Abstract
Background
Pathyashadangam kwath, a classical ayurvedic polyherbal formulation is used for the treatment of cluster head ache, migraine, upper respiratory diseases, ear ache and night blindness. Review of literature suggested that characterization parameters of Pathyashadangam kwath are not reported.
Objective
To report characteristic parameters of Pathyashadangam kwath to confirm quality and purity.
Materials and methods
The fruit pericarps of Haritaki, Bibhitaki and Amalaki, aerial parts of Bhunimba, rhizome of Haridra, stem bark of Nimba and stem of Guduchi were the ingredients of Pathyashadangam kwath. Three batches of the kwath were prepared as per standard procedures. The kwath was evaluated for organoleptic, physical, phytochemical and chromatographic parameters as per standard methods.
Results
HPTLC analysis revealed that Toluene: Ethyl Acetate: Formic acid (2.5: 2.0: 0.5) was a suitable mobile phase for characterization of the kwath. HPLC analysis revealed that andrographolide was a suitable marker for standardization of the kwath.
Conclusion
The characterization parameters presented in this paper may serve as standard reference for quality control analysis of Pathyashadangam kwath.
Keywords: Standardization, Phytochemical, HPLC, HPTLC, Pathyashadangam kwath
1. Introduction
More than 75% of world's population relies on traditional medicine for their basic health issues. India with its quintessence in ayurvedic medicine, is the coffers of more than 45,000 plant species. Of these 7500 finds praxis in convalesce. Ayurveda, a holistic science which accentuate on maintaining fitness in addition to treatment of diseases, relies mainly on healing potential of plants. The focus of Ayurveda is to restore balance by eradicating the root cause of disease using a blend of natural elements and prevent the recurrence of imbalance by creating a healthy life style. Ayurveda treatises like Sarngadhara samhita highlights the importance of combining different herbs in a particular proportion to lessen toxicity and augment therapeutic efficacy [1]. According to kwatha vidhi of S. samhita, kashayam or kwath is the filtered decoction obtained after boiling a mixture of herbs with 16 times water under low flame for a prolonged period till the volume is reduced to one eighth [2]. It is used as a starter for the preparation of different dosage forms or employed directly as drug.
On account of its natural origin and lack of side effects, the demand for ayurvedic drugs have aggrandized across the world. The commercialization of traditional medicines has led to the widespread use of adulterants and low cost substitutes, because of non-availability and high cost of standard authentic drugs. Hence it has now become imperative to testify polyherbal formulations as per modern research parameters to standardize and evaluate its quality [3].
Quality assurance which guarantees the delivery of apt quantity of standard medicament is an integral part of traditional medicine [4]. Organoleptic and physical evaluation of drugs and its comparison with standard values is helpful to authenticate top quality drugs, free of adulteration. Chromatographic fingerprinting with its ability to characterize chemical composition of herbal drug products can serve as a useful tool for appraising batch to batch consistency [5]. Physicochemical parameters, biochemical analysis, microbiological features and HPTLC fingerprint profile may be used as marker parameters for quality evaluation and standardization of polyherbal formulations [6].
Pathyashadangam kwath (Pathyadi shadangam kashayam) is a classical ayurvedic polyherbal formulation prescribed by ayurvedic physicians for the treatment of upper respiratory tract infections and different types of head ache. There is dearth of information regarding the scientific analysis of Pathyashadangam kwath. Hence characterization of Pathyashadangam kwath was attempted to confirm its quality and purity.
2. Materials and methods
2.1. Plant materials
Pathyashadangam kwath was prepared from seven ingredients, viz., Haritaki (Terminalia chebula Retz.), Bibhitaki (Terminalia bellirica (Gaertn.) Roxb.), Amalaki (Phyllanthus emblica L.), Bhunimba (Andrographis paniculata (Burm. f.) Wall. ex Nees), Haridra (Curcuma longa L.), Nimba (Azadirachta indica A. Juss.) and Guduchi (Tinospora cordifolia (Willd.) Miers.). The fruit pericarps of Haritaki, Bibhitaki and Amalaki, aerial parts of Bhunimba, rhizome of Haridra, stem bark of Nimba and stem of Guduchi were employed for preparation of the formulation [7]. Voucher specimens of all these ingredients were deposited in the herbarium of KFRI, Peechi for future reference (Accession numbers 13037-13043).
2.2. Methods
Pathyashadangam kashayam was prepared as per standard method described in kwatha vidhi of S. samhita. Three batches of kwath viz PS 15, PS 23 and PS 25 were prepared in the same manner at different times in the months of April and May.
2.2.1. Organoleptic evaluation
Organoleptic characters like colour, odor, taste and consistency of the kashayam were evaluated based on the method described by Siddiqui et al. [8].
2.2.2. Physical evaluation
Different physical parameters like total solids, specific gravity, water soluble extractive value, alcohol soluble extractive values and pH were evaluated using standard pharmacopoeial methods [9].
2.2.3. Microbial load
Total aerobic microbial count (bacterial and fungal) was determined by plate count method [10]. Test for specific organisms was carried out as per standard procedures [11].
2.2.4. Heavy metal analysis
The kashayam was digested in HNO3 and HCLO4 (5:2). The volume of the digested samples was then made up to 100 ml with deionized water and analyzed with ICP-AES system (Thermo Electron, IRIS INTREPID II XSP DUO, Munich, Germany). The limit of detection was Cd 0.01 ppm; Pb 0.05 ppm; Hg 0.1 ppm and As 0.04 ppm.
2.2.5. Phytochemical investigation
The kashayam was evaporated on a water bath for the removal of water, the dried mass was refluxed with methanol for three times and filtered. The methanol extract was subjected to preliminary phytochemical analysis. Ethanol extract was prepared in the same manner and phytochemical analysis was carried out. Dragendorff's test for alkaloids, Alkaline reagent test for flavonoids, Ferric chloride test for tannins, Froth formation test for saponins, Keller-killiani test for glycosides, Libermann Burchard test for steroids and Salkowski test for triterpenoids were conducted [12].
2.2.5.1. Quantification of total bitter
To estimate total bitter of the kashayam, 5 g of the kashayam was refluxed with 50 ml ethanol for 30 min and filtered. The process was repeated thrice. The alcohol was evaporated from the filtrate and the residue was shaken with 25 ml hot water. The extract was shaken with 25 ml ethyl acetate and the solution was transferred to a separating funnel and the ethyl acetate layer was collected in a previously weighed flask (W1).The process was repeated with 20 ml and 15 ml hot water and ethyl acetate and continued till the ethyl acetate layer turned colourless. The ethyl acetate was distilled off and the final residue was weighed (W2). The percentage of total bitter was calculated from the formula (W2—W1) × 100/Weight of the sample and was expressed as mean ± standard deviation of three values [13].
2.2.5.2. Quantification of andrographolide
Andrographolide in the three batches of the kwath was quantified. 3 g of the kwath was refluxed with 50 ml ethanol for 30 min and filtered. The process was repeated thrice. The alcohol was evaporated from the filtrate and the residue was shaken with 25 ml hot water. The above extract was shaken with 25, 15 and 10 ml petroleum ether till the ether layer turned colourless. The above solution was transferred to a separating funnel and the ether layer was discarded. The aqueous phase was shaken with 25, 20, 15 and 10 ml ethyl acetate. The ethyl acetate layer was collected in a previously weighed flask (W1). The ethyl acetate was distilled off and evaporated to dryness. Weight of the final residue was taken (W2). Percentage of andrographolide was calculated by the formula (W2—W1) × 100/Weight of sample and was expressed as mean ± standard deviation of three values [13].
2.2.6. HPTLC
The methanol extracts of three batches of kashayam were subjected to HPTLC analysis. The kashayam was dried in a water bath at a fixed temperature and was extracted with methanol. 4 micro litres of the extract was spotted on HPTLC silica gel 60F 254(Merck) plate as bands of length 6 mm at a distance of 10 mm. The plates were developed using Toluene: Ethyl Acetate: Formic acid (2.5: 2.0: 0.5) in the CAMAG twin-trough glass chamber, previously saturated with the solvent for 30 min. The mobile phase was chosen after testing different solvent systems of varying polarity. After development, the plates were dried in an oven at 60 °C and scanned using a CAMAG TLC Scanner in absorbance mode. Data processing was performed with winCATS planar chromatography manager software. The compounds were scanned at 254 and 366 nm.
2.2.7. HPLC
The methanol extract of the kashayam was subjected to HPLC using andrographolide as marker. A kashayam was prepared excluding all ingredients of Pathyashadangam kashayam other than A. paniculata and was also subjected to HPLC. The HPLC analysis was carried out using Luna 5u C18 analytical column (250 × 4.6 mm), Shimadzu LC-10 AT vp binary pump and SPD-M10A vp photo diode array detector (PDA). The mobile phase consisted of water (A) and acetonitrile (B). The elution condition was 0–25 min, 20%–55% B [14]. The injection volume was 20 μl and flow rate was 1 ml/min. UV spectral range was 190 nm–400 nm. The auto sampler and column compartment were maintained at 25 °C and 35 °C respectively.
3. Results
3.1. Organoleptic evaluation
Organoleptic evaluation of the kashayam revealed that it had bitter taste, brown colour, and characteristic odour and was a colloidal suspension.
3.2. Physical evaluation
The specific gravity of the kashayam was found to be 1.075 for all the three batches. The pH was found to be 3.46, 3.39 and 3.48 and total solids was 18.72, 17.95 and 17.34% for PS 15, PS 23 and PS 25 respectively. Water soluble extractive value of PS 15, PS 23 and PS 25 was 14.79, 15.43 and 12.50% respectively. The methanol soluble extractive value was 13.13, 11.89 and 10.43% and ethanol soluble extractive value was 9.15, 9.66 and 9.18% respectively for PS 15, PS 23 and PS 25. Thus it was found that the extractive value was highest for water followed by methanol and ethanol.
3.3. Microbial load
Analysis of the kwath for aerobic microbial count revealed that the kwath was free from bacterial and fungal contamination. Test for specific organisms revealed that pathogenic bacteria like Escherichia coli, Salmonella, Staphylococcus aureus and Pseudomonas aeruginosa were absent in the kwath.
3.4. Heavy metal analysis
Heavy metal analysis of the formulation revealed that the formulation lacked heavy metals or if present their concentration was below the permissible limits.
3.5. Phytochemical investigation
Preliminary phytochemical analysis of methanol and ethanol extracts of kashayam revealed the presence of alkaloids, flavonoids, tannins, glycosides, sterols, triterpenoids and saponins in both the extracts.
The percentage of total bitter and andrographolide in three batches of the kwath were similar. PS 15, PS 23 and PS 25 were found to contain 4.24 ± 0.11%, 4.82 ± 0.10%, 4.18 ± 0.41% total bitter and 3.89 ± 0.42%, 3.87 ± 0.18% and 3.78 ± 0.35% andrographolide respectively.
3.6. HPTLC analysis
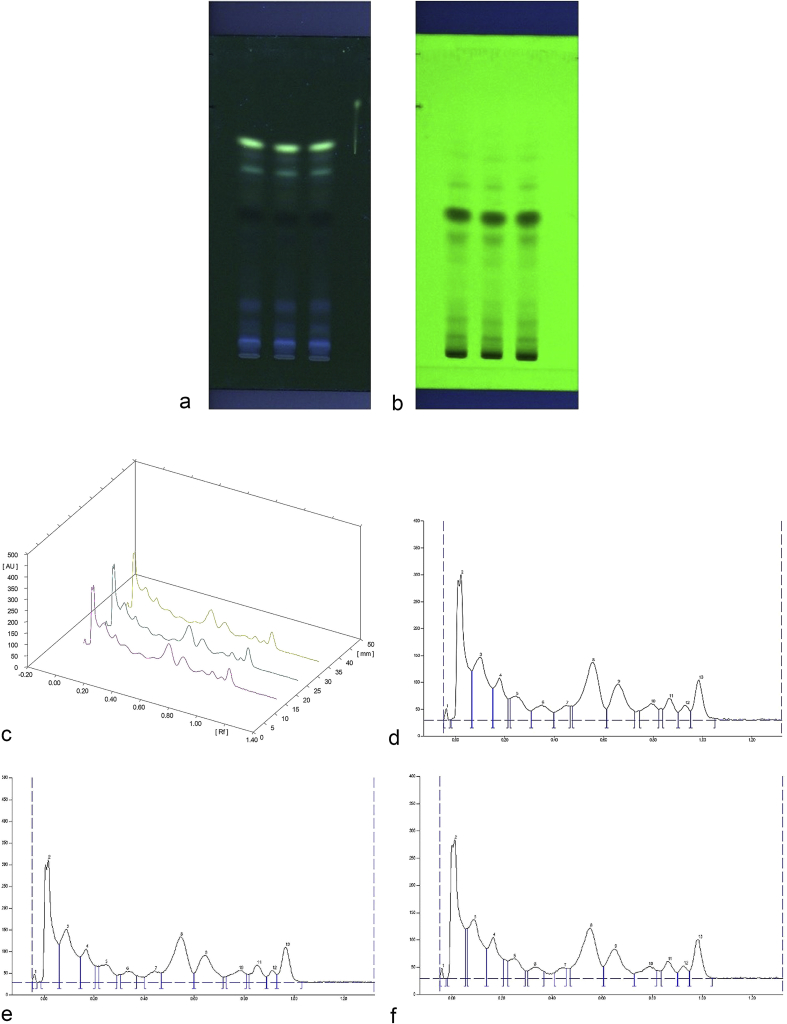
HPTLC analysis of three batches of kashayam, PS 15, PS 23 and PS 25 gave similar finger print patterns. 13 bands of similar RF value were observed for all the three batches. The intensity and colour of bands in the three batches were remarkably similar (Fig. 1). The total area under the peak was 31,359.5 for PS 15, 29,102.7 for PS 23 and 26,264.7 for PS 25 (Supplementary Table 1).
Fig. 1.
HPTLC plate of three batches of kwath under (a) UV 366 nm (b) UV 254 nm (c) Chromatogram of three batches of Pathyashadangam kwath at 366 nm (d) HPTLC chromatogram of PS 15 at 366 nm (e) HPTLC chromatogram of PS 23 at 366 nm (f) HPTLC chromatogram of PS 25 at 366 nm.
3.7. HPLC analysis
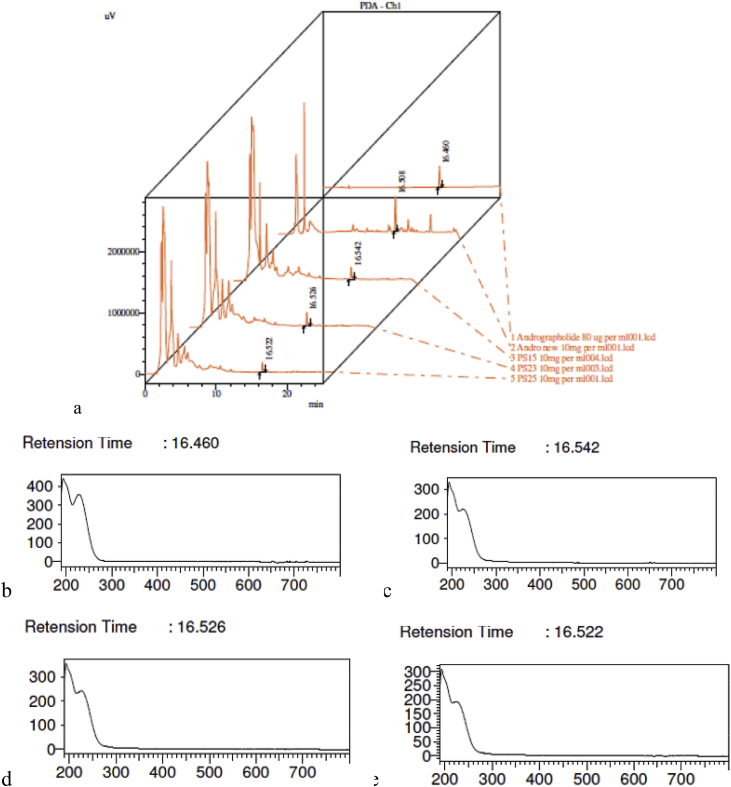
HPLC analysis of methanol extracts of three batches of Pathyashadangam kwath and Andrographis kwath was carried out along with andrographolide standard (Fig. 2a). The analysis revealed that all the three batches of pathyashadangam kwath and A. kwath showed characteristic peak corresponding to andrographolide. The RT for andrographolide was 16.46, 16.508, 16.542, 16.526 and 16.522 for standard, A. kwath, PS 15, PS 23 and PS 25 respectively. The spectral index confirmed that the peak obtained was that of andrographolide (Fig. 2b,c,d,e).
Fig. 2.
(a) HPLC profile of three batches of kwath, Andrographis kwath and andrographolide standard. (b) Spectral index of andrographolide standard and of peak corresponding to andrographolide of (c) PS 15 (d) PS 23 and (e) PS 25 at 225 nm.
4. Discussion
Organoleptic analysis is the appraisal of a product as it is perceived by sense organs and helps in the preliminary quality evaluation. Hence the organoleptic parameters of three batches of kwath were analysed as preliminary quality check which revealed that brown colour and bitter taste were characteristic of Pathyashadangam kashayam. Physicochemical analysis can be employed for routine evaluation at the sites of manufacture and established physicochemical standards can furnish information for further investigation and facilitate identification of formulations. Hence the specific gravity, pH, total solids and extractive values of the kwath were determined, which will serve as reference for future analysis. Plants are nature's drug stores. The enormous chemical compounds present in plants are to be explored for solving present day health problems. Phytochemical analysis of plants and plant products can open the keys to the goldmine of chemicals present therein. Plant derived secondary metabolites are valuable source of new drugs while some are drug precursors [15]. Flavonoids are low molecular weight secondary metabolites which possess antiageing, antibacterial and antioxidant properties and with their ability to inhibit carcinogenesis at different stages, they can emerge as effective cancer chemotherapeutic agents [16]. Tannins are water soluble polyphenols most of which have antimicrobial properties and hence can enhance the shelf life of preparations [17]. Phytochemical analysis revealed that the kashayam contained secondary metabolites like alkaloids, flavonoids, tannins, sterols, triterpenoids, and glycosides conferring cumulative pharmacological properties to the formulation.
The fear of presence of detrimental elements including heavy metals is a major apprehension causing scepticism in minds of common man regarding the safety and quality of ayurvedic formulations. Hence it is indispensable to rule out the occurrence of undesirable elements to augment the acceptance of formulations. Cadmium and Lead are reported to be of great concern since they cause adverse health issues in humans [18]. Elemental analysis of three batches of Pathyashadangam kashayam by ICP-AES revealed that toxic heavy metals like Lead and Cadmium were below detectable limits in all the three batches. In two batches of kashayam, mercury was below detectable limit and in the other batch it was below permissible limit. Arsenic was below detectable limit in two batches and very much below permissible limit in the third batch. The permissible limits for heavy metals in ayurvedic formulations is 10 ppm for lead, 1 ppm for mercury, 3 ppm for arsenic, and 0.3 ppm for cadmium [19]. So it was found that all the three batches of Pathyashadangam kashayam are safe to consume as the concentration of heavy metals was either absent or very much below permissible limits.
Herbal drugs may be contaminated with microorganisms from soil, air or water. Some of these can cause health problems to their consumers. The therapeutic activity of herbal products may be reduced or even nullified by the presence of microbial contaminants causing ill health to patients taking the drug [20]. Hence it is vital to ensure that the finished product is free from microbial contamination. Microbial load of the kwath was determined with this objective in mind. The absence of microbial growth in the kwath assures safety of its consumption.
Polyherbal formulation is a complex mixture of more than one herb in a particular proportion. The absence of any ingredient or the presence of an undesirable ingredient knowingly or unknowingly can lead to reduction of therapeutic value or even serious health problems to the consumers. Hence it is essential to ensure that only desirable drugs in precise proportion are included in the preparation of polyherbal drugs. This can be assured by quality checks and standardized manufacturing practices. For finished products quality can be ensured through fingerprinting by HPLC and HPTLC with suitable markers. HPLC/HPTLC method has been used for the estimation of markers and standardization of different ayurvedic formulations [21], [22]. Three batches of the kwath were subjected to HPTLC analysis which generated a fingerprint, wherein similarity in number, RF, intensity and colour of bands were obtained, showing that the active constituents present in all the three batches were similar and helped to establish batch to batch consistency of the formulation.
Standardization of formulation and its ingredients based on specific marker compounds and its validation is very important [23]. Andrographolide is a diterpene lactone conferring bitter taste to A. paniculata, commonly known as the king of bitters. Standardization of market preparations containing A. paniculata was achieved by RP HPLC using andrographolide as marker [24]. Methanol is a better solvent for extraction of andrographolide and dehydroandrographoilide than ethanol and aqueous acetone [25]. The fingerprint of a plant, its extracts and products will be the same under identical conditions which makes chromatographic fingerprinting an ideal method for authentication and identification of herbal drugs [26]. Co-chromatography followed by comparison of RT and absorption spectra of formulation and standards of marker compounds by which the presence of marker can be ascertained is an important parameter for quality evaluation of polyherbal formulations [27]. HPLC analysis of methanol extracts of three batches of kwath along with andrographolide standard could confirm the presence of andrographolide in the formulation. The similarity in retention time and spectral index in all the three batches of kashayam and standard andrographolide showed that andrographolide could be used as marker for the standardization of Pathyashadangam kwath.
5. Conclusion
Pathyashadangam kwath was characterized on the basis of organoleptic, physical, microbiological, heavy metal, phytochemical and chromatographic analysis. Bitter taste, brown colour, absence of heavy metal and microbial contamination, pH of 3.44 ± 0.047, specific gravity of 1.075, water soluble extractive value of 14.24 ± 1.54%, methanol soluble extractive value of 11.82 ± 1.35%, ethanol soluble extractive value of 9.33 ± 0.29%, presence of alkaloids, flavonoids, tannins, sterols, triterpenoids, saponins, glycosides and the marker compound andrographolide were found to be characteristic of the kwath. HPLTC analysis using Toluene: Ethyl acetate: Formic acid (2.5:2:0.5) mobile phase was found suitable for consistency evaluation of the kashayam. All these parameters may be employed as standard reference for quality control analysis of the formulation.
Source of funding
None.
Conflict of interest
None.
Acknowledgement
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaim.2017.10.011.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Parasuraman S., Thing G.S., Dhanaraj S.A. Polyherbal formulation: concept of ayurveda. Phcog Rev. 2014;8(16):73–80. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopalapilla S.A. 5th ed. Vol. 111. 1998. Sarngadhara samhita with hridayapriya interpretation. (Malayalam): chapter 2. Kodungalloor. [Google Scholar]
- 3.Tripathy M., Sikarwar R., Tiwari A., Dwivedi N. Pharmacognostical identification of ingredients in Laghulai curna: an Ayurvedic compound formulation. Indian J Tradit Knowl. 2015;14(4):531–536. [Google Scholar]
- 4.Mukherjee P.K., Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian systems of medicine. J Ethnopharmacol. 2006;103(1):25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Xiong H., Yu L., Qu H. Batch-to-Batch quality consistency evaluation of botanical drug products using multivariate statistical analysis of the chromatographic fingerprint. AAPS Pharm Sci Tech. 2013;14(2):802–810. doi: 10.1208/s12249-013-9966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari A., Dwivedi N., Tripathi M. Scientific evaluation and standardization of Ayurvedic compound formulation Yavanyadi curna. Indian J Tradit Knowl. 2015;14(4):544–549. [Google Scholar]
- 7.Gopalapilla S.A. 5th ed. Vol. 133. 1998. Sarngadhara samhita with hridayapriya interpretation. (Malayalam): chapter 2, verse 143-145. Kodungalloor. [Google Scholar]
- 8.Siddiqui Hakim M.A. Central Council for Research in Unani Medicine (CCRUM); New Delhi: 1995. Format for the pharmacopoeial analytical standards of compound formulation, workshop on standardization of Unani drugs. (appendix) [Google Scholar]
- 9.Anonymous . 1st ed. Vol. 1. Ministry of Health and Family Welfare. Department of AYUSH, Government of India; New Delhi: 2007. p. 141. (The Ayurvedic Pharmacopoeia of India. Part 2). [Google Scholar]
- 10.Lohar D.R. 1st ed. Vol. 88. Pharmacopoeial Laboratory for Indian Medicine, AYUSH. Ministry of Health and Family Welfare. Government of India; 2011. (Protocol for testing of ayurvedic, siddha and unani medicines). Ghaziabad. [Google Scholar]
- 11.Anonymous . 1st ed. Vol. 3. Ministry of health and family welfare. Department of AYUSH, Government of India; New Delhi: 2010. pp. 176–180. (The Ayurvedic Pharmacopoeia of India. Part 2). [Google Scholar]
- 12.Kokate C.K., Purohit A.P., Gokhale S.B. 48th ed. Nirali Prakashan; Pune: 2013. Pharmacognosy; pp. A21–A26. [Google Scholar]
- 13.Rajpal V. 2nd ed. Vol. 1. Eastern Publishers; New Delhi: 2002. p. 34. (Standardization of Botanicals (Testing and extraction methods of medicinal herbs)). 215. [Google Scholar]
- 14.Zhao Y., Kao C.P., Wu K.C., Liao C.R., Ho Y.L., Chang Y.S. Chemical compositions, chromatographic fingerprints and antioxidant activities of Andrographis herba. Molecules. 2014;19(11):18332–18350. doi: 10.3390/molecules191118332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salim A.A., Chin Y.W., Kinghorm A.D. 2008. Drug discovery from plants. In: Ramawat K.G., Mérillon J.M., editors. Bioactive molecules and medicinal plants. Springer; Berlin, Heidelberg: 2008. pp. 1–24. [Google Scholar]
- 16.Ren W., Qiao Z., Wang H., Zhu L., Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23(4):519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 17.Chung K.T., Wong T.Y., Wei C.I., Huang Y.W., Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38(6):421–464. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 18.Pilarczyk R., Wójcik J., Czerniak P., Sablik P., Pilarczyk B., Tomza-Marciniak A. Concentrations of toxic heavy metals and trace elements in raw milk of Simmental and Holstein-Friesian cows from organic farm. Environ Monit Assess. 2013;185(10):8383–8392. doi: 10.1007/s10661-013-3180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonymous . 1st ed. Vol. 2. Ministry of health and family welfare. Department of AYUSH, Government of India; New Delhi: 2008. p. 168. (The Ayurvedic Pharmacopoeia of India. Part 2). [Google Scholar]
- 20.Mukhi S., Bose A., Panda P., Rao M.M. Pharmacognostic, physicochemical and chromatographic characterization of Samasharkara churna. J Ayurveda Integr Med. 2016;7(2):88–99. doi: 10.1016/j.jaim.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra A., Mishra A.K., Tiwari O.P., Jha S. HPLC analysis and standardization of Brahmi vati - an Ayurvedic poly-herbal formulation. J Young Pharm. 2013;5(3):77–82. doi: 10.1016/j.jyp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baragi C.U., Baragi C.P., Vyas K.M., Shukla V.J. Standardization and quality control parameters of Dashanga Kwatha Ghana tablet: an Ayurvedic formulation. Int J Ayurveda Res. 2011;2(1):42–47. doi: 10.4103/0974-7788.83190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad W., Zaidi S.M.A., Mujeeb M., Ansari S.H., Ahmad S. HPLC and HPTLC methods by design for quantitative characterization and in vitro anti-oxidant activity of polyherbal formulation containing Rheum emodi. J Chromatogr Sci. 2013;52(8):911–918. doi: 10.1093/chromsci/bmt123. [DOI] [PubMed] [Google Scholar]
- 24.Sajeeb B.K., Kumar U., Halder S., Bachar S.C. Identification and quantification of andrographolide from Andrographis paniculata (burm. F.) Wall. Ex nees by RP-HPLC method and standardization of its market preparation. Dhaka Univ J Pharm Sci. 2015;14(1):71–78. [Google Scholar]
- 25.Kumoro A.C., Hasan M., Singh H. Effects of solvent properties on the Soxhlet extraction of diterpenoid lactones from Andrographis paniculata leaves. Sci Asia. 2009;35:306–309. [Google Scholar]
- 26.Sharma R.K., Arora R. 1st ed. Jaypee Brothers and Medical Publishers; New Delhi: 2006. Herbal drugs, a twenty first century perspective; p. 40. [Google Scholar]
- 27.Rajani M., Kanaki N.S. Phytochemical standardization of herbal drugs and polyherbal formulations. In: Ramawat K.G., Mérillon J.M., editors. Bioactive molecules and medicinal plants. Springer; Berlin, Heidelberg: 2008. pp. 349–369. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.