Abstract
Background
Chronic fatigue syndrome (CFS) holds a mystery for researchers due to its multifactorial nature; hence, its diagnosis is still based on symptoms and aetiology remains obscured. Number of scientific evidences regarding the role of oxidative stress, immune dysfunction in CFS and alleviation of symptoms with the help of nutritional supplements guided us to study effect of ethanolic extract of Spilanthes oleracea (SPE) in CFS.
Objectives
Present study was designed to evaluate antioxidant, immunomodulatory properties of S. oleracea flower to ameliorate CFS infirmity in mice.
Materials and method
In order to induce fatigue, experimental animals were stressed by chronic water – immersion stress model. Meanwhile, parameters like immobility period and tail withdrawal latency were assessed. On the 21st day, mice blood was collected and they were immediately sacrificed for biochemical estimations.
Results
Biochemical analysis results revealed that CFS elevates lipid peroxidation, nitrite level and diminishes the endogenous antioxidant enzyme like catalase level in stressed animal’s brain homogenate. Stressful condition developed muscle fatigue leading in alteration of lactate dehydrogenase level (LDH), Blood urea nitrogen (BUN) and Triglycerides (TG) levels. Concurrent and chronic treatment of SPE for 21 days restored all these behavioural despairs and associated biochemical adaptation in mice in dose-dependent manner.
Conclusion
The outcome of this study indicates ability of SPE in amelioration of CFS by mitigating the oxidative stress and thus provide a powerful combat against CFS which may be due to its antioxidant and immunomodulatory properties.
Keywords: Chronic fatigue syndrome, Spilanthes oleracea, Immunomodulator, Antioxidant
1. Introduction
Chronic fatigue occurs when symptoms of exhaustion or lack of energy last over 6 months. Chronic fatigue is a symptom of many chronic conditions, including rheumatoid arthritis, fibromyalgia, or lupus. Although the exact cause of chronic fatigue is unknown, certain factors can play a role, such as infection, hormone level changes, and stress. Chronic fatigue syndrome (CFS) is illness characterized by unexplained profound disabling chronic fatigue lasting for six months and more and which is not improved by bed rest and may be worsened by physical and mental activity [1]. Estimated worldwide prevalence of CFS was 0.2–2.6% [2], [3]. Pathophysiology of CFS still remains unclear due to its multifactorial nature. Ample hypotheses related to CFS have pointed out towards changes in immune system resulting in biochemical and immunological disturbances [4].
Numerous studies have speculated that, CFS arises due to infectious disease (viral-bacterial), multiple nutritional deficiencies, food intolerance, immune dysfunction, oxidative stress and excessive physical or mental stress that act as potential stressor for generation of reactive oxygen species (ROS) [5], [6], [7]. Moreover these reactive oxygen species target the lipid system and elevates the level of lipid peroxidation in CFS [8], [9]. Further the genetic studies have demonstrated that abnormal activation of T-lymphocytes and higher level of Pro-inflammatory substances are responsible for progression of CFS [10], [11]. This may be due to stress induced activation of sympathetic – adrenal medulla and hypothalamic–pituitary-adrenal (HPA) axis which stimulates secretion of catecholamine and glucocorticoid, which are capable of modulating immune cells leading to modulation of cytokine production [12], [13]. Antioxidants have been shown to trap free radical and thus delay the onset of lipid peroxidation [14]. Established evidences have denoted that use of antioxidants is beneficial in management of CFS patients [15]. This finding has emerge new path for research leading to use traditional plants which possess good antioxidant properties to balance the altered endogenous enzymatic pool and good immunostimulatory properties to prohibit the changes in immune function associated with CFS without the side effect of psychotropic drugs.
Spilanthes oleracea also known as Spilanthes acmella var oleracea belonging to family Asteraceae (common name Akarkara), is described in folk medicine, for its use in stammering, toothache, Stomatitis and throat complaints, gout and bladder pain [16]. This species is found in tropical regions all over India [17]. Flowers of S. oleracea possess the highest amount of spilanthol in it which is pungent in taste. S. acmella L. has shown analgesic and anti-inflammatory, diuretics, insecticidal, protective effect against genotoxic damage in cultured human peripheral blood lymphocytes, vasorelaxant–antioxidant activities and immunomodulatory properties [18], [19], [20], [21], [22], [23]. Up till now no research work has been made on S. oleracea regarding its effect on CFS as it possesses antioxidant and immunomodulatory activity which will beneficial to cure symptoms of CFS. Therefore the present study was designed to develop and explore the ability of S. oleracea as an antioxidant and immunomodulator to alleviate chronic fatigue induced behavioural as well as biochemical changes.
2. Material and methods
2.1. Drug
Flowers of S. oleracea were collected from local ayurvedic pharmacy Mankarnika, Chinchwad of Pune, Maharashtra, India, and authenticated by Agarkar Research Institute of, Pune, Maharashtra. India (MCP/ASP/2011/159). Flowers were shade dried, powdered coarsely and extracted with water and 99.97% Ethanol. The phytochemical constituents of each extract were determined in laboratory by performing the qualitative analytical test.
2.2. Animals
Swiss albino mice (20–30 g) were bred from National Institute of Bioscience of Pune, Maharashtra, India were used for the experimental purpose. Experimental animals had a free access to water and standard pellets. All work were conducted between 09.00 and 17.00. The experimental protocol were approved by Institutional Animal Ethic Committee and conducted according to Committee for the Purpose of Control and Supervision of Experiments on Animals. CPCSEA (Reg No. 884/ac/05/CPCSEA). Animals were divided in different groups; each group had six animals which was sufficient sample size to show statistical relationship and relevance to the study.
2.3. Experimental protocols
2.3.1. Acute toxicity study
Animals were divided in four different groups (n = 6). SPE (Ethanolic extract of Spilanthes oleracea) was administered in different doses (300–2000 mg/kg, per oral) according to OECD Guideline number 423 to investigate the lethal dose of the plant extract. The animals were carefully observed for next 8 h for the signs of toxicity, morphological, behavioural despair and mortality and then kept under observation for next 14 days period. Mice receiving different dose of SPE did not show any signs of clinical toxicity as well as mortality up to 2000 mg/kg dose range. After carrying out the acute toxicity studies two different doses 200 mg/kg and 400 mg/kg were selected for experimental work.
For experimental purpose the animals divided in four different groups as control, Standard, Test-200 and Test-400. The test and standard drug were suspended in 0.5% Tween-80, for CFS experiment standard group received Revital 100 mg/kg p.o and for immunomodulatory activity standard drug was methotrexate (0.5 mg/kg i.p).
2.4. In-vitro antioxidant studies
2.4.1. DPPH radical scavenging activity of S.oleracea extract
An antioxidant activity of the different extracts of S. oleracea flowers were determined using the method described by Dasgupt et al. Extract of different concentrations i.e. 200–1000 μg/ml (0.1 ml) were added to 3 ml of 0.004% methanolic solution of DPPH. Absorbance at 517 nm was determined after 30 min, and the percent inhibition activity was calculated using formula [24].
where,
Ao = Absorbance without extract.
Ae = Absorbance with extract.
2.4.2. Nitric oxide scavenging activity of S.oleracea extract
Nitric oxide scavenging activity of different extracts of S. oleracea flowers were determined by slightly modified procedure of Saha et al. [25].
2.4.3. Determination of reducing power of S.oleracea extract
Reducing power of different extracts of S. oleracea flowers were determined using the method described by Kumaran et al. [24].
2.4.4. Determination of total phenolic content of S.oleracea extract
Total phenolic content of S. oleracea extracts were determined by Folin Ciocalteu method (Folin phenol method) [26].
2.5. In vivo immunomodulatory activity
2.5.1. Humoural antibody (HA) and delayed type hypersensitivity (DTH) response
Humoural antibody (HA) and delayed type hypersensitivity (DTH) response were studied in mice. Animals were divided in four different groups each group contains 3 animals. Group-I (control group) received the vehicle (0.5% tween-80, 0.5 ml each orally). Group-II received standard immunosuppressant drug methotrexate (0.5 mg/kg i.p). The animals of Group-III and Group-IV received ethanolic extract (200 mg/kg and 400 mg/kg p.o respectively) for next 7 consecutive days. After 7 days of treatment the animals were immunized by injecting 0.1 ml of 20% of sheep red blood cells suspension (SRBC) i.p and the day is considered as day-0. Again the same treatment was given for next 7 days daily. Blood samples were collected in microcentrifuge tubes from individual animals by retro-orbital plexus on the 7th day after immunization and the serum was separated. Antibody levels were determined by haemagglutination technique [27].
The DTH responses were studied by measuring the thickness of right hind footpad oedema after 24 h of immunization for detection of cellular response. The Thickness of the right footpad was measured using digital vernier calliper on 7th day. The drug treatment was exactly the same as described above for HA titre. The mice were then challenged by injecting 0.03 ml 20% SRBC inn the sub plantar region of the right hind paw and the same amount of normal saline in left hind paw after 7 days of immunization with (0.1 ml of 20% SRBC i.p). The footpad reaction was observed as the difference in the thickness (mm) between the right footpad injected with SRBC and the left footpad with normal saline after 24 h [28].
2.5.2. Neutrophil adhesion test
Mice were divided in same groups as mentioned above and dosed with the same dose of drug respectively for 7 days. On 8th day of treatment the blood samples from all the groups were pooled by puncturing the retro-orbital plexus under mild ether anaesthesia. Blood was collected in vials pre-treated with Disodium EDTA and analysed for total leucocyte count (TLC) and differential leucocyte count (DLC) by fixing the blood smear and staining with Leishman's stain. After initial counts, blood samples were incubated with nylon fibre (80 mg/ml of blood sample) for 15 min at 37 °C. The incubated samples were again analysed for TLC and DLC. The product of TLC and % neutrophil gives neutrophil index (NI) of Blood samples. Percent neutrophil adhesion was calculated [29].
where,
NIu: Neutrophil index before incubation with nylon fibre.
NIt: Neutrophil index after incubation with nylon fibre.
2.6. Assessment of fatigue and its associated parameters
2.6.1. Water-immersion stress test
Chronic exposure to stress produced fatigue in animals represented chronic fatigue syndrome. Stress was induced by forcing the mice individually to swim in glass jar (25 cm × 12 cm × 25) containing 15 cm deep water at room temperature (22 ± 3 °C). After initial struggling period each animal hold a typical immobile posture. When animals stopped to struggle and made minimal hind limb movement to keep their head above the water level they were considered to be immobile. All the animals were forced swim for total 10 min daily for 21 consecutive days. The immobility time was noted for a period of initial 6 min in a total period of 10 min on day 1, 7, 14, 21. Drugs were administered to the standard and test group 1 h before the experiment each day [30].
2.6.2. Stress-induced hyperalgesia
Stress-induced hyperalgesia was assessed by tail immersion test. Mice were held individually and their tails were immersed in hot water (52 ± 0.5 °C) for not more than 10 s (cut off time) and withdrawal latency was observed on the same days on the immobility was observed [31].
2.7. Biochemical studies
2.7.1. Determination of BUN, TG, LDH
On 22nd day after exhaustion, 0.5 ml of blood was collected in the tube without anticoagulants, by extirpating the left eye ball. The level of blood urea nitrogen (BUN), Plasma triglycerides (TG) and lactate dehydrogenase were determined by using by semi-automatic biochemical analyser with commercial kits [32].
2.7.2. Preparation of brain homogenates
A 10% (W/V) Brain tissue homogenates was prepared in 0.1M phosphate buffer (pH 7.4) which was further used for estimating lipid peroxidation, nitrite and catalase assay [33].
2.7.3. Assessment of lipid peroxidation
Briefly homogenates were incubated with 15% Trichloroacetic acid (TCA), 0.375% thiobarbituric acid (TBA) and 0.25N HCl at 95 °C for 15 min period. The mixture was cooled with the help of tap water at room temperature, centrifuged and absorbance of supernatant measured at 532 nm against blank [34].
2.7.4. Assessment of nitrite level
Accumulation of nitrite was measured in cell-free supernatants from brain homogenates by spectrophotometer assay based Greiss reaction [35]. The nitrite concentration was calculated from standard curve using sodium nitrite as standard and expressed as micromolar nitrite per millilitre homogenate.
2.7.5. Assessment of catalase activity
Catalase activity was assayed by the method of Luck, 1971. The result was expressed as micromoles of H2O2 decomposed/min/mg protein [36].
2.7.6. Protein estimation
The amount of protein was estimated according to the method of Lowry et al. using commercial protein estimation kit [37].
2.8. Statistical analysis
The results were measured in seconds and expressed as mean ± SEM. The intergroup variability in results were measured by one-way analysis of variance (ANOVA) followed by Dunnett's test. The values were considered significant at p < 0.05.
3. Results
3.1. In vivo antioxidant parameters
3.1.1. DPPH radical scavenging activity
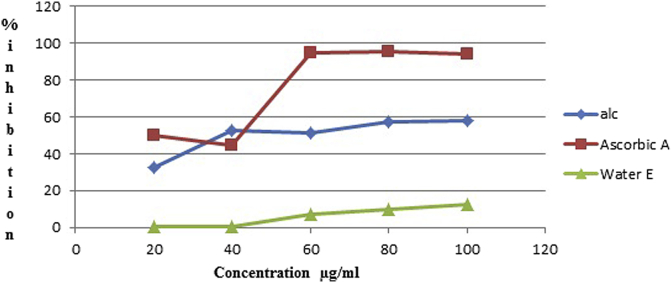
As shown in Fig. 1, at 100 μg/mL the scavenging effect of both alcoholic and water extracts were SPE (58.21%), SPW (12.34%) whereas the scavenging effect of ascorbic acid was obtained (94.2%).
Fig. 1.
DPPH radical scavenging activity in % inhibition of different dilutions of Ascorbic acid, Ethanolic and Water extract.
3.1.2. Nitric oxide scavenging activity
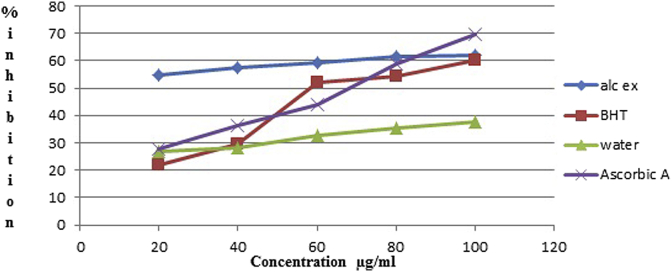
As shown in Fig. 2, nitric oxide scavenging effect of SPE (Water extract of Spilanthes oleracea), SPW and standards (BHT, ascorbic acid) at 100 μg/mL was found in following order SPE (62.08%) > BHT (60.4%), SPW (37.46%) < ascorbic acid (69.42%).
Fig. 2.
Nitric oxide scavenging activity in % inhibition of different dilutions of BHT, Ascorbic acid, Ethanolic and Water extract.
3.1.3. Determination of reducing power
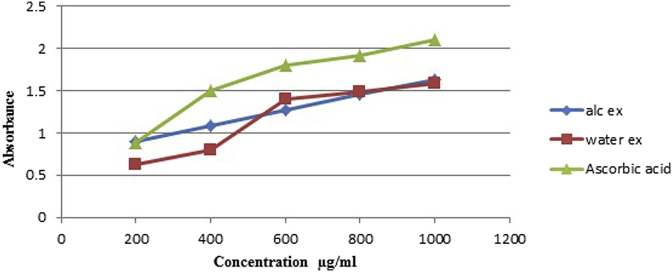
As shown in Fig. 3, reducing power of in SPE, SPW decreasing order was found to be ascorbic acid (2.1098) > SPE (1.6233) > SPW (1.5898).
Fig. 3.
Reducing power ability of different dilutions of Ascorbic acid, Ethanolic and Water extract.
3.1.4. Determination of total phenolic content
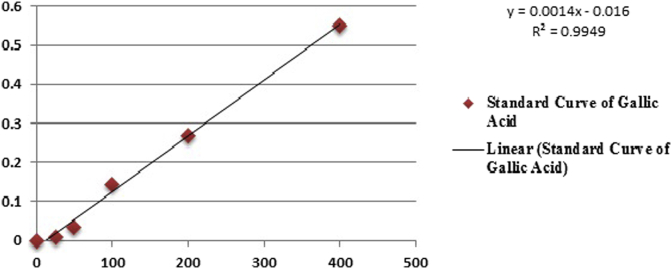
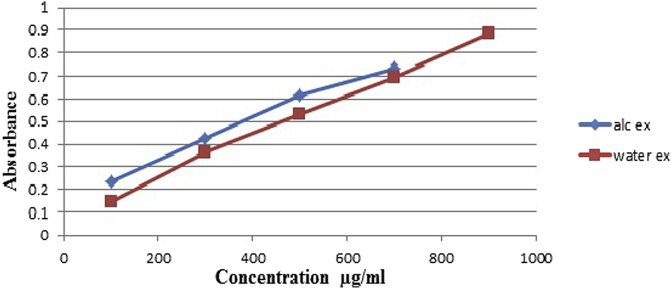
As shown in Fig. 4, Fig. 5 total phenolic content of SPE, SPW was found to be for SPE 186.21 μg/mL (Gallic acid equivalent) and for SPW 121.92 μg/ml (Gallic acid equivalent).
Fig. 4.
Standard calibration curve of Gallic acid.
Fig. 5.
Absorbance of ethanolic extract and water extract of different dilutions.
3.2. Immunological parameter
3.2.1. Haemagglutination titre and DTH response
Effect of SPE on antibody response on HA titre were shown in (Table 1). SPE treated group 200 mg, 400 mg/kg showed significant increase (p < 0.05) in HA titre when titre compared with control group. A significant decreased (p < 0.05) in the antibody titre was observed in the methotrexate treated group when compared with the control group.
Table 1.
Effect of SPE Flower extract on Haemagglutinating titre.
| Group | HA titre |
|---|---|
| Control | 4.33 ± 0.333 |
| Standard | 3.33 ± 0.33 |
| Test-200 | 6.00 ± 0.5571* |
| Test-400 | 8.33 ± 0.33** |
Values were expressed as mean ± SEM (n = 3), one way Annova followed by Dunnett's test **p < 0.05 considered significant as compared to control after 7 days of immunization. *p < 0.01, **p < 0.05.
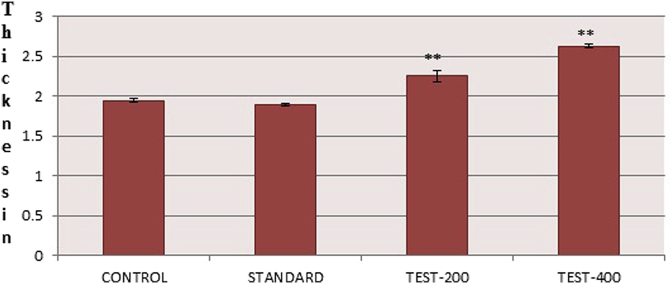
Effect of SPE on cell mediated immune response by DTH induced footpad oedema was shown in (Fig. 6). Only the methotrexate group showed decreased in footpad thickness after 24 h as compare to control whereas both the SPE treated group increased the footpad thickness and showed significant (p < 0.05) potentiated DTH responses in terms of increase in the mean difference of paw thickness when compared with control group and immunosuppressed group.
Fig. 6.
Effect of SPE on average thickness in mm of right hind paw after 24 h for SPE in 200 mg/kg and 400 mg/kg values were expressed as mean ± SEM (n = 3), one way Annova followed by Dunnett's test **p < 0.05 considered significant as compared to control.
3.2.2. Neutrophil adhesion test
Effect of SPE On neutrophil activation by neutrophil adhesion test was shown in (Table 2).
Table 2.
Effect of SPE Flower extract on neutrophil adhesion.
| Group | TLC (A) |
% Neutrophil (B) |
Neutrophil index (A × B) |
% Neutrophil adhesion | |||
|---|---|---|---|---|---|---|---|
| UB | TB | UB | TB | UB | TB | ||
| Control | 4.23 ± 0.2028 | 3.63 ± 0.2604 | 40.66 ± 1.202 | 37.00 ± 3.055 | 172.43 ± 10.927 | 133.33 ± 7.247 | 22.5046 ± 3.662 |
| Standard | 4.033 ± 0.2011 | 3.56 ± 0.1856 | 40.33 ± 1.202 | 37.33 ± 1.382 | 161.37 ± 8.480 | 129.53 ± 6.409 | 19.771 ± 0.4147 |
| Test-200 | 4.83 ± 0.0233 | 3.96 ± 0.2186 | 51.00 ± 1.528 | 40.66 ± 0.8819 | 247.13 ± 32.652 | 161.63 ± 12.058 | 34.555 ± 0.9809* |
| Test-400 | 5.33 ± 0.2186 | 4.13 ± 0.1202 | 55.667 ± 2.333 | 44.66 ± 0.8819 | 296 ± 6.429 | 184.83 ± 8.935 | 37.0287 ± 3.520** |
Values were expressed as mean ± SEM (n = 3), one way Annova followed by Dunnett's test **p < 0.05 considered significant as compared to control after 7 days of treatment. *p < 0.01, **p < 0.05.
The percentage neutrophil adhesion was significantly (p < 0.05) increased by both the SPE treated groups when compared with the control group, showed possible immunostimulant effect. This increased the neutrophil adhesion in both the SPE treated group correlates the process of margination of the cells in the blood vessels.
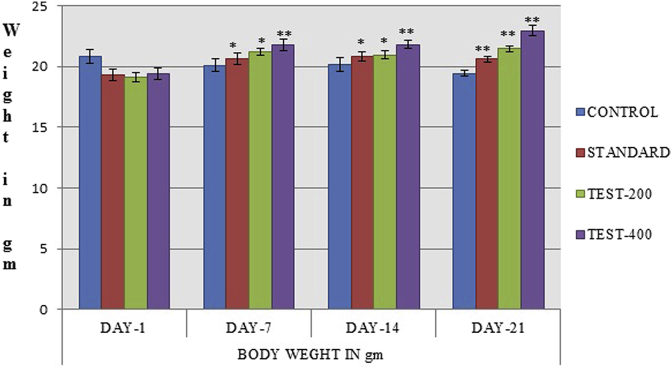
3.3. Effect of SPE extract on animal body weight
Both the standard and SPE treated group showed increased in body weight as compare to control group on day 7, 14, 21. Thus in SPE treated groups dose dependant prevention in reduction of body weight was observed (Fig. 7).
Fig. 7.
Effect of SPE extract on body weight of mice during stress condition induced CFS. The values were expressed as mean ± SEM (n = 6), one way Annova followed by Dunnett's test **p < 0.05 considered significant as compared to control.
3.4. Effect of SPE extract on immobility time and stress induce hyperalgesia
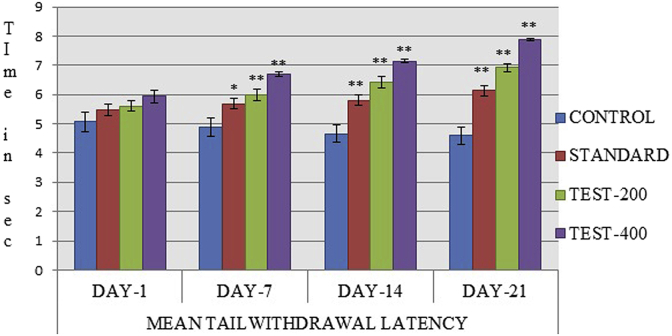
Chronic water-immersion stress for 21 consecutive days for 10 min duration produced significant increase in immobility time in control group and decreased in mean tail withdrawal latency representing severe fatigue as noted on day 1, 7, 14, 21 days. However, oral administration of both the standard (100 mg/kg) and SPE treated group (200,400 mg/kg) produced dose dependent significant decreased in immobility time along with increased mean tail withdrawal latency in mice as compared to control group (Table 3) (Fig. 8).
Table 3.
Effect of SPE extract on immobility period on day 1, 7, 14, 21 days of chronic fatigue syndrome.
| Group | Immobility time in sec |
|||
|---|---|---|---|---|
| Day-1 | Day-7 | Day-14 | Day-21 | |
| Control | 48.66 ± 1.498 | 56 ± 1.789 | 63.5 ± 1.803 | 96.1667 ± 3.619 |
| Standard | 46.16 ± 2.496 | 49.16 ± 1.167* | 53 ± 2.060* | 57.1 ± 1.183** |
| Test-200 | 48.16 ± 2.372 | 50.33 ± 2.044* | 52.33 ± 1.453* | 58.66 ± 1.202** |
| Test-400 | 42.166 ± 1.302 | 41.66 ± 0.9845** | 45.33 ± 1.647** | 49.3 ± 2.338** |
Values were expressed as mean ± SEM (n = 6), one way Annova followed by Dunnett's test **p < 0.05 considered significant as compared to control. *p < 0.01, **p < 0.05.
Fig. 8.
Effect of SPE on mean tail withdrawal latency value were expressed as mean ± SEM (n = 6), one way Annova followed by Dunnett's test shows **p < 0.05 considered significant as compared to control.
3.5. Effect of SPE extracts on blood parameters BUN, TG, LDH
The results were shown in Table 4, respectively for various parameters. After 21 days treatment with standard and SPE treated groups showed significant increase in LDH value in dose dependant manner while BUN and triglyceride level were decreased significantly as compare to control group (Table 4).
Table 4.
Effect of SPE extract on various blood parameters.
| Group | Blood parameter |
|||
|---|---|---|---|---|
| BUN mg/dL | TG U/L | LDH mg/dL | Catalase (μg of H2O2/min/mg of protein) | |
| Control | 131.55 ± 19.668 | 352 ± 38.428 | 714.05 ± 20.986 | 0.19765 ± 0.0528 |
| Standard | 51.066 ± 2.587** | 213.667 ± 40.563** | 851.7667 ± 15.081** | 0.0294 ± 0.02694** |
| Test-200 | 62.0016 ± 3.764** | 157.667 ± 17.938** | 914.0667 ± 21.274** | 0.00617 ± 0.00617** |
| Test-400 | 65.6083 ± 2.540** | 155 ± 6.861** | 907.3267 ± 21.989** | 0.0098 ± 0.0098** |
Values were expressed as mean ± SEM (n = 6), one way Annova followed by Dunnett's test **p < 0.05 considered significant as compared to control after 21 days.
3.6. Effect of SPE extract on biochemical alteration associated with CFS
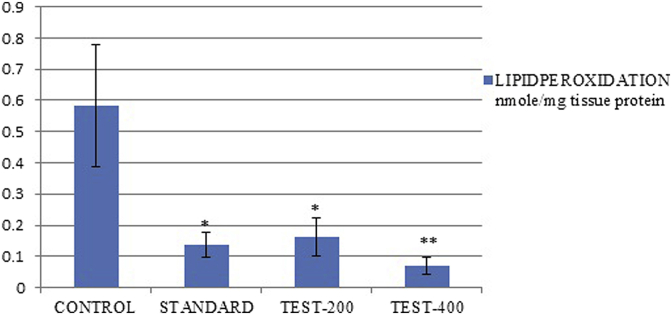
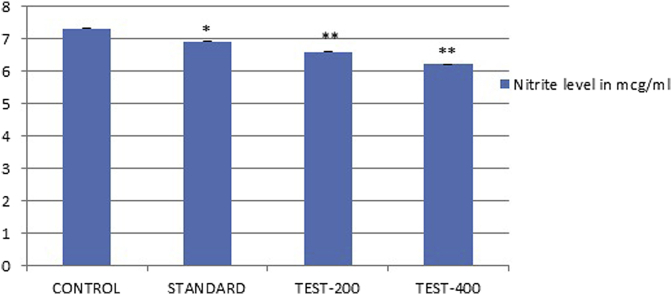
Lipid peroxidation and nitrite level were significantly increased in the brain homogenates of control group. Chronic treatment with SPE and Standard drug produced a significant (p < 0.05) decreased in lipid peroxidation and nitrite levels in the brain homogenates dose dependently (Fig. 9) (Fig. 10). However, endogenous antioxidant enzyme level like catalase in control group was reduced significantly in brain homogenates (Table 4). Whereas treated groups were significantly (p < 0.05) elevated catalase level in dose dependent manner as compare to control group.
Fig. 9.
Effect of SPE extract on lipid peroxidation values were expressed as mean ± SEM (n = 6), one way Annova followed by Dunnett's test **p < 0.05 considered significant as compared to control after 21 days treatment.
Fig. 10.
Effect of SPE extract on Nitrite level values were expressed as mean ± SEM (n = 6), one way Annova followed by Dunnett's test, **p < 0.05 considered significant as compared to control after 21 days of treatment.
4. Discussion
Present study was design to evaluate antioxidant, immunomodulatory properties of S. oleracea flowers to ameliorate the symptoms of chronic behaviour despair and biochemical alteration associated with chronic fatigue syndrome infirmity. Several studies have confirmed the relation between oxidative stress and CFS [38]. Moreover ample of studies have focused on involvement of reactive oxygen species which were responsible increase for in oxidative stress and find out the correlation in the pathophysiology of fatigue [39] which results in increase in lipid peroxidation, nitrite level and decrease in catalase level. In this study CFS was induced by exposing the experimental animals to stressful condition [40] The results of study revealed that with concurrent exposure to stress by force swim water immersion showed increased in immobility time that indicates development of CFS with decrease in mean tail withdrawal latency in mice of control group. Mice treated with standard and SPE extract for 21 days showed significant reduction in immobility period and rise in mean tail withdrawal latency period as compare to control group. These results suggest that SPE may act by altering the HPA axis function in similar way to other anti-stress agents [41]. As it was established that dysfunction of HPA axis have important role behind pathogenesis of CFS [42].
Results of in vitro antioxidant studies on SPE extract have indicated that it possesses antioxidant activity. Free radicals and ROS cause dysfunction of HPA axis and developed fatigue in mice. This was overcome by standard and SPE treated group after 21 days treatment significantly because of SPE's ability to neutralize or scavenge the free radicals. This will ultimately reduce the nitrite concentration and inhibit oxidation of lipid in brain leading to decrease in lipid peroxidation, nitrite value and increased activity of antioxidant enzyme catalase in brain homogenates of mice as compared to control group. Thus the results of antioxidant studies support the role of oxidative stress in CFS and suggest the use of antioxidants in its management [43]. Patient suffering from CFS have shown increased in neutrophil cell apoptosis which support the hypothesis of immune dysfunction during CFS [44]. SPE extract showed increase the neutrophil adhesion significantly in dose dependant manner due to its ability to increase neutrophil count. Further in HA titre and DTH assay SPE showed increase in agglutination in number of wells significantly and also increased the footpad thickness as compare to control group. Thus the results of HA Titre and DTH have denoted that SPE only possess the good immunostimulant properties not only in humoural immunity but also in cellular (innate) immunity. These results of immunomodulation have suggested that treatment with SPE help to mitigate immune dysfunction associated with CFS condition.
Chronic exposure to stress developed muscle fatigue in mice with altered level of serum enzyme, BUN which increase due to elevation in protein metabolism along with increase TG clearance and LDH value due to increase in lactic acid [32]. Mice treated with SPE and standard for 21 days showed significant amelioration in altered serum enzyme level as compare to control group. SPE reduced the BUN value may be by inhibiting its accumulation, lower the clearance TG may be by increasing fat utilisation and enhance the LDH level by reducing the accumulation of lactic acid in muscle tissue and thus protect from muscle tissue from damage and development of fatigue.
From the above studies and by phytochemical analysis it can be concluded that SPE extract have polyphenols and flavonoid in it which may be responsible for its antioxidant and immunomodulatory activity. Finally it is suggested that use of SPE extract can be beneficial to ameliorate the symptoms of CFS and its associated parameters.
5. Conclusion
The results of behavioural and biochemical parameters indicates that SPE possesses good free radical scavenging activity and potent immunomodulator activity that not only stimulates humoural immunity models but also stimulates cellular immunity this may be due to presence of flavonoids or polyphenols. Thus, SPE helps to maintain the hampered antioxidant level during fatigue and overcomes the situation causing the immune dysfunction during CFSproviding a new powerful combat to against CFS. Further there is a need to find out the exact immunological mechanism by which SPE act, like whether it is targeting the TNF-α level or altering the IL-6 to mitigate the symptoms of CFS.
Sources of funding
Not disclosed.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Fukuda K., Straus S.E., Hichie I., Sharpe M.C., Dobbins J.G., Komarroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic fatigue syndrome study group. Ann Inter Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Reid S., Chalder T. Chronic fatigue syndrome. BMJ. 2000;320:292–296. doi: 10.1136/bmj.320.7230.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afari N., Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatr. 2003;160:221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 4.Reeves W.C., Wagner D., Nisenbaum R. Chronic fatigue syndrome- a clinical empirical approach to its definition and study. BMC Med. 2005;3:19. doi: 10.1186/1741-7015-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wearden A.J., Apple L. Research on cognitive complaints and cognitive functioning in patient with chronic fatigue syndrome: what conclusion can we draw? J Psychosom Res. 1996;41:197–211. doi: 10.1016/0022-3999(96)00131-6. [DOI] [PubMed] [Google Scholar]
- 6.Gaab J., Rohleder N., Heitiz V., Engert V., Schad T., Schurmeyer T.H. Stress-induced changes in LPS –induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Klimas N.G., Koneru A.O. Chronic fatigue syndrome: inflammation, immune function and neuroendocrine interaction. Curr Rheumatol Rep. 2007;9:482–487. doi: 10.1007/s11926-007-0078-y. [DOI] [PubMed] [Google Scholar]
- 8.Manuel K.B., Moorkens G., Vertommen J., De Leeuw I. Antioxidants status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci. 2001;68:2037–2049. doi: 10.1016/s0024-3205(01)01001-3. [DOI] [PubMed] [Google Scholar]
- 9.Nijs J., De Meirleir K. Oxidative stress might reduce the essential fatty acids in erythrocytes membrane of chronic fatigue syndrome patients. Nutr Neurosci. 2004;7:251–253. doi: 10.1080/10284150400004148. [DOI] [PubMed] [Google Scholar]
- 10.Fang H., Xie Q., Boneva R., Fostel J., Perkins R., Tong W. Gene expression profile exploration of large dataset on chronic fatigue syndrome. Pharmacogenommics. 2006;7:429–440. doi: 10.2217/14622416.7.3.429. [DOI] [PubMed] [Google Scholar]
- 11.Kaushik N., Fear D., Richards S.C.M., McDermott C.R., Nuwaysir E.F., Kellam P. Gene expression in peripheral blood mononuclear cells from patients with chronic fatigue syndrome. J Clin Pathol. 2005:826–832. doi: 10.1136/jcp.2005.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad J.J., Saade N.E., SAfieh- Garabedian B. Cytokine and neuroimmune-endocrine interaction: a role of hypothalamic –pituitary-adrenal axis revolving axis. J Neuroimmunol. 2002;13:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 13.Sekiyama A., Ueda H., Kashiwamura S., Nishida K., Yamaguchi S., Sasaki H. Role of the adrenal gland in stress-induced up regulation of cytokines in plasma. J Neuroimmunol. 2006;171:38–44. doi: 10.1016/j.jneuroim.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B., Aeschbach R., Loliger J., Aruoma O.I. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-v. [DOI] [PubMed] [Google Scholar]
- 15.Logan A.C., Wong C. Chronic fatigue syndrome: oxidative stress and dietary modification. Altern Med Rev. 2001;6:450–455. [PubMed] [Google Scholar]
- 16.Nadkarni K.M., Nadkarni A.K. vol. 2. Popular Prakashan, Mumbai; 1994. p. 1164. (Indian materia medica). [Google Scholar]
- 17.Khare C.P. Springer Science Business Media; 2007. Indian medicinal plants: an illustrated dictionary; p. 622. LLC. [Google Scholar]
- 18.Nakatani N., Nagashima M. Pungent amides from Spilanthes acmella var oleracea clark. Biosci Biotechnol Biochem. 1992;56:759. doi: 10.1271/bbb.56.759. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty A., Devi R.K.B., Rita S., Sharatchandra K.H., Singh T.H. Preliminary studies on anti-inflammatory and analgesic activities of Spilanthes acmella in experimental animals models. Indian J Pharmacol. 2004;36(3):148–150. [Google Scholar]
- 20.Ratnasooriya W.D., Pieris K.P.P., Samaratunga U., Jayakody J.R.A.C. Diuretic activity of Spilanthes acmella flowers in rats. J Ethanopharmacology. 2004;91:317–320. doi: 10.1016/j.jep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Siddique Y.H., Ara G., Mohammad F., Mohammad A. Protective effect the Spilanthes acmella extract against the genotoxic damage induced by cytoproteroe acetate in cultured human peripheral blood lymphocytes. Glob J Pharmacol. 2011;5(3):136–142. [Google Scholar]
- 22.Orapin W., Supaluk P., Chartchalerm I.N.A., Jutammad S., Somsak R., Virapong P. Vasorelaxant and antioxidant activities of Spilanthes acmela Murr. Int J Mol Sci. 2008;9:2724–2744. doi: 10.3390/ijms9122724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav R., Kharya D.M., Yadav N., Savadi R. Immunomodulatory potential of ethanol extract of Spilanthes acmella leaves. Int J Bio Med Res. 2011;2(3):631–635. [Google Scholar]
- 24.Kumaran A., Karunakaran R.J. In-vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT. 2007;40:344–352. [Google Scholar]
- 25.Saha M.R., Jahangir R., Biva I.J. In-vitro nitric oxide scavenging activity of ethanol leaf extracts of four Bangladeshi medicinal plants. S J Pharm Sci. 2008;1:57–62. [Google Scholar]
- 26.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other substrates and antioxidants by mean of Folin Ciocalteu reagent. Meth Enzym. 1999;299:152–178. [Google Scholar]
- 27.Satpute K.L., Jadhav M.M., Karodi R.S., Katare Y.S., Patil M.J., Rukhsana R. Immunomodulatory activity of fruits of Randia dumetorum Lamk. J Pharmacogn Phytother. 2009;1 [Google Scholar]
- 28.Gayathri V., Asha V.V., Subramoniam A. Preliminary studies on the immunomodulatory and antioxidant properties of Selaginella species. Indian J Pharmacol. 2005;37:381–385. [Google Scholar]
- 29.Dasputre N.L., Naikwade N.S. Immunomodulatory activity of Abutil on indicum Linn.on albino mice. Int J Pharma Sci Res. 2010;1(3):178–184. [Google Scholar]
- 30.Gupta A., Vij G., Sharma S., Tirkey N., Rishi P., Chopra K. Curcumin, a polyphenolic antioxidants, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunology. 2009;214:33–39. doi: 10.1016/j.imbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Kaur S., Anurag A., Tirkey N., Chopra K. Reversal of LPs-induced central and peripheral hyperalgesia by green tea extract. Phytother Res. 2005;19:39–43. doi: 10.1002/ptr.1621. [DOI] [PubMed] [Google Scholar]
- 32.Huang L., Huang B., Ye Q., Qin L. Bioactivity guided fractionation for anti-fatigue property of Acantho panax senticosus. J Ethnopharmacol. 2011;133:213–219. doi: 10.1016/j.jep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 33.Sachdeva K.A., Kushad A., Tiwari V., Chopra K. Epigallo cathechin gallate ameliorates chronic fatigue syndrome in mice: behavioral and Biochemical evidences. Behav Brain Res. 2009;205:414–420. doi: 10.1016/j.bbr.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Kumar P., Devala R.G., Lakshmayya Ramachandra S.S. Ethanol extract of Momordicatuberosa tubers protects liver in Paracetamol-induced damage. Arch Biol Sci. 2010;62:999–1003. [Google Scholar]
- 35.Green L.C., Wagner D.A., Glagowski J. Analysis of nitrate nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A., Garg R. Protective effect of antidepressants against chronic fatigue syndrome-induced behavioural changes and biochemical alterations. Fundam Clin Pharmacol. 2008:1–7. doi: 10.1111/j.1472-8206.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurements with the folin phenol reagents. J Biol Chem. 1951;6:450–455. [PubMed] [Google Scholar]
- 38.Gupta A., Garima V., Chopra K. Possible role of oxidative stress and immunological activation in mouse model of chronic fatigue syndrome and its attenuation by olive extract. J Neuroimmunol. 2010;226:3–7. doi: 10.1016/j.jneuroim.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira L.F., Reid M.B. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol. 2008;104:853–860. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- 40.Singh A., Garg V., Gupta S., Kulakarni S.K. Role of antioxidant in chronic fatigue syndrome in mice. Indian J Exp Bio. 2002;40:1240–1244. [PubMed] [Google Scholar]
- 41.Kulkarni S.K., Verma A. Protective effect of Mentat (BR-16 A®) a herbal preparation on alcohol abstinence-induced anxiety and convulsions. Probe. 1994;2:147–154. [PubMed] [Google Scholar]
- 42.Cleare A.J. The HPA axis and the genesis of chronic fatigue syndrome. Trends Endocrinol Metab. 2004;15(2):55–59. doi: 10.1016/j.tem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Jammes Y., Steinberg J.G., Mambrini O., Bregeon F., Delliux S. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med. 2005;257:299–310. doi: 10.1111/j.1365-2796.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy G., Spence V.A., McLaren M., Hill A., Underwood C., Belch J.J. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. 2005;395:584–589. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]