Abstract
Background
Rasashastra needs to be upgraded using the technological advances, with regards to drug processing, development and therapeutics. The potential of Rasaaushadhis need to be explored by subjecting them against newer life threatening diseases like cancer where contemporary medicine has limitations. Abhrak Bhasma, one of the drugs of Rasashastra, has some peculiar attributes. According to classical Rasashastra texts, Shataputi Abhrak Bhasma is regarded as a Rasayan, whose efficacy is in direct proportion to the number of Putas. Thus increasing number of Putas not only has a significant effect on the physical, analytical aspects but also the therapeutic effect of the Abhrak Bhasma.
Objectives
To screen in vitro anticancer activity of Abhrak Bhasma at various stages of Putas (20, 50, 100). To evaluate and thus validate the principle from classical Rasashastra texts, which explains direct relation of number of Putas with therapeutic efficacy.
Materials and methods
Shataputi Abhrak Bhasma, at various stages of its preparation was subjected to in vitro anticancer activity on three different cancer cell lines (LungHOP62, LeukemiaU937, ProstateDU145) at Tata Memorial Centre- Advanced Centre for Treatment, Research Education in Cancer, Navi Mumbai. SRB assay was followed to evaluate the anti-proliferative activity.
Results
It was found that Abhrak Bhasma shows concentration dependent positive in vitro anticancer activity on all three cell lines with highly significant activity on prostate cancer cell lines. Anticancer activity of Abhrak Bhasma is in the order 100 Puti > 50 Puti > 20 Puti. Shataputi Abhrak Bhasma had maximum activity on prostate cancer cell lines almost equivalent to positive control drug adriamycin.
Conclusion
The in vitro anticancer activity of Shataputi Abhrak Bhasma increases with increasing number of Putas, thus revalidating the direct relation between number of Putas and efficacy of the drug.
Keywords: Adriamycin, Anticancer activity, Prostate cancer, Puta, Rasayan, Shataputi Abhrak Bhasma
1. Introduction
Today, the world is reeling on the verge of an epidemic. The magnitude of mortality or morbidity of this epidemic on humans seems to be unclear and unpredictable. This epidemic is termed as ‘CANCER’.
The latest surveys carried out by the WHO, worldwide, establish and reveal some alarming facts about the incidences of cancer and compels the medical fraternity to take immediate steps to curb this growing menace the mankind is facing. Some of the key facts as stated by WHO, updated in February 2015, are as follows [1].
Cancers figure among the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases and 8.8 million cancer related deaths in 2012. The number of new cases is expected to rise by about 70% over the next 2 decades. Among men, the 5 most common sites of cancer diagnosed in 2012 were lung, prostate, colo-rectum, stomach, and liver cancer. Among women the 5 most common sites diagnosed were breast, colo-rectum, lung, cervix, and stomach cancer. It is expected that annual cancer cases will rise from 14 million in 2012 to 22 within the next 2 decades.
In the Indian System of Health, earliest and foremost record of cancer could be traced in Atharvaveda, of which Ayurveda is an Upaveda, where the disease was given the nomenclature as ‘Apacit’. Swellings or lumps situated in the deep structures or as chronic ulcers have been categorized under the heading of “ARBUDA”, where as non-healing ulcer as “ASADHYA VRANA”.
As a parallel can be drawn between the various types of cancers and the similar noted references in the Ayurvedic classical texts, there is a lot of potential to unravel the various treatment modalities mentioned in Ayurveda, which have stood the test of time. With the advent of newer and potentially life threatening disorders, it is the need of the hour to unearth and research various ways to tackle the ailments of the 21st century, keeping in view the principles of Ayurveda, which were propagated for maintaining the health of healthy and curing the diseased.
Rasashastra, one of the branches of Ayurveda, dealing with the pharmaceutical aspects, has a number of formulations having some unique attributes attached to them. It involves harnessing the therapeutic potential of herbs, metals and minerals by subjecting them to various procedures converting them into bio-assimilable form. The concepts of Rasashastra, need to be validated using the scientific and technological advances of today's world, which shall open up new avenues for drug processing and development in Ayurveda. This shall pave the way for exploring the Siddhantas (principles) given in the form of Sutras (verse) and re-establish the efficacies of the Rasa aushadhis which have been claimed to have been the saviour of human race from various ailments in the medieval and pre-medieval period.
Abhrak (Mica) is one such entity mentioned in classical Rasashastra texts having unique attributes. Shataputi and Sahastraputi Abhrak Bhasma are indeed unique attributes of Abhrak. Abhrak Bhasma is repeatedly subjected to Puta (incineration in a closed earthen vessel). Also process like Lohitikaran where Shataputi Abhrak Bhasma is tritutated with some distinct herbs before incinerating and process of Amrutikaran where Abhrak Bhasma is fried in Goghruta (cow ghee) and Triphala kwath, have a role in its therapeutic efficacy which needs evaluation. As many as 100 incinerations are mentioned for the preparation of Shataputi Abhrak Bhasma which underlines the amount of Agni Sanskaar (heat processing) Abhrak Bhasma is subjected to before being used therapeutically. Abhrak Bhasma is a Rasayan (which promotes life) [2] and its therapeutic properties undergo changes with incinerations. This is evident from the Siddhant mentioned in Rasendra Saar Sangraha [3]. Repeated incinerations lead to fineness in particle size. Recent studies show nanoparticle size range of Bhasmas [4]. Thus Bhasmas have emerged as Ayurveda's Nanomedicine. Properties of Bhasmas like Yogavahitwa (which promotes passage and movement of a formulation or drug) and Shighra vyapti (fast spreading) [5] further underline their similarities with nanoparticles in nanomedicine, having unique properties like fast action and target specific drug delivery. Nanomedicine is emerging as an important field for cancer drug research. The property of Rasayan, as mentioned in the classical text Charak Samhita can be correlated with the concept of Immunomodulation as is seen from the recent researches [6]. As also, the recent researches have shown that a number of Rasayan dravyas mentioned in Ayurveda classics have proven anti-cancer properties [7].
The need to screen more drugs of Rasa Shastra to find an innovative drug therapy for cancer management was one of the reasons of screening Shataputi Abhrak Bhasma on various cancer cell lines. A survey among the various renowned Vaidyas, established the fact that Abhrak Bhasma is widely used in combination with other dravyas in the management of various types of cancers in various stages. Some references of Abhrak Bhasma indicate its use in diseases like Shopha [8], Granthi [9], Gulma [10], Vrana [9], Pandu [10] which can be considered analogous with some form of benign or malignant tumors. Clinically, Abhrak Bhasma works predominantly on Pranavaha, Raktavaha and Mutravaha strotasas (channels of circulation). Hence, analogous, lung, leukemia and prostate cancer cells lines were selected for screening. Recently a lot of research has been done and is in progress regarding the role of Nanomedicines in cancer management, due to their unique properties like target specificity through drug delivery [11] and minimal dosages thus reducing the ill effects of the chemotherapeutic agents [12]. Shataputi Abhrak Bhasma being a Rasayan, with reduced particle size, this study was an attempt to screen its anti-cancer activity through preclinical assays and also evaluate the importance of Putas in pharmaceutics of Abhrak Bhasma.
The aim and objectives of the present study was to screen in vitro activity of Krushnavajra Abhrak Bhasma on lung (Pranavaha strotas), leukemia (Raktavaha strotas) and prostate cancer (Mutravaha strotas) cell lines and to study the effect of Puta on the in vitro anticancer activity of Krushnavajra Abhrak Bhasma. Also, to validate the Siddhanta given in Rasashastra texts which says ‘As the number of Putas is increased the qualities of the drug increases manifold (thousand times)’ [3].
2. Materials and methods
2.1. Material
Shataputi Abhrak Bhasma was prepared at the D.Y. Patil School of Ayurveda pharmacy after authentication of the raw drug to be Krushnavajra Abhrak from University of Pune. Standard Operative Procedure which was standardized by Dr. Deepali Korde and Prof. Dr. Kulwant Singh at ICPT&R, Jamnagar in 2003 was adopted for preparation. The prepared Abhrak Bhasma showed positive results when screened for classical parameters like Nischandratva, Varitaratwa, Rekhapurnatva, Apsumajjanam. It was also subjected to advanced analytical tests like XRD, ICP-AES, SEM-EDS, FEG-SEM, and TGA-DTA. During the process of preparation of the drug, samples of the Abhrak Bhasma were collected after 20 Putas (AB20), 50 Putas (AB50), 100 Putas (AB100), after Lohitikaran (ABL) and after Amrutikaran (ABA). These samples were subjected to in vitro anticancer activity.
In vitro anticancer activity of Shataputi Abhrak Bhasma was performed at Tata Memorial Centre- Advanced Centre for Treatment, Research Education in Cancer, Navi Mumbai. For the study 3 cells lines were selected ie. lung (HOP62), leukemia (U937), prostate (DU145). Instrument and materials used were SRB calorimeter, 96 well microtitre plates, dimethyl sulfoxide, 10%TCA, sulphorhodamine B dye, 1% acetic acid and plate reader.
2.2. Method
2.2.1. Cancer cell line culture
The cell lines were grown in RPMI 1640 medium containing 10% fetal bovine serum and 2 mM l-glutamine. For present screening experiment [13], [14], [15], cells were inoculated into 96 well microtiter plates in 100 μl at plating densities as shown in the study details above, depending on the doubling time of individual cell lines. After cell inoculation, the microtiter plates were incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of experimental drugs.
2.2.2. Preparation of solution of test drug
Experimental drugs were initially solubilized in dimethyl sulfoxide at 100 mg/ml and diluted to 1 mg/ml using water and stored frozen prior to use [13]. At the time of drug addition, an aliquot of frozen concentrate (1 mg/ml) was thawed and diluted to 100 μg/ml, 200 μg/ml, 400 μg/ml and 800 μg/ml with complete medium containing test article. Aliquots of 10 μl of these different drug dilutions were added to the appropriate microtiter wells already containing 90 μl of medium, resulting in the required final drug concentrations i.e.10 μg/ml, 20 μg/ml, 40 μg/ml, 80 μg/ml.
2.2.3. Plate preparation and drug addition
After compound addition, plates were incubated at standard conditions for 48 h and assay was terminated by the addition of cold TCA. Cells were fixed in situ by the gentle addition of 50 μl of cold 30% (w/v) TCA (final concentration, 10% TCA) and incubated for 60 min at 4 °C. The supernatant was discarded; the plates were washed five times with tap water and air dried [13], [16].
2.2.4. SRB assay
Sulforhodamine B (SRB) solution (50 μl) at 0.4% (w/v) in 1% acetic acid was added to each of the wells, and plates were incubated for 20 min at room temperature [16], [17]. After staining, unbound dye was recovered and the residual dye was removed by washing five times with 1% acetic acid. The plates were air dried. Bound stain was subsequently eluted with 10 mM trizma base, and the absorbance was read on a plate reader at a wavelength of 540 nm with 690 nm reference wavelength.
2.2.5. End point measurement
Percentage growth was calculated on a plate by plate basis for test wells relative to control wells [13], [17]. Percentage growth was expressed as the ratio of average absorbance of the test well to the average absorbance of the control wells × 100.
Using the six absorbance measurements [time zero (Tz), control growth (C) and test growth in the presence of drug at the four concentration levels (Ti)]; the percentage growth was calculated at each of the drug concentration levels. Percentage growth inhibition was calculated as [(Ti−Tz)/(C−Tz)] 100 for concentrations for which Ti>/ = Tz (Ti−Tz) positive or zero [(Ti−Tz)/Tz] × 100 for concentrations for which Ti.
The dose response parameters were calculated for each test article. Growth inhibition of 50% (GI50) was calculated from [(Ti−Tz)/(C−Tz)] × 100 = 50, which is the drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation. The drug concentration resulting in total growth inhibition (TGI) was calculated from Ti = Tz. The LC50 (concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning) indicating a net loss of cells following treatment is calculated from [(Ti−Tz)/Tz] × 100 = −50.
Values were calculated for each of these three parameters if the level of activity was reached; however, if the effect was not reached or was exceeded, the values for that parameter were expressed as greater or less than the maximum or minimum concentration tested. The summary of the parameters is as follows.
GI50 Growth inhibition of 50% (GI50) calculated from [(Ti−Tz)/(C−Tz)] × 100 = 50, drug concentration resulting in a 50% reduction in the net protein increase.
TGI Drug concentration resulting in total growth inhibition (TGI) will calculated from Ti = Tz.
LC50 Concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning, indicating a net loss of cells following treatment is calculated from [(Ti−Tz)/Tz] × 100 = 7−50.
Values obtained were analysed, their graphs were plotted and the final results were expressed in terms of LC50, TGI and GI50 values in comparison to standard or positive control drug taken as adriamycin.
3. Results
There are very few Rasa dravyas mentioned in Classical texts of Rasashastra where Acharyas have mentioned to give 100 Putas. Abhrak is one among them. Various Siddhantas like the one given in Rasendra Chudamani which says that when subjected to many Putas the dravya becomes finer in particle size and acquires newer unpredictable properties (Vichitra gunadipti) [12]. The effect of Puta was analysed by screening Abhrak Bhasma of 20, 50, 100 Putas and after process of Lohitikaran and Amrutikaran.
Before establishing the activity of the drug clinically, it has to be subjected to test preclinically. In the present study Abhrak Bhasma was prepared in D.Y.Patil School of Ayurveda, Navi Mumbai and was screened for in vitro anticancer activity at Tata Memorial Centre- Advanced Centre for Treatment, Research Education in Cancer, Navi Mumbai.
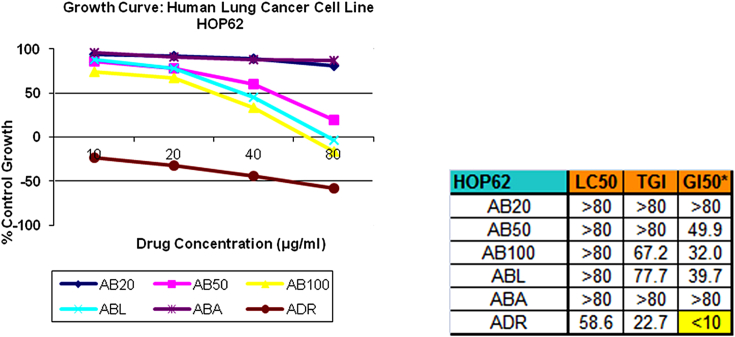
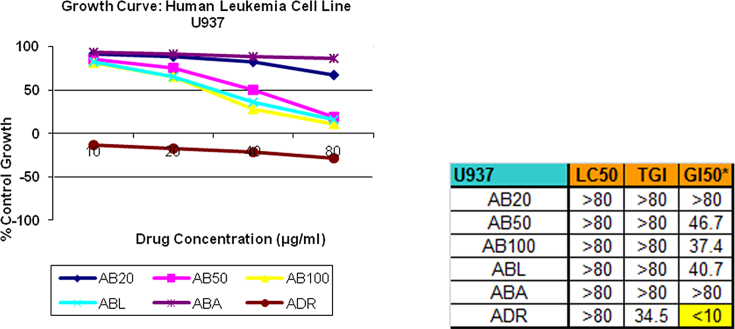
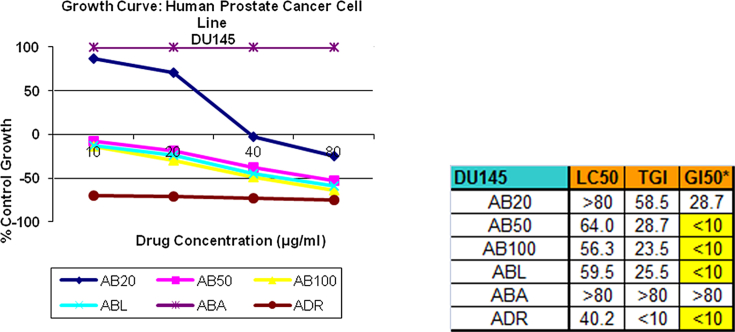
SRB assay [17] was selected as a preclinical assay for in vitro anticancer screening. This anti-proliferative assay was performed to assess growth inhibition. All 5 samples were subjected to screening at 4 different concentrations, viz. 10,20,40,80 μg/ml for 3 times and the average readings of the same were noted. All the samples were tested on 3 different cell lines, namely, lung (HOP62), leukemia (U937), prostate (DU145). The findings were represented in the form of graphs and final conclusions were drawn (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Drug concentration (μg/ml) calculated from graph.
Fig. 2.
Drug concentration (μg/ml) calculated from graph.
Fig. 3.
Drug concentration (μg/ml) calculated from graph.
3.1. 20 Puti Abhrak Bhasma (AB20)
From Fig. 1, Fig. 2, Fig. 3 it can be seen that Abhrak Bhasma after 20 Putas shows anti-proliferative/growth inhibitory activity on all the three cell lines.
3.1.1. Lung cancer cell line (HOP62)
On lung cancer cell line AB20 inhibitory average results are 93.5, 91.3, 89.1 and 80.3 as against adriamycin results −23.2, −32.2, −44.4 and −58.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB20 shows little or negligible growth inhibitory activity in concentration dependent manner as against adriamycin.
3.1.2. Leukemia cell line (U937)
On Leukemia cell line AB20 inhibitory average results are 91.5, 88.1, 82.9 and 67.4 as against adriamycin results −13.8, −17.4, −21.7 and −28.6 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB20 shows little or negligible growth inhibitory activity in concentration dependent manner as against adriamycin.
3.1.3. Prostate cancer cell line (DU145)
On prostate cancer cell line AB20 inhibitory average results are 87.0, 70.4, −2.2 and −24.7 as against adriamycin results −69.6, −70.5, −72.7 and −75.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB20 shows mild to moderate growth inhibitory activity in concentration dependent manner as against adriamycin.
3.2. 50 Puti Abhrak Bhasma (AB50)
From Fig. 1, Fig. 2, Fig. 3 it can be seen that Abhrak Bhasma after 50 Putas shows anti-proliferative/growth inhibitory activity on all the three cell lines.
3.2.1. Lung cancer cell line (HOP62)
On lung cancer cell line AB50 inhibitory average results are 86.2, 77.4, 59.7 and 19.4 as against adriamycin results −23.2, −32.2, −44.4 and −58.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB50 shows mild to moderate growth inhibitory activity in concentration dependent manner as against adriamycin.
3.2.2. Leukemia cell line (U937)
On Leukemia cell line AB50 inhibitory average results are 85.5, 75.0, 50.3 and 18.4 as against adriamycin results −13.8, −17.4, −21.7 and −28.6 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB50 shows mild to moderate growth inhibitory activity in concentration dependent manner as against adriamycin.
3.2.3. Prostate cancer cell line (DU145)
On prostate cancer cell line AB50 inhibitory average results are −7.5, −18.7, −37.8, and −52.5 as against adriamycin results −69.6, −70.5, −72.7 and −75.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB50 shows significant growth inhibitory activity in concentration dependent manner as against adriamycin.
3.3. 100 puti Abhrak Bhasma (AB100)
From Fig. 1, Fig. 2, Fig. 3 it can be seen that Abhrak Bhasma after 100 Putas shows anti-proliferative/growth inhibitory activity on all the three cell lines.
3.3.1. Lung cancer cell line (HOP62)
On lung cancer cell line AB100 inhibitory average results are 74.0, 67.1, 32.7 and −16.9 as against adriamycin results −23.2, −32.2, −44.4 and −58.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB100 shows mild to moderate growth inhibitory activity in concentration dependent manner as against adriamycin.
3.3.2. Leukemia cell line (U937)
On Leukemia cell line AB100 inhibitory average results are 81.4, 65.3, 28.2 and 10.7 as against adriamycin results −13.8, −17.4, −21.7 and −28.6 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB100 shows mild to moderate growth inhibitory activity in concentration dependent manner as against adriamycin.
3.3.3. Prostate cancer cell line (DU145)
On prostate cancer cell line AB100 inhibitory average results are −14.2, −29.9, −49.3, and −63.8 as against adriamycin results −69.6, −70.5, −72.7 and −75.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus AB100 shows highly significant growth inhibitory activity in concentration dependent manner as against adriamycin.
3.4. Abhrak Bhasma after Lohitikaran (ABL)
From Fig. 1, Fig. 2, Fig. 3 it can be seen that Abhrak Bhasma after Lohitikaran shows anti-proliferative/growth inhibitory activity on all the three cell lines.
3.4.1. Lung cancer cell line (HOP62)
On lung cancer cell line ABL inhibitory average results are 87.4, 78.2, 45.3 and −3.7 as against adriamycin results −23.2, −32.2, −44.4 and −58.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus ABL shows mild to moderate growth inhibitory activity in concentration dependent manner as against adriamycin.
3.4.2. Leukemia cell line (U937)
On leukemia cell line ABL inhibitory average results are 82.6, 65.4, 36.2 and 16.0 as against adriamycin results −13.8, −17.4, −21.7 and −28.6 for 10, 20, 40, 80 μg/ml concentration respectively. Thus ABL shows mild to moderate growth inhibitory activity in concentration dependent manner as against adriamycin.
3.4.3. Prostate cancer cell line (DU145)
On prostate cancer cell line ABL inhibitory average results are −12.8, −23.7, −44.9, and −58.8 as against adriamycin results −69.6, −70.5, −72.7 and −75.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus ABL shows highly significant growth inhibitory activity in concentration dependent manner as against adriamycin.
3.5. Abhrak Bhasma after Amrutikaran (ABA)
From Fig. 1, Fig. 2, Fig. 3 it can be seen that Abhrak Bhasma after Amrutikaran shows negligible anti-proliferative/growth inhibitory activity on lung and leukemia cell lines and no activity on prostate cell line.
3.5.1. Lung cancer cell line (HOP62)
On lung cancer cell line ABA inhibitory average results are 95.8, 90.4, 88.0 and 86.3 as against adriamycin results −23.2, −32.2, −44.4 and −58.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus ABA shows negligible inhibitory activity in concentration dependent manner as against adriamycin.
3.5.2. Leukemia cell line (U937)
On Leukemia cell line ABA inhibitory average results are 93.4, 91.2, 88.5 and 86.8 as against adriamycin results −13.8, −17.4, −21.7 and −28.6 for 10, 20, 40, 80 μg/ml concentration respectively. Thus ABA shows negligible inhibitory activity in concentration dependent manner as against adriamycin.
3.5.3. Prostate cancer cell line (DU145)
On prostate cancer cell line ABA inhibitory average result is 100 as against adriamycin results −69.6, −70.5, −72.7 and −75.1 for 10, 20, 40, 80 μg/ml concentration respectively. Thus ABA shows no growth inhibitory activity as against adriamycin.
4. Discussion
The above findings are an indicator that Abhrak Bhasma at different stages of preparation when put to SRB test showed anti-proliferative, growth inhibitory activity on all the 3 cell lines selected. The mechanism of in-vitro growth inhibition achieved by Abhrak Bhasma needs to be evaluated with further studies. Shataputi Abhrak Bhasma AB100 showed highest activity among all 5 samples on prostate cancer cell lines. The activity was almost equivalent to the activity of control group standard chemotherapeutic agent adriamycin. This finding needs further evaluation in invivo studies for its mechanism of action.
Putas or incinerations done repeatedly lead to increase in fineness of the bhasma, brings about changes in the structure, composition, arrangement of molecules and thus formation of newer compounds. Hence, the activity varied with number of Putas. The more the incinerations with Agni the better is the activity (anti-proliferative in this case).
However, in vitro studies have to be supported with invivo studies to further substantiate the findings. Another limitation of the study is decrease in solubility of Abhrak Bhasma after Amrutikaran thus hampering its anti-proliferative activity.
5. Conclusion
The present study signifies the importance of various pharmaceutical processes of Rasashastra like Puta and the effect they have on the efficacy of the drug. It validates the Siddhanta that the activity of bhasma increases with increase in Putas. Thus, the number of Putas is directly proportional to the efficacy of the drug and inversely proportional to the particle size and the dosage required. Hence, it may be concluded that Shataputi Abhrak Bhasma, highly regarded as a Rasayan showed positive in vitro anticancer activity on prostate cancer cell lines, thus leading to scope for exploring the mechanism of its activity through animal and cclinical studies.
Source of funding
None.
Conflicts of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Shastri Pandit Kashinath., editor. Rasa Tarangini of Sadananda Sharma. XI ed. Motilal Banarsidas; Varanasi: 1979. p. 227. [Chapter 10], Verse 29. [Google Scholar]
- 2.Rasendra Saar Samgraha with Rasavidyotini Commentary by Indradev Tripathi. II ed. Chaukhambha Orientalia; Varanasi: 1998. [Chapter 1], Verse 313. [Google Scholar]
- 3.http://www.who.int/mediacentre/factsheets/fs297/en/.
- 4.Bhatia Babita, Kale Purushottam G. Analytical evaluation of an ayurvedic formulation- Abhrak Bhasma. Int J Pharm Sci Rev Res. Nov–Dec.2013;23(1) nº04,17–23, ISSN 0976-044X. [Google Scholar]
- 5.Mishra Siddhinandan. 1st ed. Chaukhambha Orientalia; Varanasi: 2011. Rasa Ratna Samuchhaya, Vaghbhattacharya; pp. 242–243. (edited with Hindi commentary) [Chapter 10] (Rasavarga), Verse 48–50. [Google Scholar]
- 6.Tripathi J.S. the concept and practice of immunomodulation in ayurveda and the role of Rasayanas as immunomodulators. Anc Sci Life. July–Oct 1999;XIX(1&2) [PMC free article] [PubMed] [Google Scholar]
- 7.Dornala Sathya N., Dornala Snehalata SN. Scope of ayurveda in integrative oncology. Ann Ayurvedic Med. 2012;1(4):158–165. [Google Scholar]
- 8.Gaidhani S.N., Singh Arjun, Kumari Suman, Lavekar G.S., Juvekar A.S., Sen S. Dept of Ayush, Govt of India; 2009. Report on screening of single herb extracts for potential anticancer activity by Advanced Centre for Treatment, Research and Education in Cancer (ACTREC) and Central Council for Research in Ayurveda and Siddha (CCRAS) [Google Scholar]
- 9.Macleod Kenneth G., Langdon Simon P. Essential techniques for cancer cell culture, methods in molecular medicine. In: Langdon S.P., editor. vol. 88. Humana Press Inc; Totowa, NJ: 2004. (Cancer cell culture: methods and protocols). [DOI] [PubMed] [Google Scholar]
- 10.Lovitt Carrie J., Shelper Tod B., Avery Vicky M. Review: advanced cell culture techniques for cancer. Drug Discov Biol. 2014;3(2):345–367. doi: 10.3390/biology3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skehn P., Storeng R., Scudiero A., Monks J., McMohan D., Vistica D. New colorimetric cytotoxicity assay for anticancer drug screening. J Natl Cancer Inst. 1990;82:1107. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 12.Vichai Vanicha, Kirtikara Kanyawim. National Centre for Genetic Engineering and Biotechnology (BIOTEC); 17 Aug 2006. Sulforhodamine B colorimetric assay for cytotoxicity screening. published online. [DOI] [PubMed] [Google Scholar]
- 13.Chaubey Dattaram. 3rd ed. Uparasa prakaran, Chaukhambha Orientalia; 2000. Bruhat Rasaraj Sunder; p. 135. [Google Scholar]
- 14.Shrishivsharma, editor. Ayurveda Prakash of Shri Gulraj Sharma Mishra. Chaukhambha Bharati Academy; 1943. p. 283. [Chapter 2], Verse 101. Reprint 1999. [Google Scholar]
- 15.Mookerji Bhudeb. Revised 1st ed. vol. 2. Parimal Publications; Delhi: 2001. p. 16. (Rasa Jala Nidhi with English translation by author). [Chapter 1], Verse 19 (Unwinsho vidhi) [Google Scholar]
- 16.Khanna Vinod Kumar. ISRN Pharmacology; 2012. Targeted delivery of nanomedicines. Article ID 571394, 9 pages, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vajpayee Rameshwar Dayal. Chaukhambha Krushnadas Academy; Varanasi: 2004. Rasendra Chudamani of Somdev; p. 112. [Chapter 10] (Maharasa), Verse 36. Reprint. [Google Scholar]