Abstract
Background
Ayurveda, the Indian system of medicine offers many herbs and formulations for management of obesity. Baidyanath Bhawan Pvt. Ltd has designed a formulation, HFO-02, based on Ayurvedic literature.
Objective
To evaluate the efficacy of Herbal Formulation for Obesity (HFO-02) in overweight individuals.
Materials & methods
With approval from the Institutional Ethics Committee, a proof of concept study was carried out in overweight individuals (Body Mass Index, BMI ≥25.0 and ≤ 30.0 kg/m2), devoid of any endocrinological disorders. Tablet HFO-02 (500 mg) was administered to these individuals twice daily for 90 days, during which they were called at study site fortnightly. After stopping the treatment, they were further followed up for 30 days off-medication and the last follow up was scheduled on day 120. Anthropometric parameters were assessed at every visit, while biochemical parameters viz. lipid profile, blood sugar & insulin levels (both fasting and post prandial), C- reactive protein and adipocytokines (leptin & adiponectin) were estimated monthly.
Results
Of the 18 participants recruited in the study; 14 completed the study. HFO-02 did not show reduction in weight, however a significant decrease in the body circumference and skin fold was demonstrated. This decrease was maintained till day 120. The levels of all biochemical parameters were maintained and no adverse events were reported throughout the study.
Conclusion
Tablet HFO-02 reduced body circumferences and skinfold thickness indicating its potential for obesity management.
Clinical trial registration number
CTRI/2016/07/007067.
Keywords: Circumferences, Obesity, Overweight, Skinfolds
1. Introduction
Obesity is a disease, developed due to complex interaction between biological psychosocial and environmental factors that affect quality of life of an individual [1]. This can be attributed to the multiple co-morbidities like hypertension, diabetes mellitus, stroke, osteoarthritis, and even cognitive impairment. The current treatment of obesity includes increased physical activity and reduced calorie intake. When the lifestyle modifications fall short, drug treatment is recommended. Though numerous modern anti-obesity drugs have been approved so far; most of them have been withdrawn from the market because of their adverse effects. Orlistat is the only available antiobesity drug at present. Hence, search for effective and safer anti-obesity drugs is need of the hour.
Ayurveda, the Indian System of Medicine describes many herbs and formulations for the management of obesity. Baidyanath Bhawan Pvt. Ltd. has come up with a formulation coded as Herbal Formulation for Obesity (HFO)-02 that consists of Triphala, Trimad, Guggul and Vrikshamla. As per Ayurvedic Pharmacology, all these herbs have been described to be beneficial in obesity. Further, there are reports highlighting anti-obesity and anti hyperlipidemic activity of each of the ingredient present in this formulation. In addition, these herbs are also known to possess anti-oxidant activity [2], [3], [4], [5], [6], [7], [8], [9], [10], [11].
With this background, the present proof-of-concept study was planned to evaluate efficacy of the new formulation Tab. HFO-02, in overweight individuals.
2. Materials and methods
2.1. Ethics
After obtaining approval from the Ethics Committee of Bharati Vidyapeeth Deemed (to be) University, College of Ayurved (BVDUCOA/EC/1553/15–16), the study was carried out over a period of 7 months (February 2016 to August 2016). It was registered retrospectively in the Clinical Trial Registry of India (CTRI/2016/07/007067). An informed written consent was obtained from all participating individuals on recruitment.
2.2. Study design
An open label prospective study.
2.3. Sample size
This was a proof of concept study, so a sample size of 12 completed patients was considered adequate.
2.4. Eligibility criteria
Overweight [Body Mass Index, BMI between ≥25.0 and ≤ 30.0 kg/m2] individuals of either sex, without or with controlled co-morbid conditions such as dyslipidemia, hypertension, type 2 diabetes mellitus were included in the study.
Participants with known endocrinological disorders such as hyper/hypothyroidism, Poly Cystic Ovarian Syndrome (PCOS) were excluded from the study. Participants with current/past one-month usage of Ayurvedic medications, dietary supplements causing weight gain/loss were also excluded. Participants with a history of major depressive or other severe psychiatric disorders or an eating disorder [judged by frequency and quantity of food], pregnant and lactating females were not included in the study.
2.5. Study intervention
Two tablets of HFO-02 (weighing 500 mg) were administered orally twice a day before meals with water, for a duration of 3 months (90 days). Each tablet comprised of Triphala powder [150 mg-combination of dried fruits of Terminalia chebula, Emblica officinalis and Terminalia belerica], Trimad powder [60 mg-combination of tubers of Cyperus rotundus, fruits of Embelia ribes and roots of Plumbago zeylanica], latex of Guggul [50 mg -Commiphora mukul] and Vrikshamla fruit powder [250 mg - Garcinia cambogia].
The drug was dispensed in bottles containing 120 tablets each. Study participants were asked to return the empty containers at follow up to ensure compliance. After 90 days of intervention period, the participants were followed for another 30 days, to assess rebound weight gain or additional weight loss. Thus the total duration of the study was 4 months (120 days).
2.6. Study conduct
At baseline, demographic details and baseline characteristics such as age, sex, concomitant disease history, surgical and family history were recorded from all participants. Anthropometric measures viz. height, weight, Waist Circumference (WC), Hip Circumference (HC), Mid Upper Arm Circumference (MUAC), Mid Thigh Circumference (MTC), Neck Circumference (NC) and Skinfold thicknesses at 4 sites viz. Biceps, Triceps, Subscapular & Suprailiac were measured. Wall mounted Stadiometer was used to record the height. To measure weight, participants were asked to stand in an upright position with hands on sides on the digital scale without footwear.
BMI was calculated according to the formula: BMI (kg/m2) = body weight (kg)/squared height (m2). WC was measured at the upper level of umbilicus HC was recorded around the widest portion of the buttock region. The midpoint between shoulder and elbow on the left arm was noted to record MUAC while MTC was measured at the midpoint between the inguinal region and knee. The point above thyroid cartilage was noted to measure neck circumference. All circumferences were recorded using inextensible measuring tape. Waist to hip ratio (WHR) was calculated by dividing WC by HC. Skinfold thicknesses (SFT) were measured on the right side of the body using a Harpenden's Skinfold Caliper following standard protocol.
Following this, 10 mL blood (8 mL in fasting state and 2 mL in post-prandial state) was collected from each of the study participant for estimation of biochemical parameters viz. blood glucose and insulin (both in fasting and post prandial state), lipid profile (Total cholesterol, Triglycerides, LDL & HDL) and C Reactive Protein (CRP) along with adipocytokines (leptin and adiponectin).
The participants were then provided with the study drug and asked to report at study site every 15 days (for a period of 3 months), i.e on days 15, 30, 45, 60, 75, 90. The treatment was stopped on day 90 and the participants were called for last follow up directly on day 120. Anthropometry was repeated every 15 days while biochemical investigations were done monthly.
The participants were asked to refrain from any other obesity management drugs/programmes and advised to continue their existing lifestyle (diet and physical activities) during the study period.
2.7. Statistical analysis
The parametric data was expressed as mean ± standard deviation (SD) and the non-parametric data was presented as Median (Range). The baseline values were compared with follow up values using Repeated Measures ANOVA test (in case of parametric data) and Friedman's test (in case of non-parametric data). A p value of <0.05 was considered as level of significance.
3. Results
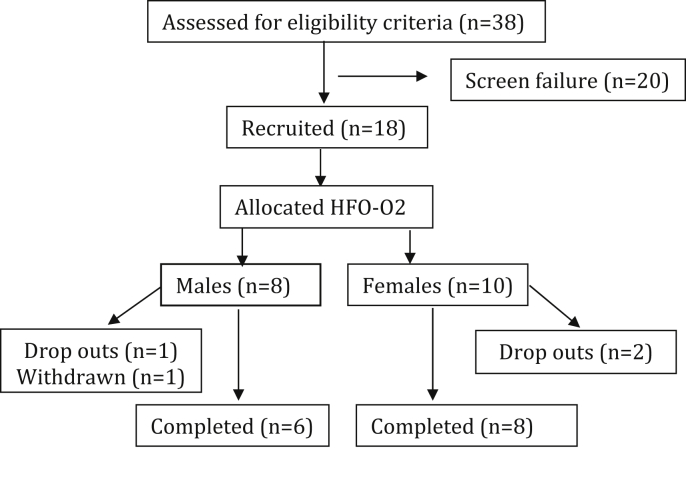
A total of 18 participants were recruited in the study. Of which, 14 participants (6 males and 8 females) completed the study. There were 3 dropouts and 1 withdrawal. One patient dropped out of the study at the end of 1st month due to dissatisfaction about the drug effect in terms of weight reduction. The other two dropped out patients failed to report to the study site on the scheduled follow up visits. There was one withdrawal as the participant was diagnosed with nephrotic syndrome during follow up visits. The participant flow has been shown in (Fig. 1).
Fig. 1.
Participant flow.
The average age of the recruited participants was 38.7 ± 9.42 years. There was no statistically or clinically significant difference observed pre and post treatment in case of both weight and BMI. Only one participant showed weight loss of 2.3 kg, however the weight loss observed in rest of the participants was negligible.
The circumferential measures (waist, hip and neck) showed gradual reduction throughout the study period, which was statistically significant on day 90 (HC and NC) and day 120 (all three). The significant decrease in waist and hip did not reflect in corresponding decrease in waist-to-hip ratio. The other circumferential measures (MUAC and MTC) although showed reduction, but did not reach to statistical significance.
All the skinfold thicknesses, showed gradual decrease during the study period, that was statistically significant on both day 90 and day 120 as compared to day 0. Suprailiac skinfold thickness showed significant decrease only on day 120.
No statistically significant change was seen in glycemic parameters as well as in lipid profile (data not shown). There was a steady decrease in the values of C-reactive protein till day 90 i.e. during treatment. However, on day 120 (off medication period), an increase was seen in the same. No significant changes or specific trend were noted in case of both the adipokines (Table 1).
Table 1.
Effect of Tab HFO–O2 on anthropometric variables and biochemical markers.
| Parameters | Day 0 | Day 30 | Day 60 | Day 90 | Day 120 |
|---|---|---|---|---|---|
| Anthropometric variables | |||||
| Weight (kg) | 73.45 ± 11.70 | 73.57 ± 11.3 | 73.36 ± 11.22 | 73.18 ± 11.24 | 73.12 ± 11.19 |
| BMI (kg/m2) | 28.51 ± 1.50 | 28.58 ± 1.51 | 28.50 ± 1.48 | 28.43 ± 1.49 | 28.40 ± 1.44 |
| Waist circumference (cm) | 98.071 ± 8.99 | 98.42 ± 8.58 | 96.67 ± 8.07 | 96.10 ± 9.77 | 95.03 ± 8.83* |
| Hip circumference (cm) | 104.89 ± 8.62 | 104.92 ± 9.13 | 103.32 ± 8.14 | 103.03 ± 8.37* | 102.57 ± 8.03** |
| WHR | 0.93 ± 0.08 | 0.93 ± 0.07 | 0.93 ± 0.07 | 0.93 ± 0.07 | 0.92 ± 0.08 |
| Neck circumference (cm) | 37.5 ± 5.16 | 36.92 ± 4.82 | 36.53 ± 4.70 | 36.07 ± 4.24** | 35.92 ± 4.16*** |
| Mid upper arm circumference (cm) | 31.84 ± 2.84 | 31.67 ± 3.15 | 31.17 ± 2.79 | 31.5 ± 3.0 | 31.57 ± 2.73 |
| Mid thigh circumference (cm) | 53 ± 6.26 | 52.89 ± 5.69 | 51.03 ± 8.19 | 52.35 ± 5.37 | 51.78 ± 4.92 |
| Skinfold thickness | |||||
| Biceps (mm) | 116.78 ± 34.48 | 116.07 ± 34.84 | 109.57 ± 31.99 | 101.85 ± 30.48*** | 99.71 ± 31.21*** |
| Triceps (mm) | 236.14 ± 49.24 | 234.14 ± 47.19 | 229.85 ± 49.97 | 224 ± 49.56** | 222 ± 48.93*** |
| Subscapular (mm) | 328.42 ± 49.68 | 325.42 ± 49.38 | 315.5 ± 49.79** | 306.71 ± 48.39*** | 300.64 ± 47.06*** |
| Suprailiac (mm) | 215.21 ± 83.91 | 213 ± 86.60 | 205.21 ± 81.72 | 201.42 ± 81.09 | 198.07 ± 76.74* |
| Biochemical markers | |||||
| CRP | 4.6 (1.2–11.3) | 4 (1.3–13) | 3.7 (1.4–12.1) | 2.8 (1.7–13.6) | 3.5 (1.4–9) |
| Leptin (ng/mL) | 18.76 (2.11–26.49) | 17.07 (1.01–26.28) | 18.47 (2.54–26.49) | 20.44 (2.91–26.06) | 20.86 (2.01–25.93) |
| Adiponectin (μg/mL) | 7.33 (2.11–26.49) | 5.81 (1.01–26.28) | 6.45 (2.54–26.49) | 7.74 (2.91–26.06) | 7.52 (2.01–25.93) |
*p < 0.05, **p < 0.01, ***p < 0.001 as compared to day 0.
There were no adverse events reported by any of the patients throughout the study, indicative of safety of the formulation.
4. Discussion
The present study was aimed to evaluate the efficacy of Tab HFO-02 in overweight individuals. We observed that HFO-02 significantly reduced the circumferential measures and skin fold thickness though it did not show any statitistically or clinically significant change in weight and corresponding BMI. It is however interesting to note that HFO-02 did not allow further increase in both these parameters. No significant changes were observed in biochemical parameters except the decrease in C-reactive protein.
HFO-02 contains Triphala and Trimad. We have reported anti-obesity activity of both these formulations earlier in a clinical study (in press). It was observed that the effect of Triphala was pronounced on weight and BMI while Trimad was more effective in reducing circumferential measures. In a previous study reported in Iranian individuals between the age group of 16–60 years, Triphala in a dose of 5 g (one tea spoon) of confection twice a day for three months had resulted in mean weight loss of 4.82 kg, mean decrease of 4.01 cm and 3.21 cm in waist and hip circumferences respectively after 90 days' treatment [12]. The decrease in all the above mentioned parameters observed in our study was lesser than that reported in the Iranian study, probably because of differences in ethnicity, age and baseline weight of the study participants, as well as dose of Triphala. It should also be noted that our study formulation had ingredients other than Triphala such as guggul and vrikshamla (garcinia) which may have affected the overall efficacy. There are 9 clinical studies available on guggul supporting its anti-obesity and anti-hyperlipidaemic activity. The anti-obesity activity of Garcinia is well established in animal studies though the clinical studies are limited.
In our study it was observed decrease in circumferential measures of various parts including Neck Circumference. Neck Circumference has been reported as a marker of upper body subcutaneous (SC) fat. Neck fat is responsible for larger proportion of systemic free fatty acid release as compared to visceral fat [13]. A cut off of Neck Circumference >37 cm in men and >34 cm in women has been reported to determine individuals with central obesity [14].All the participants in this study, both males and females, had elevated baseline Neck Circumference values and post intervention, a significant decrease was seen.
Skinfold thickness (SFT) has been specified to be a sensitive predictor for obesity [15]. Subscapular SFT for males and biceps SFT for females have been mentioned as adiposity predictors [16].HFO-02 has significantly reduced the thickness of these two skinfolds along with triceps and suprailiac in all participants. There was no change seen on glycemic profile and lipid profile indicating that HFO-02 may not be acting through glucose homeostasis. It is surprising that in spite of decrease in circumferences and SFT (i.e. action on fat deposits), the lipid profile remains unchanged.
We also evaluated adipocyte specific inflammatory markers like leptin and adiponectin along with C-reactive protein. . CRP though a non-specific marker for inflammation, has been reported to get modulated directly by adipose tissue [17], [18]. The most significant roles of leptin include regulation of energy homeostasis, neuroendocrine function, and metabolism [19]. Adiponectin, an adipocyte specific protein is negatively correlated with adiposity. The expression of adiponectin decreases with increase in the adiposity and is suggestive of insulin resistance [20]. HFO-02 decreased the CRP levels but had no effect on leptin and adiponectin levels. In short, HFO-02 reduced circumferential measures and skinfold thickness with no change in any other parameters. This indicates that HFO-02 might be responsible for fat redistribution or lipolysis of subcutaneous fat (beta oxidation) or decrease in adipose hypertrophy (inflammatory changes). However, these mechanisms are in hypothetical realm and need to be confirmed with appropriate parameters.
5. Limitation
The main limitation of this study was the sample size which made it difficult to draw conclusions regarding various assessment parameters.
6. Conclusion
HFO-02 proved to be a safe therapeutic agent and effectively decreased the anthropometric parameters like body circumferences and skinfold thickness in overweight individuals.
Source of Funding
Siddhayu Ayurvedic Research Foundation Pvt Ltd, Nagpur funded the entire study and provided the study drug.
Conflict of interest
Dr Veena Deo and Dr Bharat Bhushan Shrikhande, from Siddhayu initiated the study concept and are also co-authors. Dr Supriya Bhalerao is a part of JAIM's editorial board and is the corresponding author for this article. Dr Bhalerao was not involved in any review or editorial processes of the manuscript.
Acknowledgement
The authors acknowledge the technical help received from Ms. Shital Giramkar during processing and storage of blood samples.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Forhan M., Gill S.V. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metabol. 2013;27:129–137. doi: 10.1016/j.beem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Singh R., Singh B., Kumar N., Arora S. Antioxidant activity of triphala a combination of Terminalia chebula, Terminalia bellerica and emblica officinalis. J Food Biochem. 2010;34(1):222–232. [Google Scholar]
- 3.Perera M.G., Soysa S.S., Abeytunga D.T., Ramesh R. Antioxidant and cytotoxic properties of three traditional decoctions used for the treatment of cancer in Sri Lanka. Pharmacogn Mag. 2008;4(15):172–181. [Google Scholar]
- 4.Pfundstein B., El Desouky S.K., Hull W.E., Haubner R. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry (Oxf) 2010;71(10):1132–1148. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Peterson C.T., Denniston K., Chopra D. Therapeutic uses of triphala in ayurvedic medicine. J Altern Complement Med. 2017;23(8):607–614. doi: 10.1089/acm.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurjar S., Pal A., Kapur S. Triphala and its constituents ameliorate visceral adiposity from a high-fat diet in mice with diet-induced obesity. Altern Ther Health Med. 2012;18(6):38–45. [PubMed] [Google Scholar]
- 7.Chaudhari H.S., Bhandari U., Khanna G. Preventive effect of embelin from embelia ribes on lipid metabolism and oxidative stress in high-fat diet-induced obesity in rats. Planta Med. 2012;78(07):651–657. doi: 10.1055/s-0031-1298379. [DOI] [PubMed] [Google Scholar]
- 8.Pendurkar S.R., Mengi S.A. Antihyperlipidemic effect of aqueous extract of Plumbago zeylanica roots in diet-induced hyperlipidemic rat. J Pharm Biol. 2009;47(10):1004–1010. [Google Scholar]
- 9.Nazish I., Ansari S.H., Arora P. Antiobesity actions of Embelia ribes. Pharmacogn J. 2012;4(32):73–80. [Google Scholar]
- 10.Ulbricht C., Basch E., Szapary P., Hammerness P., Axentsev S., Boon H. Guggul for hyperlipidemia: a review by the natural standard Research collaboration. Complement Ther Med. 2005;13(4):279–290. doi: 10.1016/j.ctim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Chuah L.O., Ho W.Y., Beh B.K., Yeap S.K. Updates on antiobesity effect of Garcinia origin (−)-HCA. Evid Based Complement Altern Med. 2013:1–14. doi: 10.1155/2013/751658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamali S.H., Khalaj A.R., Hasani-Ranjbar S., Esfehani M.M., Kamalinejad M., Soheil O. Efficacy of ‘Itrifal Saghir’, a combination of three medicinal plants in the treatment of obesity; A randomized controlled trial. DARU. 2012;20(1):33. doi: 10.1186/2008-2231-20-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aswathappa J., Garg S., Karthiyanee K., ShShankar V. Neck circumference as an anthropomertric measure of Obesity in diabetics. N Am J Med Sci. 2013;5(1):28–31. doi: 10.4103/1947-2714.106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang G.R., Yuan S.Y., Fu H.J., Wan G., Zhu L.X., Bu X.L. Neck circumference positively related with central obesity, overweight, and metabolic syndrome in Chinese subjects with type 2 diabetes: beijing community diabetes study 4. Diabetes Care. 2010;33:2465–2467. doi: 10.2337/dc10-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams R.L., Wood L.G., Collins C.E., Callister R. Effectiveness of weight loss interventions – is there a difference between men and women: a systematic review. Obes Rev. 2015;16(2):171–186. doi: 10.1111/obr.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nooyens A.C., Koppes L.L., Visscher T.L., Twisk J.W., Kemper H.C., Schuit A.J. Adolescent skinfold thickness is a better predictor of high body fatness in adults than is body mass index: the Amsterdam Growth and Health Longitudinal Study. Am J Clin Nutr. 2007;85:1533–1539. doi: 10.1093/ajcn/85.6.1533. [DOI] [PubMed] [Google Scholar]
- 17.Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. J Am Med Assoc. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 18.Yudkin J.S., Stehouwer C.D.A., Emeis J.J., Coppack S.W. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 19.Park H.K., Ahima R.S. Physiology of Leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav A., Kataria M.A., Saini V., Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;18(417):80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]