B and T cell-mediated immune (CMI) response against HPV-16 and HPV-18 from day 0 to month 12 in a subset of 100 subjects (50 with and 50 without HIV).
See Table A1 for description of primary and secondary endpoints.
2.5. Statistical methods
Statistical analysis of baseline characteristics, immunogenicity and safety outcomes was conducted. Demographic characteristics of each study cohort were tabulated. Cohorts for analysis and withdrawal status were summarized per treatment group. HIV mode of transmission, WHO clinical staging, HIV viral load, CD4 cell count and ARV use of the subjects at baseline are presented as a whole and by treatment group.
For immunogenicity primary and secondary outcomes, between-group comparisons to assess non-inferiority were done on the more restrictive ATP cohort for immunogenicity (by PBNA, regardless of HPV serostatus at baseline). The ATP cohort for immunogenicity included all subjects who met all eligibility criteria and complied with procedures, received all three allocated vaccine doses and for whom immunogenicity endpoints were available. A second analysis on the TVC was performed to support the primary analysis. Primary and secondary between-group comparisons to assess superiority were performed on the TVC (by PBNA; regardless of HPV serostatus at baseline). The TVC for analysis of immunogenicity included subjects who received at least one dose of the vaccines and for whom data concerning immunogenicity endpoint measures were available. Two-sided 95% CIs of anti-HPV-16 and anti-HPV-18 GMT ratios (AS04-HPV-16/18 over 4vHPV), at month 7, were computed using an analysis of variance (ANOVA) model on the log10 transformation of the titers for WLWH (primary objective) and for subjects without HIV (secondary objectives). The ANOVA model included the vaccine group and country as fixed effect. For CMI, T-cells response (frequency of d-CD40L, d-IL2, d-TNFγ, d-IFNγ or all doubles positive CD4/CD8) by intracellular cytokine staining and B cells response by enzyme linked immunospot were measured in a subset of 100 subjects.
The primary safety analysis was performed on the TVC. The following analyses were done on WLWH subjects (primary objectives) and on subjects without HIV (secondary objectives): percentage of subjects with solicited local and general and grade 3 AEs during the solicited follow-up period (days 0 – 6) and during the 30-day follow-up period, with relationship to vaccination and median duration; medically significant conditions or a potentially immune-mediated disease (pIMD) from first vaccination up to 12 months after the last vaccine dose (i.e., month 18); SAEs, withdrawal due to AE(s); proportion of subjects who received at least one concomitant medication during the entire study period; in WLWH, CD4 cell counts, HIV viral load, WHO HIV clinical staging and the use of ARVs.
A post-hoc analysis using a general linear model was performed on neutralizing antibodies at Month 7 on TVC. The final model included age, HAART status and CD4 cell count at baseline and vaccine type.
See Table A2 for statistical methods used for all immunogenicity and safety outcomes.
3. Role of funding
GlaxoSmithKline Biologicals S.A. funded this study (NCT01031069) and all costs related to the development of the related publications.
4. Results
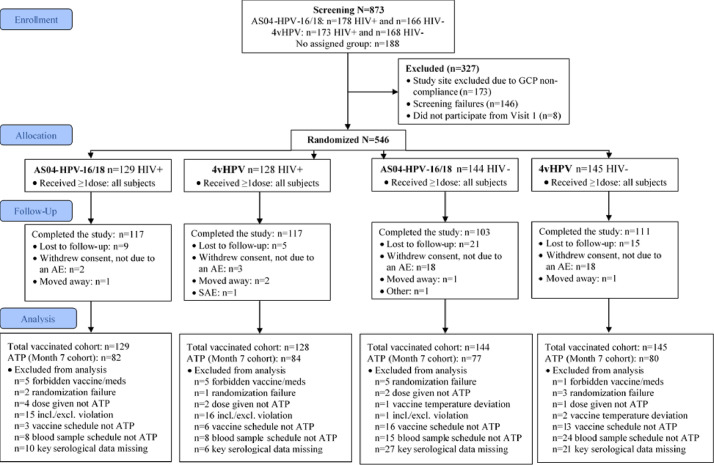
Among 873 subjects recruited between 26 October 2010 and 14 May 2015, 173 from the same study site were excluded due to GCP non-compliance, 146 were screening failures and 1 subject did not participate after Visit 1. The remaining 546 subjects were randomized (1:1) and received at least one dose of the allocated vaccine (TVC): 257 were WLWH (129 AS04-HPV-16/18 and 128 4vHPV) and 289 were without HIV (144 AS04-HPV-16/18 and 145 4vHPV). Overall, 448 subjects completed the study (117 and 117 WLWH and 103 and 111 subjects without HIV, in the AS04-HPV-16/18 and 4vHPV groups, respectively). The main reasons for withdrawal from the study were withdrawal of consent not due to an AE, and loss to follow-up (Fig. 1). The ATP cohort for immunogenicity included 323 subjects at month 7: 82 and 84 WLWH and 77 and 80 subjects without HIV in the AS04-HPV-16/18 and 4vHPV groups, respectively.
Fig. 1.
Study flow diagram (following CONSORT).AE: adverse event; ATP: according to protocol; GCP: good clinical practice; HIV: human immunodeficiency virus; HPV: human papillomavirus; SAE: serious adverse event; WLWH: women living with HIV.
The mean age of women at baseline was 19·8 years (SD: 3·2) and 63·5% (347/546) were of Asian heritage. Among WLWH, HIV was sexually-transmitted in 48·2% (124/257) and maternally-transmitted in 42·8% (110/257) of cases. Overall, 93·4% (240/257) had WHO HIV clinical stage 1 and 61·5% (158/257) were using HAART. Median CD4 cell count was 569 (interquartile range [IQR]: 444–780) and 609 (IQR: 488–783) cells/mm3 in the AS04-HPV-16/18 and 4vHPV groups, respectively. The groups were comparable in terms of clinical stage and HIV viral load (median 40 copies/mL in both groups; IQRs: 9–2531 and 9–3343 for the AS04-HPV-16/18 and 4vHPV groups, respectively). At baseline, 94·8% (254/268) of subjects without HIV (in both vaccine groups) were seronegative for HPV-16 and HPV-18 antibodies vs. 70·3% (83/118) and 81·0% (94/116) of WLWH (AS04-HPV-16/18 and 4vHPV groups, respectively) (Table 2).
Table 2.
Baseline demographic and HIV clinical characteristics (TVC).
| WLWH |
Women without HIV |
Total | |||
|---|---|---|---|---|---|
| AS04-HPV-16/18 | 4vHPV | AS04-HPV-16/18 | 4vHPV | ||
| Randomized, N (TVC) | 129 | 128 | 144 | 145 | 546 |
| Female, n | 129 | 128 | 144 | 145 | 546 |
| Mean age, years (SD) | 20·4 (3·4) | 20·1 (3·5) | 19·3 (3·0) | 19·6 (3·0) | 19·8 (3·2) |
| Asian: | |||||
| Central/South Asian heritage, n (%) | 20 (15·5) | 18 (14·1) | 67 (46·5) | 68 (46·9) | 173 (31·7) |
| South East Asian heritage, n (%) | 40 (31·0) | 44 (34·4) | 39 (27·1) | 42 (29·0) | 165 (30·2) |
| East Asian heritage, n (%) | 3 (2·3) | 2 (1·6) | 1 (0·7) | 0 | 6 (1·1) |
| Japanese heritage, n (%) | 0 | 0 | 1 (0·7) | 2 (1·4) | 3 (0·5) |
| White: | |||||
| Caucasian/European heritage, n (%) | 33 (25·6) | 37 (28·9) | 24 (16·7) | 19 (13·1) | 113 (20·7) |
| Other: | |||||
| Arabic / North African heritage, n (%) | 17 (13·2) | 12 (9·4) | 7 (4·9) | 6 (4·1) | 42 (7·7) |
| African heritage / African American, n (%) | 10 (7·8) | 6 (4·7) | 4 (2·8) | 4 (2·8) | 24 (4·4) |
| Other, n (%) | 6 (4·7) | 9 (7·0) | 1 (0·7) | 4 (2·8) | 20 (3·7) |
| HPV Seropositive at baseline, n (%) | |||||
| Anti-HPV-16 and 18 antibody | 5 (3·9) | 3 (2·3) | 1 (0·7) | 1 (0·.7) | 10 (1·8) |
| Anti-HPV-16 antobody only | 16 (12·4) | 11 (8·6) | 5 (3·5) | 5 (3·4) | 37 (6·8) |
| Anti-HPV-18 antibody only | 14 (10·9) | 8 (6·3) | 1 (0·7) | 1 (0·7) | 24 (4·4) |
| Seronegative | 83 (64·3) | 94 (73·4) | 127 (88·2) | 127 (87·6) | 431 (78·9) |
| Missing n | 11 (8·5) | 12 (9·4) | 10 (6·9) | 11 (7·6) | 44 (8·1) |
| HIV Mode of transmission, n (%): | – | – | |||
| Sexual | 66 (51·2) | 58 (45·3) | 124 (48·2) | ||
| Blood transfusion | 0 (0) | 2 (1·6) | 2 (0·8) | ||
| Drug user by needles | 4 (3·1) | 1 (0·8) | 5 (1·9) | ||
| From mother | 50 (38·8) | 60 (46·9) | 110 (42·8) | ||
| Other | 7 (5·4) | 5 (3·9) | 12 (4·7) | ||
| Unknown | 2 (1·6) | 1 (0·8) | 3 (1·2) | ||
| Multiple modes | 0 (0) | 1 (0·8) | 1 (0·4) | ||
| WHO HIV Clinical stage, n (%): | – | – | |||
| Stage 1 | 120 (93·0) | 120 (93·8) | |||
| Stage 2 | 7 (5·4) | 3 (2·3) | |||
| Stage 3 | 1 (0·8) | 1 (0·8) | |||
| Stage 4 | 1 (0·8) | 4 (3·1) | |||
| HIV viral load n (%) | |||||
| Detectable | 45 (34 9) | 44 (34 4) | |||
| Not detectable (≤400 copies/mL) | 84 (65 1) | 84 (65 6) | |||
| Antiretroviral (HAART) use, n (%): | – | – | |||
| Yes | 80 (62·0) | 78 (60·9) | 158 (61·5) | ||
| No | 49 (38·0) | 50 (39·1) | 99 (38·5) | ||
| CD4 cell count (cells/mm3) | |||||
| n | 124 | 122 | – | – | 246 |
| n Missing | 5 | 6 | 11 | ||
| n (%) with CD4 counts 350–500 | 39 (30·2) | 39 (30·5) | |||
| n (%) with CD4 counts >500 | 90 (69·8) | 89 (69·5) | |||
| Median | 569·1 | 609·0 | 586·5 | ||
| Q1 | 444·0 | 488·0 | 448·0 | ||
| Q3 | 780·0 | 783·0 | 782·2 | ||
| Mean | 655·7 | 667·2 | 661·4 | ||
| Minimum | 196·0 | 123·0 | 123·0 | ||
| Maximum | 2703·0 | 1598·0 | 2703·0 | ||
| HIV viral load (copies/mL) | |||||
| n | 129 | 128 | – | – | 257 |
| Median | 40·0 | 40·0 | 40·0 | ||
| Q1 | 9·0 | 9·0 | 9·0 | ||
| Q3 | 2531·0 | 3343·0 | 2900·0 | ||
| Mean | 11,002·1 | 10,417·9 | 10,711·2 | ||
| Minimum | 0·0 | 0·0 | 0·0 | ||
| Maximum | 293,840·0 | 600,000·0 | 600,000·0 | ||
HAART: highly-active antiretroviral therapy; HIV: human immunodeficiency virus; HPV: human papillomavirus; n: number of subjects in a category; N: total number of subjects; Q: quartile; SD: standard deviation; TVC: total vaccinated cohort; WHO: World Health Organization; WLWH: women living with HIV.
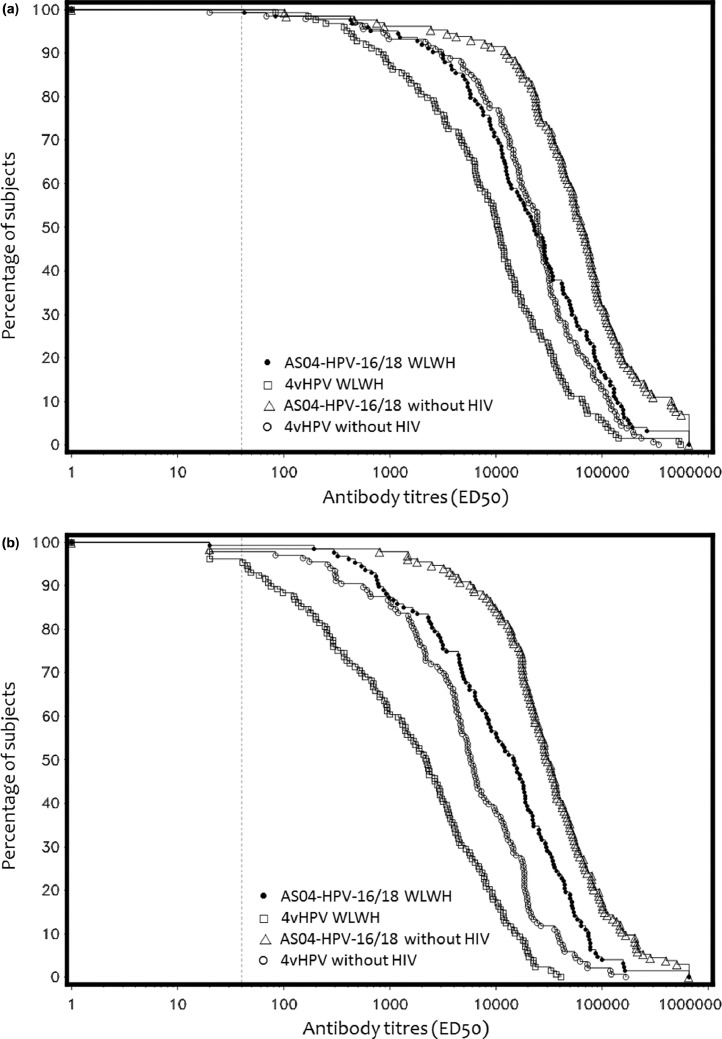
The primary immunogenicity endpoint was met, with demonstration of non-inferiority and immunological superiority of AS04-HPV-16/18 versus 4vHPV in WLWH at month 7; anti-HPV-16 and anti-HPV-18 PBNA titers were 2·74-fold and 7·44-fold higher (p<0·0001), with AS04-HPV-16/18. Immunological superiority with AS04-HPV-16/18 versus 4vHPV was also demonstrated in subjects without HIV at month 7; anti-HPV-16 and anti-HPV-18 PBNA titers were 3·05-fold and 5·38-fold higher with AS04-HPV-16/18 (both p<0·0001), respectively (Table 3). In addition, the immunogenicity of AS04-HPV-16/18 in WLWH subjects was comparable and non-inferior to 4vHPV in subjects without HIV (ATP cohort, by PBNA) (Fig. 2 and Table 3).
Table 3.
Immunogenicity of AS04-HPV-16/18 and 4vHPV vaccines, by PBNA at month 7.
| Non-inferiority analysis in WLWH subjects (Month 7 ATP cohort) | ||||||
|---|---|---|---|---|---|---|
| AS04-HPV-16/18 | 4vHPV | Adjusted GMT ratio (95%CI) | ||||
| anti-HPV-16 PBNA titers | n = 80 | 22,253·4 | n = 83 | 7542·9 | 2·95 (1·92; 4·52) | |
| anti-HPV-18 PBNA titers | n = 80 | 11,855·2 | n = 83 | 1514·9 | 7·83 (4·84; 12·66) | |
| Superiority analysis in WLWH subjects (TVC) | ||||||
|---|---|---|---|---|---|---|
| AS04-HPV-16/18 | 4vHPV | Adjusted GMT ratio (95%CI) | p value | |||
| anti-HPV-16 PBNA titers | n = 109 | 20,279·6 | n = 110 | 7400·8 | 2·74 (1·83; 4·11) | <0·0001 |
| anti-HPV-18 PBNA titers | n = 109 | 11,128·1 | n = 110 | 1496·5 | 7·44 (4·79; 11·54) | <0·0001 |
| Superiority analysis in subjects without HIV (TVC) | ||||||
|---|---|---|---|---|---|---|
| AS04-HPV-16/18 | 4vHPV | Adjusted GMT ratio (97·5%CI) | p value | |||
| anti-HPV-16 PBNA titers | n = 105 | 60,249·2 | n = 112 | 19,726·8 | 3·05 (1·84; 5·06) | <0·0001 |
| anti-HPV-18 PBNA titers | n = 105 | 32,016·1 | n = 112 | 5947·8 | 5·38 (3·20; 9·06) | <0·0001 |
| Non-inferiority analysis in AS04-HPV-16/18-WLWH subjects vs. 4vHPV- subjects without HIV (ATP) | ||||||
|---|---|---|---|---|---|---|
| AS04-HPV-16/18-WLWH | 4vHPV-women without HIV | Adjusted GMT ratio (95%CI) | ||||
| anti-HPV-16 PBNA titers | n = 80 | 22,515·2 | n = 80 | 27,234·7 | 0·83 (0·57–1·20) | |
| anti-HPV-18 PBNA titers | n = 80 | 12,397·4 | n = 80 | 7002·5 | 1·77 (1·20–2 61) | |
ATP: according to protocol; CI: confidence interval; HIV: human immunodeficiency virus; HPV: human papillomavirus; PBNA: Pseudovirion-Based Neutralization Assay; TVC: total vaccinated cohort; WLWH: women living with HIV.
Adjusted GMT = geometric mean antibody titer adjusted for country.
n = Number of subjects with post-vaccination results available.
Fig. 2.
Reverse cumulatitive distribution curves for anti-HPV-16 and anti-HPV-18 neutralizing antibody titers (by ED50 level) at month 7 on initially seronegative subjects (TVC, PBNA). (a) Anti-HPV-16 PBNA titers. (b) Anti-HPV-18 PBNA titers.Figure 2 shows the reverse cumulative distribution curves for anti-HPV-16 and anti-HPV-18 neutralizing antibody titers in initially seronegative subjects (measured by PBNA) at Month 7 in each study group.
ATP: according to protocol; ED50: highest dilution that caused at least 50% reduction in enzymatic activity; HIV: human immunodeficiency virus; HPV: human papillomavirus; PBNA: Pseudovirion-Based Neutralization Assay; WLWH: women living with HIV
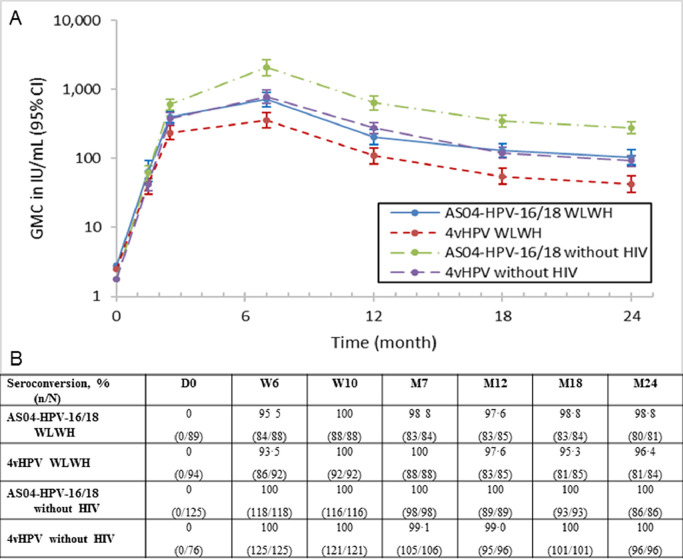
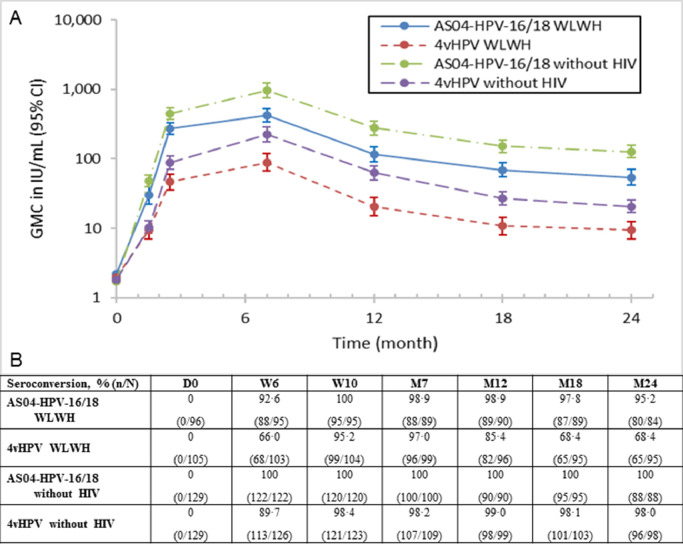
Serum antibody response to HPV-16 and HPV-18 was assessed throughout the study duration for both vaccines by ELISA. A correlation was shown at month 7 in WLWH between PBNA and ELISA. Pearson's correlation coefficient for HPV-16 was 77·5% for AS04-HPV-16/18 and 88·1% for 4vHPV. For HPV-18, the coefficients were 84·5% and 95·4%, respectively. Antibodies showed a peak in concentration one month after vaccination and started reach a plateau phase by month 24 (Fig. 3, Fig. 4, Table A3). In the TVC, nearly all initially seronegative subjects had seroconverted for HPV-16 antibodies at month 7, with seropositivity rates at month 24 remaining above 96%. At month 7, most subjects (≥97%) had seroconverted for HPV-18 antibodies. However, by month 18 seropositivity rate dropped to 68·4% (65/95 subjects) for 4vHPV while AS04-HPV-16/18 maintained a high seroconversion rate (97·8%, 87/89 subjects).GMCs in the AS04-HPV-16/18 groups remained above those in the corresponding 4vHPV groups for both antigens at month 24. WLWH receiving AS04-HPV-16/18 had non-inferior antibody responses to subjects without HIV receiving 4vHPV (Table 3). Within both AS04-HPV-16/18 and 4vHPV WLWH groups, antibody response to HPV-16 and HPV-18 was higher in subjects with maternally-transmitted vs. sexually-transmitted HIV. Antibody responses to both antigens also appeared higher with AS04-HPV-16/18 vs. 4vHPV, regardless of HIV transmission mode (Tables A4 and A5). When comparing WLWH according to baseline CD4 cell count, antibody responses to both HPV-16 and HPV-18 appeared higher in WLWH with higher CD4 counts (i.e., >500 vs. 350–500 at baseline), and appeared higher with AS04-HPV-16/18 vs. 4vHPV for both antigens, regardless of baseline CD4 cell count. In addition, WLWH with lower CD4 counts who received AS04-HPV-16/18 had comparable and higher antibody titers, to HPV-16 and HPV-18 respectively, than WLWH with higher CD4 counts who received 4vHPV (Tables A6 and A7).
Fig. 3.
(A) Persistence of HPV-16 antibody titers (International units per mL[IU/mL]) and (B) seroconversion rates in all subjects who received at least one dose of vaccine (TVC).
Seroconversion: percentage of subjects with an anti-HPV-16 VLP IgG antibody concentrations ≥3.1 IU/mL (by ELISA) ATP: according to protocol; D: Day; CI: confidence interval; ELISA: Enzyme-linked Immunosorbent Assay; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IgG: immunoglobulin G; M: Month; n: number of subjects seroconverted; N: total number of subjects; VLP: virus-like particle; W: Week; WLWH: women living with HIV
Fig. 4.
(A) Persistence of HPV-18 antibody titers (International Units per mL [IU/mL]) and (B) seroconversion rates in all subjects who received at least one dose of the vaccine (TVC). Seroconversion: percentage of subjects with an anti-HPV-18 VLP IgG antibody concentrations ≥3.2 IU/mL (by ELISA) ATP: according to protocol;D: Day; CI: confidence interval; ELISA: Enzyme-linked Immunosorbent Assay; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IgG: immunoglobulin G; M: Month; n: number of subjects seroconverted; N: total number of subjects; VLP: virus-like particle; W: Week; WLWH: women living with HIV.
In a subset of post-menarcheal subjects living with HIV, HPV-16 and HPV-18 antibody titers were assessed in CVS samples by ELISA. At month 24, anti-HPV-16 antibodies were still detected in 55·6% (n = 5/9) of AS04-HPV-16/18 and 50·0% (n = 6/12) of 4vHPV subjects (seronegative at baseline), while anti-HPV-18 antibodies were detected in 55·6% (n = 5/9) of AS04-HPV-16/18 and 15·4% (n = 2/13) of 4vHPV subjects (TVC). Despite the small sample size, these data appear to be in line with the serum antibody titers showing higher responses with AS04-HPV-16/18 versus 4vHPV.
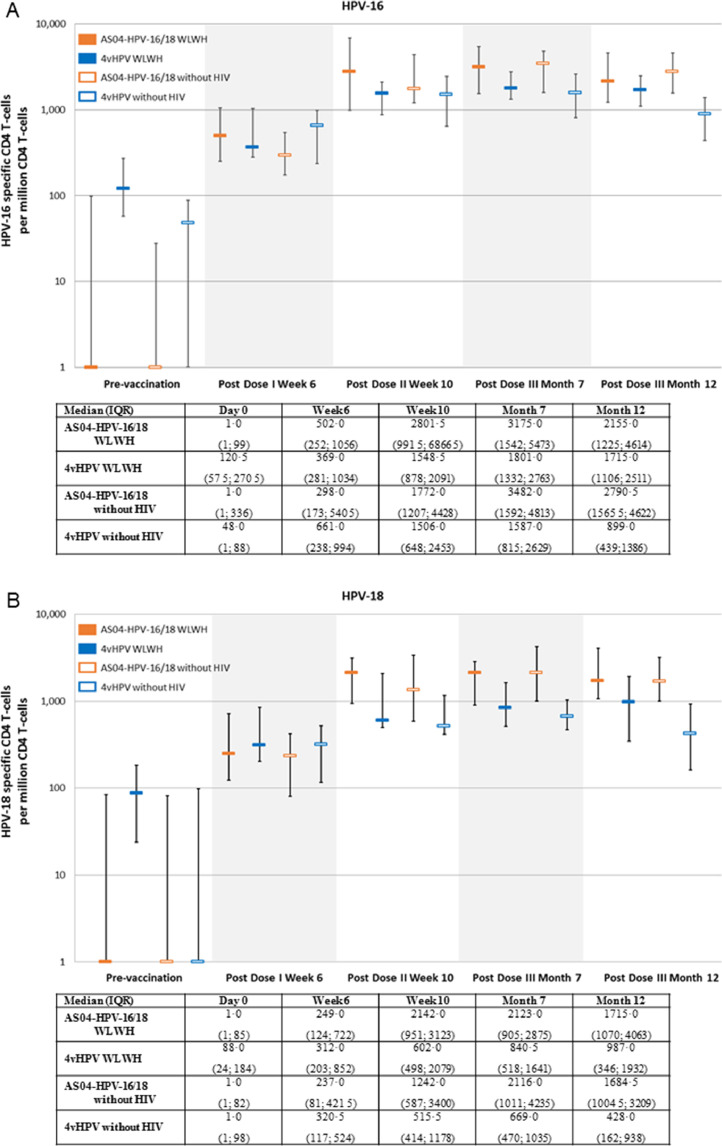
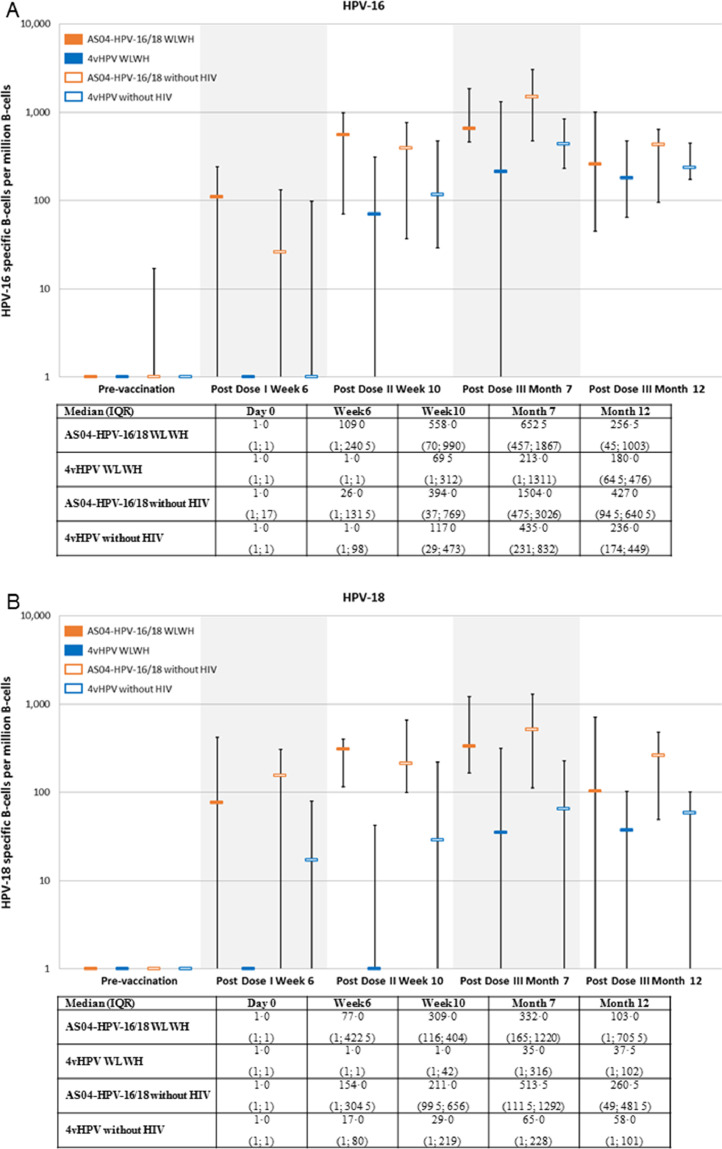
Cell-mediated immunity was assessed in a subset of 102 subjects; CD4 T cell responses (i.e., median frequency of HPV-16 and HPV-18 specific CD4 T cells per million CD4 T cells expressing at least two different immune markers) were detected in all groups, and were comparable in subjects with or without HIV for both antigens. There was a trend for higher responses in AS04-HPV-16/18 versus 4vHPV groups from the second dose (Fig. 5). No substantial HPV-16 and HPV-18 specific CD8 T cell responses were detected. Overall there was a trend for better memory B-cell responses with AS04-HPV-16/18 versus 4vHPV. Memory B cell response against HPV-18 in WLWH receiving 4vHPV were poor (Fig. 6). A summary of key results is shown in Table A8.
Fig. 5.
HPV-16 (A) and HPV-18 (B) specific CD4 T-cell mediated immune responses (subjects seronegative at baseline, TVC; median and IQR 25% and 75%). ATP: according to protocol; HIV: human immunodeficiency virus; HPV: human papillomavirus; IQR: interquartile range; WLWH: women living with HIV.
Fig. 6.
HPV-16 (A) and HPV-18 (B) specific B-cell mediated immune responses (subjects seronagtive at baseline, TVC; median and IQR 25% and 75%). ATP: according to protocol; HIV: human immunodeficiency virus; HPV: human papillomavirus; IQR: interquartile range; WLWH: women living with HIV.
The post-hoc modeling of the response in terms of neutralizing antibodies at Month 7 comparing the two vaccines and adjusting for HAART, CD4 and age in WLWH showed a GMT ratio of 2·95 (1·99, 4·35) for HPV-16 and 8·02 (5·32, 12·08) for HPV-18 in favor of AS04 HPV-16/18.
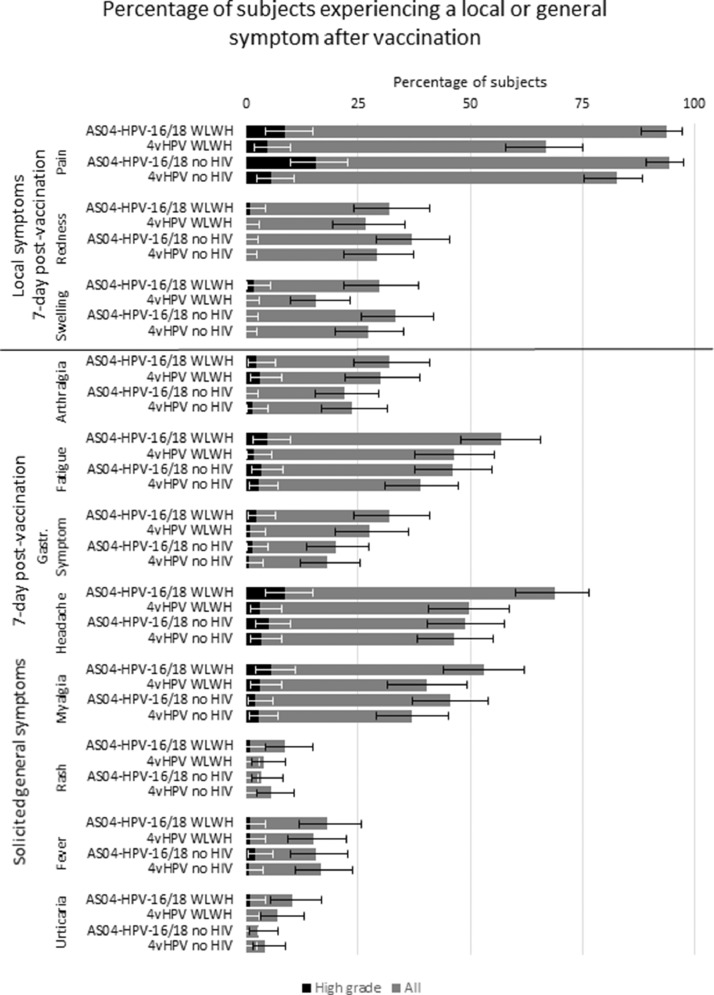
Solicited local and general symptoms were in line with approved product labels. Within seven days of vaccination, the most frequently reported solicited local symptom in all groups was pain at the injection site, occurring in 93·8% (120/128) and 94·3% (133/141) of AS04-HPV-16/18 subjects and 66·9% (85/127) and 82·6% (119/144) of 4vHPV subjects, (subjects with and without HIV, respectively) (Fig. 7). The most frequently reported grade 3 symptom was pain, occurring in 8·6% (11/128) and 15·6% (22/141) of AS04-HPV-16/18 subjects and, 4·7% (6/127) and 5·6% (8/144) of 4vHPV subjects (subjects with and without HIV, respectively). The most frequently reported solicited general symptom in all groups was headache, occurring in 68·8% (88/128) and 48·9% (69/141) of AS04-HPV-16/18 subjects and 49·6% (63/127) and 46·5% (67/144) of 4vHPV subjects (subjects with and without HIV, respectively). Individual grade 3 solicited general symptoms were reported in not more than 8·6% (11/128) and 5·0% (7/141) of AS04-HPV-16/18 subjects and 3·1% (4/127) and 3·5% (5/144) of 4vHPV subjects (subjects with and without HIV, respectively).
Fig. 7.
Percentage of subjects experiencing a local (above solid black line) or general (below solid black line) symptom after vaccination.
Gastr. Symptom: gastrointestinal symptom; high grade: grade 3, or >50 mm (redness, swelling), or >39 °C (fever); WLWH: women living with HIV.
Within 30 days post vaccination, the most frequently reported unsolicited symptoms were headache (5·4% [7/129] of subjects in the WLWH AS04-HPV-16/18 group), dysmenorrhea (2·8% [4/144] of subjects in AS04-HPV-16/18 group without HIV) and nasopharyngitis (3·1% [4/128] and 4·1% [6/145] of subjects in the 4vHPV groups with and without HIV, respectively). At least one grade 3 unsolicited symptom was reported by 5·4% (7/129) and 0·7% (1/144) of AS04-HPV-16/18 subjects, and 3·1% (4/128) and 2·1% (3/145) of 4vHPV subjects (with and without HIV, respectively). None of the grade 3 unsolicited symptoms were reported by more than one subject in any group. At least one unsolicited symptom considered by the investigator to have a possible causal relationship to vaccination was reported by 7·0% (9/129) and 2·8% (4/144) of AS04-HPV-16/18 subjects and, 6·3% (8/128) and 1·4% (2/145) of 4vHPV subjects (with and without HIV, respectively): the most frequently reported were headache and dizziness, reported by two subjects (1·6%) in the AS04-HPV-16/18 group. One subject (0·8%) in the AS04-HPV-16/18 WLWH group reported one grade 3 unsolicited symptom (immune thrombocytopenic purpura also reported as a SAE). This was considered by the investigator to be due to underlying HIV infection which may have been aggravated by the study vaccine, and was therefore classed as having a possible causal relationship to vaccination.
There were more SAEs and MSCs reported in WLWH vs women without HIV. Apart from the case mentioned above with immune thrombocytopenic purpura, no other SAEs were considered to be related to vaccination. By month 7, six subjects each in the AS04-HPV-16/18 and 4vHPV groups with HIV reported at least one SAE while among subjects without HIV, one subject in the AS04-HPV-16/18 group reported two SAEs. During the entire study period, 23 subjects reported a total of 29 SAEs (WLWH: nine subjects with 11 SAEs in the AS04-HPV-16/18 group, nine subjects with 12 SAEs in the 4vHPV group and, without HIV: four subjects with five SAEs in the AS04-HPV-16/18 group and, one subject with one SAE in the 4vHPV group). Two non vaccine related fatal SAEs (bacterial pneumonia and pulmonary tuberculosis) were reported in one subject in the 4vHPV group with HIV 1 month after receiving the third dose of the 4vHPV vaccine. All non-fatal SAEs resolved without sequelae. Apart from the subject with fatal SAEs, no subjects were withdrawn from the study due to an AE or SAE.
Up to month 18 (12 months after the last dose of vaccination), at least one MSC was reported by 25 (19·4%) and 37 (28·9%) WLWH (AS04-HPV-16/18 and 4vHPV groups, respectively), and by 10 (6·9%) and 17 (11·7%) subjects without HIV (AS04-HPV-16/18 and 4vHPV groups, respectively). The most frequently reported MSCs (based on an incidence of ≥2 subjects) were herpes zoster, pain, anemia and vulvovaginal pruritus in the WLWH AS04-HPV-16/18 group, reported by two subjects (1·6%) each, and diarrhea, reported by five subjects (3·9%) in the WLWH 4vHPV group. None of the MSCs were reported by more than one subject in vaccine groups without HIV.
5. Discussion
AS04-HPV-16/18 demonstrated immunological superiority to 4vHPV in WLWH at month 7, with significantly higher anti-HPV-16 and HPV-18 neutralizing antibody titers (PBNA). At month 7, nearly all initially seronegative subjects had seroconverted for HPV-16 and HPV-18 antibodies. In the 4vHPV group, seropositivity rates for HPV-18 sharply decreased to 68·4% by month 18, whereas in other groups and for HPV-16 seropositivity rates remained close to 100%. Antibody responses were overall lower in WLWH vs subjects without HIV, however immunogenicity levels achieved in WLWH with AS04-HPV-16/18 were comparable to those achieved in subjects without HIV having received 4vHPV. More of the WLWH vs. subjects without HIV were already seropositive to HPV-16 and/or HPV-18 at baseline, in both vaccine groups. Among WLWH, antibody responses tended to be higher in subjects with maternally-transmitted HIV versus sexually-transmitted HIV, and for subjects with higher CD4 cell counts. AS04-HPV-16/18 was however able to induce a comparable antibody response in subjects with mild immunodeficiency status (CD4 counts of 350–500 cells/mm³) to the response produced by 4vHPV in subjects with normal CD4 counts (>500 cells/mm³). Regarding cell-mediated immunity, CD4 T cell responses were detected in all groups and were comparable for both antigens, while there was a trend for higher memory B cell responses with AS04-HPV-16/18 vs. 4vHPV. The 4vHPV group of WLWH showed a poor B-cell response for HPV-18 with a median value of 35·0 cells per million memory B cells at month 7, vs. 332·5 cells per million memory B cells for the AS04-HPV-16/18 vaccine. Together with the rapid waning of serum antibody seropositivity rates at month 24 (68·4%) and the observation of HPV-18 breakthrough infections in an independent efficacy trial, these findings raise questions on the potential of waning of protection against HPV-18 infection in the long term for 4vHPV [23]. Safety and reactogenicity profiles of both HPV vaccines in all subjects were acceptable and in accordance with labels.
These results are in line with other findings in both healthy populations and WLWH [11,25]. Results from a large phase III randomized trial in healthy women aged 18 to 45 years also showed a significantly higher immune response with AS04-HPV-16/18 vs. 4vHPV, in terms of serum neutralizing antibodies to HPV-16 and HPV-18 at month 7, which was sustained after five years (i.e., 2·3- to 7·8-fold differences in immune response between the vaccines, depending on subjects’ age). Based on these data, statistical models predicted that antibody responses could persist for a longer duration with AS04-HPV-16/18 vs. 4vHPV [11]. In a randomized controlled double-blind trial in 61 men and 30 women living with HIV (older adults), three doses of AS04-HPV-16/18 and 4vHPV were immunogenic and well tolerated, with superior immune responses noted for AS04-HPV-16/18 among WLWH and, higher neutralizing anti-HPV-18 antibody titers at seven and 12 months in men and women living with HIV [21]. A follow-up study assessing cell-mediated immunity against HPV in a subset of 30 adults living with HIV on long-term antiretroviral treatment reported that both vaccines were able to induce significant and comparable frequencies of HPV-antigen specific CD4 T cells [22].
Humoral and cell-mediated immunity are both likely to be responsible for vaccine-induced protection [14]. In this study, the AS04-HPV-16/18 vaccine showed immunological superiority vs 4vHPV and the ability to elicit a similar response in WLWH when compared to the 4vHPV in subjects without HIV. A higher immune response induced by vaccination has the potential to induce longer-lasting protection in subjects with altered immunocompetence. The adjuvant system in AS04-HPV-16/18 is likely to play a key role in the immunogenicity of the vaccine; in addition to aluminum hydroxide, AS04 contains MPL which is a potent immune-system stimulant that induces high antibody and T cell responses [17]. In phase II trials, the AS04-adjuvanted vaccine was able to induce significantly higher T cell and antibody responses to both HPV-16 and HPV-18 antigens compared to the same antigens formulated with aluminum hydroxide only [26]. AS04 is also thought to play a key role in the broader than expected vaccine efficacy beyond vaccine types HPV-16 and HPV-18, first observed in phase III clinical trials and more recently in real life settings [14,27].
This study was limited in the interpretation of immunogenicity given that there is no accepted immune correlate of protection for HPV vaccines, although it is believed that antibodies play a key role in protection. Another potential limitation of this study was the use of an ELISA using the L1-based VLPs present in AS04-HPV-16/18 as coating antigen to assess some (non-primary) endpoints. However, for the co-primary immunogenicity endpoint, we used PBNA, which was developed at the US National Cancer Institute [28] and for which the L1L2-based HPV pseudovirions are independent of either vaccine L1-based VLPs. In a previous randomized trial of these vaccines, a strong correlation was found in immune responses whether measured by PBNA or ELISA [10], and the same was shown in the present study for both vaccines. Interestingly, the correlation was found slightly better for 4vHPV than for AS04-HPV-16/18 in this trial.
To date, this is the largest head to head study that looked at HPV vaccine immunogenicity in WLWH. Both vaccines were well tolerated and immunogenic in subjects living with HIV. The AS04-HPV-16/18 vaccine was shown to be immunologically superior to 4vHPV in WLWH with higher HPV-16/18 antibody response and higher seropositivity rates to month 24. Interestingly, the AS04-HPV-16/18 vaccine induced a similar response in WLWH than 4vHPV in subjects without HIV. Of potential concern for long term protection is the apparent low humoral response -both in terms of elicited antibodies and seroconversion rates- and the nearly lack of cellular immune memory response for the HPV-18 component of the 4vHPV vaccine, in line with the observation of breakthrough infections in an independent efficacy study [23]. Overall, the difference in immune response observed after vaccination with the two vaccines highlights the potential benefits of the AS04 adjuvantation. Previous and present studies suggest a potential for a more robust and longer lasting protection with the adjuvanted vaccine, hopefully ongoing trials may help providing an answer to that question (NCT03180034 and NCT03309033).
Contributors
All authors contributed to the manuscript, having reviewed and approved its content. NF was responsible for the study at GSK and was involved in study design and conduct, data collection, analysis, and interpretation of results. JT, SJ, LG, KS, PB, TC, PC, KR, CRM, BG, SQ, NK, SP, VK were investigators for the study. They collected data and participated in the interpretation of results. MD, DF, NK, SP, BS performed analysis and participated to interpretation of the results. LL, SD, GD, MH, DMC,FTDS, FTJ, FS were involved in study design and conduct, and interpretation of results.
Declaration of Competing Interest
N Folschweiller is an employee of the GSK group of companies and holds shares in the GSK group of companies. J Teixeira, S Joshi, K Supparatpinyo, and K Ruxrungtham report grants from the GSK group of companies for the conduct of the study. K Ruxrungtham also received honoraria or consultation fees from Merck, Roche, Jensen-Cilag, Tibotec, Myland and GPO (Governmental pharmaceutical organization, Thailand) and participated in a company sponsored speaker's bureau from Abbott, Gilead, Bristol-Myers Squibb, Merck, Roche, Jensen-Cilag, GSK, and Thai GPO. L Goldani, P Basu, C Roteli-Martins, B Grinsztejn, S Quintana, N Kumarasamy, S Poongulali and V Kulkarni report no conflict of interest. T Chotpitayasunondh reports grant for the study conduct and speaker honorarium outside of the present work, from the GSK group of companies. P Chetchotisakd reports grant from the GSK group of companies for the study conduct and from Gilead outside of the present work. L Lin, S Datta, D Descamps, F Tavares da Silva, D Friel, S Poncelet, and B Salaun are employees of the GSK group of companies and hold shares in the GSK group of companies. M Dodet, N Karkada are employees of the GSK group of companies. G Dubin was an employee of the GSK group of companies during the study conduct, holds shares in the GSK group of companies, and is currently an employee of Takeda Pharmaceuticals. F Struyf was an employee of the GSK group of companies during the study conduct, holds shares in the GSK group of companies, and is currently an employee of Janssen, Pharmaceutical Companies of Johnson & Johnson. M Hezareh reports consulting fees for her institution from the GSK group of companies. D Meric Camilleri was an employee of the GSK group of companies and held shares in the company at the time of the study. F Thomas-Jooris was an employee of the GSK group of companies at the time of the study and hold shares in the GSK group of companies.
Acknowledgments
Acknowledgments
The authors thank the study participants, investigators and site staff, as well as the IDMC members. They also thank A Avihingsanon, R Awedikian, B Brasseur, C Cartier, M-P David, L Declerck, K Dobbelaere, S Emmadi, A Modi, P Moris, H Mulle, P Suryakirian, A-S Vilain, K Zilmer, and J Zima for their contribution to the study and D Borys for her critical review of the study data. Authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and publication coordination, on behalf of GSK. Jonathan Ghesquiere coordinated publications development and editorial support. The authors also thank Kavi Littlewood (Littlewood Writing Solutions, on behalf of GSK) for providing medical writing support.
Data sharing
The results summary for this study (GSK study number 109823 – NCT01031069) is available on the GSK Clinical Study Register and can be accessed at www.gsk-clinicalstudyregister.com. Within 6 months of this publication, anonymized individual participant data, the annotated case report form, protocol, reporting and analysis plan, data set specifications, raw dataset, analysis-ready dataset, and clinical study report will be available for research proposals approved by an independent review committee. Proposals should be submitted to www.clinicalstudydatarequest.com. A data access agreement will be required.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100353.
Appendix
Tables A1, A2, A3, A4, A5, A6, A7, and A8,
Table A1.
Primary and secondary endpoint description
| Co-primary immunogenicity endpoint | To demonstrate non-inferiority of AS04-HPV-16/18 versus (vs.) 4vHPV in terms of geometric mean titers (GMTs) against HPV-16 and HPV-18 measured by Pseudovirion-based neutralization assay (PBNA) one month after administration of the third dose of vaccine in HIV+ subjects. Criterion: Non-inferiority was to be demonstrated if the lower limit of the 95% confidence interval (CI) for the ratio of GMTs (AS04-HPV-16/18 over 4vHPV) was above 0·5 for both HPV types. |
If non-inferiority was demonstrated, demonstrate superiority of AS04-HPV-16/18 over 4vHPV in terms of GMTs against HPV-16 and HPV-18 measured by PBNA in HIV+ subjects assessed in a sequential approach:
Superiority was to be demonstrated if the lower limit of the 95% CI for the ratio of GMTs (AS04-HPV-16/18 over 4vHPV) was above 1 for HPV-16 type with a statistically significant p-value. | |
| Co-primary safety endpoint | Safety: safety and reactogenicity of both vaccines in HIV+ subjects for up to one month after the third dose of vaccine (up to month 7).
|
| Secondary immunogenicity endpoints | To demonstrate superiority of AS04-HPV-16/18 vs. 4vHPV in terms of GMTs against HPV-16 or HPV-18 measured by PBNA one month after the administration of the third dose of vaccine in HIV- subjects. Criterion: Superiority of AS04-HPV-16/18 vs. 4vHPV was to be demonstrated if the lower limit of the 97·5% CI for the ratio of GMTs (AS04-HPV-16/18 over 4vHPV) was above 1 for the antigen considered with a statistically significant p-value. |
| To evaluate the antibody response of both vaccines with respect to HPV-16 and HPV-18 antibody titers and total IgG titers by Enzyme-Linked Immunosorbent Assay (ELISA) in serum at Day 0, Week 6, Week 10, Months 7, 12, 18 and 24 in all (HIV+ and HIV-) subjects. | |
| To evaluate the antibody response with respect to HPV-16 and HPV-18 antibody titers and total IgG titers, by ELISA in cervico-vaginal secretions (CVS) at Day 0, Week 6, Week 10, Months 7, 12 and 24 in post-menarcheal subjects who volunteer for this procedure. | |
| To evaluate the memory B and T cell-mediated immune (CMI) response (frequencies of HPV-16 and HPV-18 specific B-cells and T cells) at Day 0, Week 6, Week 10, Months 7 and 12 in a subset of approximately 100 subjects (50 HIV+ and 50 HIV-). | |
| Secondary safety endpoints | Safety: Safety and reactogenicity of both vaccines in HIV- subjects for up to one month after the third dose of vaccine. Safety and reactogenicity of both vaccines in all subjects for up to 24 months after the first vaccine dose |
Table A2.
Statistical methods for all immunogenicity and safety analyses.
| Immunogenicity: between group and within group assessment | between-group comparisons to assess non-inferiority were done on the according-to-protocol (ATP) cohort for immunogenicity (by Pseudovirion-based neutralization assay [PBNA], regardless of HPV serostatus at baseline). A second analysis on Total Vaccinated cohort (TVC) was performed to support the primary analysis. The within-group comparisons were performed on the ATP cohort for analysis of immunogenicity. A second analysis based on the TVC was performed to complement the ATP analysis. |
|
| PBNA and ELISA | For each group, at each time point with a blood sample result available (Months 0 and 7 for PBNA; Day 0, Week 6, Week 10, Months 7, 12, 18 and 24 for enzyme-linked immunosorbent assay [ELISA]), the following analyses were conducted: |
|
| CMI | in a subset of approximately 100 subjects from selected countries (Day 0, Week 6, Week 10, Months 7 and 12; T cell by intracellular cytokine staining [ICS]; B cell by enzyme-linked immunospot [ELISPOT]), the following analyses were performed: |
|
| Safety analysis | The primary safety analysis was performed on the TVC. A second analysis based on the ATP cohort for safety was performed to complement the TVC analysis. The following analyses were done on HIV+ subjects (primary objectives) and on HIV- subjects (secondary objectives): |
|
Table A3.
Geometric mean antibody concentration (GMC) for HPV-16 and HPV-18 antibodies in subjects seronegative at baseline by ELISA (TVC).
| anti-HPV-16 antibody (IU/mL) | AS04-HPV-16/18 WLWH |
4vHPV WLWH |
AS04-HPV-16/18 without HIV |
4vHPV without HIV |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GMC | 95%CI | GMC | 95%CI | GMC | 95%CI | GMC | 95%CI | |||||
| VISIT1 (D0) | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 |
| VISIT2 (W6) | 30.3 | 22.6 | 40.5 | 22.9 | 17.2 | 30.6 | 53.2 | 44.2 | 63.9 | 35.2 | 30.0 | 41.2 |
| VISIT3 (W10) | 343.5 | 275.6 | 428.2 | 194.9 | 151.9 | 250.2 | 578.0 | 472.5 | 707.0 | 357.1 | 293.7 | 434.3 |
| VISIT5 (M7) | 752.3 | 566.2 | 999.5 | 354.9 | 269.5 | 467.4 | 2123.6 | 1621.0 | 2782.1 | 777.9 | 611.2 | 990.1 |
| VISIT6 (M12) | 189.1 | 140.2 | 255.1 | 92.2 | 68.1 | 124.8 | 642.7 | 504.6 | 818.8 | 274.9 | 226.2 | 334.1 |
| VISIT7 (M18) | 119.1 | 89.9 | 157.7 | 43.5 | 32.1 | 58.9 | 348.3 | 284.6 | 426.4 | 118.6 | 98.8 | 142.4 |
| VISIT8 (M24) | 96.8 | 71.7 | 130.7 | 33.7 | 25.0 | 45.5 | 281.8 | 226.8 | 350.2 | 91.5 | 76.2 | 109.9 |
| anti-HPV-18 antibody (IU/mL) | AS04-HPV-16/18 WLWH |
4vHPV WLWH |
AS04-HPV-16/18 without HIV |
4vHPV without HIV |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GMC | 95%CI | GMC | 95%CI | GMC | 95%CI | GMC | 95%CI | |||||
| VISIT1 (D0) | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 |
| VISIT2 (W6) | 19.8 | 15.2 | 25.8 | 7.0 | 5.3 | 9.3 | 45.2 | 37.2 | 54.9 | 9.8 | 8.1 | 11.7 |
| VISIT3 (W10) | 280.6 | 225.7 | 348.8 | 40.5 | 30.9 | 53.2 | 457.4 | 373.4 | 560.3 | 85.1 | 68.7 | 105.3 |
| VISIT5 (M7) | 486.6 | 381.5 | 620.7 | 82.8 | 60.9 | 112.6 | 1010.8 | 777.7 | 1313.7 | 217.4 | 170.2 | 277.7 |
| VISIT6 (M12) | 121.2 | 91.7 | 160.2 | 18.0 | 13.1 | 24.6 | 278.7 | 219.2 | 354.2 | 60.9 | 48.3 | 76.9 |
| VISIT7 (M18) | 69.9 | 52.9 | 92.5 | 9.1 | 6.8 | 12.3 | 156.3 | 126.3 | 193.3 | 25.8 | 20.8 | 32.0 |
| VISIT8 (M24) | 53.4 | 39.2 | 72.8 | 8.0 | 6.0 | 10.6 | 132.8 | 106.9 | 164.9 | 19.7 | 15.9 | 24.5 |
ATP: according to protocol;D: Day; CI: confidence interval; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IU: International unit; M: Month; W: Week; WLWH: women living with HIV.
Table A4.
Percentage of WLWH (seronegative at baseline) with seroconversion and anti-HPV-16 VLP IgG antibody GMCs by ELISA, by HIV transmission mode (TVC).
| AS04-HPV-16/18 |
4vHPV |
|||||||
|---|---|---|---|---|---|---|---|---|
| HIV transmission mode | Sexual |
Mother |
Sexual |
Mother |
||||
| % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | |
| VISIT1 (D0) | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 |
| VISIT2 (W6) | 97.8 | 42.6 | 92.1 | 19.8 | 91.9 | 25.7 | 93.5 | 20.0 |
| VISIT3 (W10) | 100 | 268.4 | 100 | 457.0 | 100 | 113.6 | 100 | 285.6 |
| VISIT5 (M7) | 97.8 | 505.7 | 100 | 1219.7 | 100 | 182.8 | 100 | 575.9 |
| VISIT6 (M12) | 95.6 | 113.3 | 100 | 333.2 | 97.0 | 47.0 | 97.7 | 143.4 |
| VISIT7 (M18) | 97.7 | 72.7 | 100 | 206.1 | 93.8 | 22.6 | 95.5 | 65.7 |
| VISIT8 (M24) | 97.7 | 59.2 | 100 | 161.7 | 96.9 | 18.5 | 95.3 | 49.4 |
D: Day; CI: confidence interval; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IgG: immunoglobulin G; IU: International unit; M: Month; W: Week; WLWH: women living with HIV.
Table A5.
Percentage of WLWH (seronegative at baseline) with seroconversion and anti-HPV-18 VLP IgG antibody GMCs by ELISA, by HIV transmission mode (TVC).
| AS04-HPV-16/18 |
4vHPV |
|||||||
|---|---|---|---|---|---|---|---|---|
| HIV transmission mode | Sexual |
Mother |
Sexual |
Mother |
||||
| % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | |
| VISIT1 (D0) | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 |
| VISIT2 (W6) | 93.6 | 20.4 | 89.5 | 16.0 | 61.7 | 6.3 | 70.8 | 7.6 |
| VISIT3 (W10) | 100 | 211.8 | 100 | 354.2 | 91.7 | 26.2 | 97.9 | 60.4 |
| VISIT5 (M7) | 97.7 | 338.7 | 100 | 736.1 | 95.7 | 43.0 | 97.8 | 159.8 |
| VISIT6 (M12) | 97.8 | 75.7 | 100 | 198.4 | 77.3 | 9.0 | 93.2 | 34.4 |
| VISIT7 (M18) | 95.5 | 42.0 | 100 | 113.6 | 50.0 | 4.7 | 84.4 | 16.2 |
| VISIT8 (M24) | 90.5 | 31.2 | 100 | 87.9 | 48.8 | 4.2 | 84.1 | 13.8 |
D: Day; CI: confidence interval; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IgG: immunoglobulin G; IU: International unit; M: Month; W: Week; WLWH: women living with HIV.
Table A6.
Percentage of WLWH (HPV seronegative at baseline) with seroconversion and anti-HPV-16 VLP IgG antibody GMCs by ELISA, by baseline CD4 cell count.
| AS04-HPV-16/18 |
4vHPV |
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4 baseline | CD4 350–500 |
CD4 >500 |
CD4 350–500 |
CD4 >500 |
||||
| % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | |
| VISIT1 (D0) | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 |
| VISIT2 (W6) | 95.8 | 20.0 | 95.3 | 35.3 | 90.0 | 16.3 | 95.2 | 27.0 |
| VISIT3 (W10) | 100 | 229.0 | 100 | 399.9 | 100 | 120.9 | 100 | 242.9 |
| VISIT5 (M7) | 96.0 | 481.6 | 100 | 908.7 | 100 | 203.8 | 100 | 459.8 |
| VISIT6 (M12) | 96.0 | 133.4 | 98.3 | 218.7 | 96.3 | 57.7 | 98.3 | 114.7 |
| VISIT7 (M18) | 96.0 | 83.0 | 100 | 138.8 | 92.6 | 26.7 | 96.6 | 54.6 |
| VISIT8 (M24) | 96.0 | 63.1 | 100 | 117.2 | 92.6 | 21.4 | 98.2 | 41.8 |
D: Day; CI: confidence interval; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IgG: immunoglobulin G; IU: International unit; M: Month; W: Week; WLWH: women living with HIV.
Table A7.
Percentage of WLWH (HPV seronegative at baseline) with seroconversion and anti-HPV-18 VLP IgG antibody GMCs by ELISA, by baseline CD4 cell count.
| AS04-HPV-16/18 |
4vHPV |
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4 baseline | CD4 350–500 |
CD4 >500 |
CD4 350–500 |
CD4 >500 |
||||
| % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | % | GMC (IU/mL) | |
| VISIT1 (D0) | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 | 0.0 | 1.6 |
| VISIT2 (W6) | 93.3 | 12.6 | 92.3 | 24.4 | 54.8 | 5.5 | 70.8 | 7.8 |
| VISIT3 (W10) | 100 | 167.0 | 100 | 356.5 | 90.3 | 21.9 | 97.3 | 52.6 |
| VISIT5 (M7) | 96.6 | 344.9 | 100 | 574.7 | 96.7 | 43.0 | 97.1 | 110.2 |
| VISIT6 (M12) | 96.6 | 78.3 | 100 | 149.1 | 75.9 | 10.1 | 89.6 | 23.1 |
| VISIT7 (M18) | 96.6 | 45.5 | 98.3 | 86.1 | 46.4 | 4.9 | 77.6 | 11.9 |
| VISIT8 (M24) | 93.1 | 32.6 | 96.4 | 69.3 | 48.3 | 4.3 | 77.3 | 10.5 |
D: Day; CI: confidence interval; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IgG: immunoglobulin G; IU: International unit; M: Month; W: Week; WLWH: women living with HIV.
Table A8.
Summary table of key results.
|
Superiority of AS04-HPV-16/18 vs 4vHPV in WLWH (TVC) by PBNA at month 7 | ||||||
| AS04-HPV-16/18 |
4vHPV |
Adjusted GMT ratio (95%CI) |
p value |
|||
| N | GMT | N | GMT | |||
| anti-HPV-16 PBNA titers |
109 | 20,279.6 | 110 | 7400.8 | 2.74 (1.83; 4.11) | <0·0001 |
| anti-HPV-18 PBNA titers |
109 | 11,128.1 | 110 | 1496.5 | 7.44 (4.79; 11.54) | <0·0001 |
|
Seroconversion (GMC ≥ 3.1 IU/mL) at month 24 in WLWH (TVC) | ||||||
| AS04-HPV-16/18 |
4vHPV |
|||||
| n/N | % | 95% CI | n/N | % | 95% CI | |
| anti-HPV-16 antibody |
80/81 | 98.8 | 93.3–100 | 81/84 | 96.4 | 89.9–99.3 |
| anti-HPV-18 antibody |
80/84 | 95.2 | 88.3–98.7 | 65/95 | 68.4 | 58.1–77.6 |
|
GMC (IU/mL) at month 24 in initially seronegative WLWH (TVC) | ||||||
| AS04-HPV-16/18 |
4vHPV |
|||||
| N | GMC | 95%CI | N | GMC | 95%CI | |
| anti-HPV-16 antibody |
81 | 96.8 | 71.7–130.7 | 84 | 33.7 | 25.0–45.5 |
| anti-HPV-18 antibody |
84 | 53.4 | 39.2–72.8 | 95 | 8.0 | 6.0–10.6 |
|
Non-inferiority of AS04-HPV-16/18 in WLWH vs 4vHPV in HIV-negative (ATP) by PBNA at month 7 | ||||||
| AS04-HPV-16/18 |
4vHPV |
Adjusted GMT ratio (95%CI) |
95%CI (LL-UL) |
|||
| N | GMT | N | GMT | |||
| anti-HPV-16 PBNA titers |
80 | 22,515.2 | 80 | 27,234.7 | 0.83 | 0.57–1.20 |
| anti-HPV-18 PBNA titers |
80 | 12,397.4 | 80 | 7002.5 | 1.77 | 1.20–2.61 |
|
CD4 T-cell response median frequency in initially seronegative WLWH at month 12 (TVC) | ||||||
| AS04-HPV-16/18 |
4vHPV |
|||||
| N | Median | IQR | N | Median | IQR | |
| HPV-16 | 14 | 2155.0 | 1225.0–4614.0 | 10 | 1715.0 | 1106.0–2511.0 |
| HPV-18 | 11 | 1715.0 | 1070.0–4063.0 | 13 | 987.0 | 346.0–1932.0 |
| Memory B-cell response in initially seronegative WLWH at month 12 (TVC) | ||||||
| AS04-HPV-16/18 |
4vHPV |
|||||
| N | Median | IQR | N | Median | IQR | |
| HPV-16 | 18 | 256.5 | 45.0–1003.0 | 8 | 180.0 | 64.5–476.0 |
| HPV-18 | 16 | 103.0 | 1.0–705.5 | 10 | 37.5 | 1.0–102.0 |
Appendix B. Supplementary materials
References
- 1.World Health Organization (WHO). Cancer fact sheets: cervical cancer. 2012. http://gco.iarc.fr/today/fact-sheets-cancers?cancer=16&type=0&sex=2 (accessed 21-08-2018).
- 2.Walboomers J.M., Jacobs M.V., Manos M.M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N., Bosch F.X., de Sanjose S. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New England J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.de Sanjose S., Quint W.G., Alemany L. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Human papillomavirus vaccines: Who position paper, May 2017. http://www.who.int/wer/2017/wer9219/en/ (accessed 18-08-2018).
- 6.Denny L.A., Franceschi S., de Sanjose S., Heard I., Moscicki A.B., Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30(Suppl 5):F168–F174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Moscicki A.B., Ellenberg J.H., Farhat S., Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190(1):37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency (EMA). Cervarix: summary of product characteristics. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000721/WC500024632.pdf (accessed 21-08-2018).
- 9.European Medicines Agency (EMA). Gardasil: summary of product characteristics. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000703/WC500021146.pdf (accessed 21-08-2018).
- 10.Einstein M.H., Baron M., Levin M.J. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 11.Einstein M.H., Takacs P., Chatterjee A. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother. 2014;10(12):3435–3445. doi: 10.4161/hv.36121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung T.F., Liu A.P., Lim F.S. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and 4vHPV vaccine administered according to two- or three-dose schedules in girls aged 9-14years: results to month 36 from a randomized trial. Vaccine. 2018;36(1):98–106. doi: 10.1016/j.vaccine.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Herrin D.M., Coates E.E., Costner P.J. Comparison of adaptive and innate immune responses induced by licensed vaccines for human papillomavirus. Hum Vaccin Immunother. 2014;10(12):3446–3454. doi: 10.4161/hv.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryser M., Berlaimont V., Karkada N., Mihalyi A., Rappuoli R., van der Most R. Post-hoc analysis from Phase III trials of human papillomavirus vaccines: considerations on impact on non-vaccine types. Expert Rev Vaccines. 2019;18(3):309–322. doi: 10.1080/14760584.2019.1579647. [DOI] [PubMed] [Google Scholar]
- 15.Advisory Committee on Immunization Practices (ACIP). ACIP recommendations: general recommendations on immunization. 2006. http://www.cdc.gov/mmwr/PDF/rr/rr5515.pdf (accessed 21-08-2018).
- 16.Palefsky J. CHAPTER 5 hpv infection and HPV-associated neoplasia in immunocompromised women. Int J Gynaecol Obstet. 2006;94(Suppl 1):S56–S64. doi: 10.1016/S0020-7292(07)60011-3. [DOI] [PubMed] [Google Scholar]
- 17.Denny L., Hendricks B., Gordon C. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. 2013;31(48):5745–5753. doi: 10.1016/j.vaccine.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Levin M.J., Moscicki A.B., Song L.Y. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin M.J., Huang S., Moscicki A.B. Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: effect of a fourth dose of vaccine. Vaccine. 2017;35(13):1712–1720. doi: 10.1016/j.vaccine.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojic E.M., Kang M., Cespedes M.S. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin infect dis. 2014;59(1):127–135. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toft L., Storgaard M., Muller M. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis. 2014;209(8):1165–1173. doi: 10.1093/infdis/jit657. [DOI] [PubMed] [Google Scholar]
- 22.Zurek Munk-Madsen M., Toft L., Kube T. Cellular immunogenicity of human papillomavirus vaccines Cervarix and Gardasil in adults with HIV infection. Hum Vaccin Immunother. 2018;14(4):909–916. doi: 10.1080/21645515.2017.1407896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClymont E., Lee M., Raboud J. The efficacy of the quadrivalent human papillomavirus vaccine in girls and women living with human immunodeficiency virus. Clin Infect Dis. 2018;68(5):788–794. doi: 10.1093/cid/ciy575. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). WHO HPV labnet. 2012. https://www.who.int/biologicals/areas/human_papillomavirus/WHO_HPV_LabNet/en/ (accessed 17-04-2019).
- 25.Kojic E.M., Rana A.I., Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccin. 2016;15(1):105–117. doi: 10.1586/14760584.2016.1110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannini S.L., Hanon E., Moris P. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33–34):5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Kavanagh K., Pollock K.G., Cuschieri K. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17(12):1293–1302. doi: 10.1016/S1473-3099(17)30468-1. [DOI] [PubMed] [Google Scholar]
- 28.Pastrana D.V., Buck C.B., Pang Y.Y. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.