Abstract
After failure of first-line chemotherapy with fluoropyrimidines and platinum compounds for advanced gastric cancer, second-line chemotherapy with ramucirumab plus paclitaxel, which elicits a durable response, and third-line or later chemotherapy with nivolumab have been shown to lead to a more favorable prognosis in advanced gastric cancer patients. As new and more effective drugs are now available, sequential chemotherapy would contribute to prolonged survival. From this point of view, the patient’s disease course should be frequently monitored in order to adapt treatment regimens. This review summarizes the points to note in regard to radiological assessment, and discusses the integration of prognostic factors, tumor markers, and clinical symptoms that need to be taken into account to change treatment at an appropriate timing.
Keywords: Gastric cancer, Prognostic factor, Tumor marker, RECIST
Introduction
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer mortality worldwide [1]. Platinum compounds plus fluoropyrimidines are the most common first-line treatment for patients with unresectable advanced gastric cancer, with a median survival ranging from 8–10 months for patients with human epidermal growth factor receptor 2 (HER2)-negative disease [2–9].
In the second-line setting, taxanes (docetaxel or paclitaxel), or irinotecan are the validated therapeutic options for patients in good general condition [10–12]. More recently, two phase III trials have demonstrated that ramucirumab (an anti-vascular endothelial growth factor receptor 2 [VEGFR2] monoclonal antibody), as a single agent or in combination with paclitaxel, is associated with a survival benefit [13, 14].
Evidence showing an overall survival (OS) benefit of therapy in third- or later lines of chemotherapy in patients with advanced gastric cancer suggests that salvage therapy may indeed become the standard of care. The Asian ATTRACTION-02 phase III randomized trial comparing nivolumab (an anti-PD-1 antibody) to placebo in patients with unresectable advanced gastric cancer pretreated with two or more chemotherapy regimens has recently been published [15]. OS was significantly increased in the nivolumab group compared to the control group. A phase III trial conducted in China demonstrated a benefit with apatinib, a novel, orally administered VEGFR inhibitor, in the third-line setting [16]. Trifluridine/tipiracil (TAS-102) has also shown comparable efficacy as a third-line treatment [17].
In cross-comparisons of first-line treatment trials, treatment arms with a higher proportion of patients receiving subsequent treatments showed better OS compared to treatment arms in which less patients received subsequent lines of therapy (Table 1) [2–14, 18]. This has demonstrated the positive impact that subsequent treatments can have. This phenomenon was evident in the comparison between the JCOG9205 and JCOG9912 trials, in which similar progression-free survival (PFS) was achieved with the same first-line treatment consisting of 5-FU monotherapy, yet OS was longer in the JCOG9912 trial in which 80% of patients received later lines of treatment [19]. OS has improved with the development of drugs that are effective not only in first-line but also in later lines of treatment. In a systematic review of 25 phase III trials for gastric cancer, it was reported that a higher proportion of patients receiving subsequent chemotherapy correlates with a longer overall survival [20].
Table 1.
Pivotal phase 3 trials in advanced gastric cancer
| Trial | Arm | PFS (months) | OS (months) | RR (%) | Subsequent chemotherapy (%) |
|---|---|---|---|---|---|
| First-line chemotherapy | |||||
| JCOG9912 | 5-FU | 2.9 | 10.8 | 9 | 83 |
| S-1 | 4.2 | 11.4 | 28 | 74 | |
| SPIRITS | S-1 | 4.0 | 11.0 | 31 | 75 |
| SP | 6.0 | 13.0 | 54 | 74 | |
| ML17032 | FP | 5.0 | 9.3 | 32 | 24 |
| XP | 5.6 | 10.5 | 46 | 24 | |
| AVAGAST | XP | 5.3 | 10.1 | 37.4 | 45 |
| G-SOX | SP | 5.4 | 13.1 | 52.2 | 84.7 |
| SOX | 5.5 | 14.1 | 55.7 | 84.3 | |
| JCOG1013 | SP | 6.5 | 15.3 | 56 | 79 |
| DCS | 7.4 | 14.2 | 59 | 77 | |
| ToGA* | XP/FP | 5.5 | 11.1 | 35 | 43 |
| XP/FP + Tmab | 6.7 | 13.8 | 47 | 38 | |
| JACOB* | XP + Tmab | 7.0 | 14.2 | 48.3 | 42 |
| XP + Tmab + pertuzumab | 8.5 | 17.5 | 56.7 | 43 | |
| Second-line chemotherapy | |||||
| COUGAR-02 | ASC | – | 3.6 | – | 19 |
| DTX | – | 5.2 | 7 | 8.3 | |
| WJOG4007 | PTX | 3.6 | 9.5 | 20.9 | 89.8 |
| IRI | 2.3 | 8.4 | 13.6 | 72.1 | |
| REGARD | BSC | 1.3 | 3.8 | 3 | 39.3 |
| RAM | 2.1 | 5.2 | 3 | 31.5 | |
| RAINBOW | PTX | 2.9 | 7.4 | 16 | 46 |
| PTX + RAM | 4.4 | 9.6 | 28 | 48 | |
| ABSOLUTE | PTX | 3.8 | 10.9 | 24 | 77 |
| nab-PTX (q1w) | 5.3 | 11.1 | 33 | 70 | |
| nab-PTX (q3w) | 3.8 | 10.3 | 25 | 72 | |
| Third- or later-line chemotherapy | |||||
| ATTRACTION-2 | BSC | 1.45 | 4.14 | 0 | 44.2 |
| Nivolumab | 1.61 | 5.26 | 11.2 | 47.0 | |
| TAGS | BSC | 1.8 | 3.6 | 2 | 25 |
| FTD/TPI | 2.0 | 5.7 | 4 | 26 | |
PFS: progression-free survival; OS: overall survival; RR: response rate; 5-FU: fluorouracil; SP: S-1 plus cisplatin; FP: 5-FU plus cisplatin; XP: capecitabine plus cisplatin; SOX: S-1 plus oxaliplatin; DCS: docetaxel and cisplatin plus S-1; ASC: active symptom control; DTX: docetaxel; PTX: paclitaxel; IRI: irinotecan; BSC: best supportive care; RAM: ramucirumab; nab-PTX: nab-paclitaxel; Tmab: trastuzumab; FTD/TPI: trifluridine/tipiracil
*Patients with HER2-positive metastatic gastric or gastroesophageal junction cancers were included in these trials
A post hoc analysis of the Japanese subpopulation from the RAINBOW trial showed that patients with measurable disease who received second-line ramucirumab plus paclitaxel had a response rate of 41.2% and a disease control rate of 94.1% [21]. This is surprisingly comparable to the results achieved with first-line chemotherapy such as a fluoropyrimidine plus a platinum compound (regardless of HER2 status) [22].
Data from the Japanese subpopulation in Attraction 2 trial shows objective response rate of 14% and duration of response of 14.5 months that is better than the intention-to-treat population. Japanese patients who treated with prior ramucirumab therapy indicated higher objective response rate (22.2%) and better OS (hazard ratio of 0.57) [23]. These data suggest importance of treatment sequence and treatment choice.
In the light of such findings, it is no longer true that first-line therapy is the last line of treatment for advanced gastric cancer. Many patients can now expect similar efficacy and benefit not only from first-line but also from later lines of therapy.
Figure 1a shows the conceptual diagram of tumor response according to the “Response evaluation criteria in solid tumors (RECIST)” guidelines and baseline progressive disease (PD) [24]. In the example shown, the therapy was started at baseline and a favorable effect was observed initially; however PD occurred gradually or diminished efficacy was observed. The initial 30% tumor regression is defined as “partial response (PR)” in RECIST. The presence of a subsequent 20% increase from the minimum size and an increase by at least 5 mm in absolute value is defined as “PD” in RECIST. The return to baseline is defined as “baseline PD”. Medical oncologists are taught to make the most of RECIST when evaluating the effect of therapy and when considering the timing of therapy change. However, excessive adherence to RECIST may result in missing the appropriate timing for switching to second- or third-line therapy in the treatment of advanced gastric cancer.
Fig. 1.
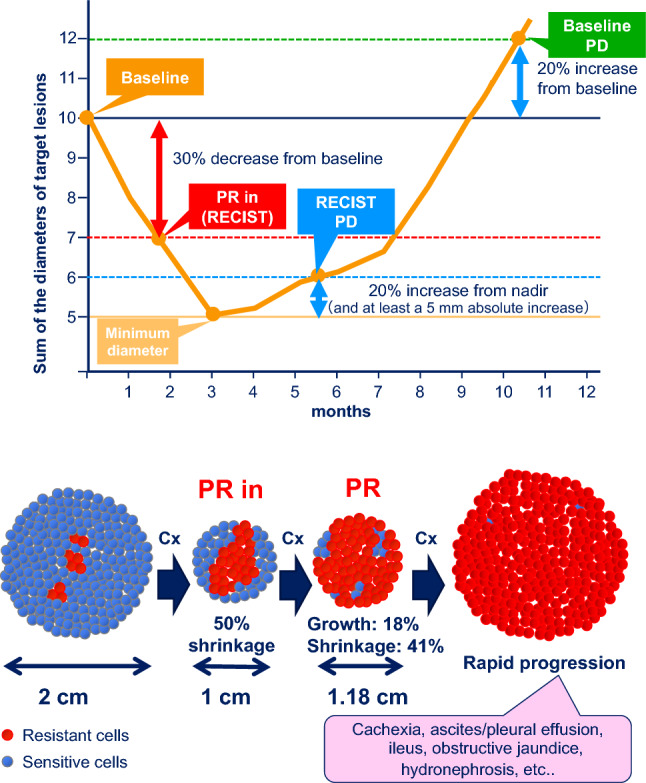
Tumor assessment and the relationship between sensitive and resistant cells. a Tumor response according to RECIST. b Important points of tumor assessment. PR: partial response, PD: progressive disease, PR in: initial partial response, Cx: chemotherapy
When the patient starts treatment, there are few resistant cells, and a PR state is achieved through chemotherapy. With continued PR, the tumor initially regresses (for example from 2 cm to 1 cm) before an increase (for example to 1.18 cm) is recorded at the next evaluation of efficacy, and the PR state is maintained. Since the tumor is smaller, the patient is categorized as having stable disease (SD). However, it is critical to know if this smaller tumor is now comprised mainly of resistant cells that emerged as a direct result of treatment (Fig. 1b). Otherwise, a sudden progression of the cancer may be observed prior to or at the next scanning occasion, which is more frequently observed in gastric cancer than in other cancer types.
Although there is mounting evidence of the importance of second-line and salvage chemotherapy, there is little published guidance on how to utilize subsequent treatment at the appropriate timing. In this review, we discuss ways to increase the proportion of patients getting the most benefit from subsequent lines of treatment. We focus on the timing of radiographic assessment, and the evaluation of tumor markers, prognostic factors and clinical symptoms, all of which may be useful in determining disease progression and clinical outcomes.
Prognostic factors
There are several prognostic factors that might be useful for monitoring patients during chemotherapy.
Two major reports analyzing prognostic factors during first-line treatment have been published. The first identifies the Royal Marsden Hospital prognostic Index (RMH-Index); performance status (PS) ≥ 2, liver metastases, peritoneal metastases, and alkaline phosphatase (ALP) ≥ 100 U/L as poor prognostic factors [25]. A second report identifies the JCOG-Index; PS ≥ 1, number of metastatic sites ≥ 2, no prior gastrectomy, and ALP ≥ 100 U/L as risk factors [26]. External validation of these factors has been obtained from phase III clinical trials. Three reports from Korea [27–29] identified several prognostic factors, which are summarized in Table 2. There were three overlapping factors in these studies, namely PS, gastrectomy, and ALP levels.
Table 2.
Prognostic factors in advanced gastric cancer
| Chau et al. [25] | Takahari et al. [26] | Lee et al. [27] | Kim et al. [28] | Koo et al. [29] | Fanotto et al. [31] | Fuchs et al. [41] | |
|---|---|---|---|---|---|---|---|
| Host status | |||||||
| ECOG PS | ● | ● | ● | ● | ● | ● | ● |
| Tumor status | |||||||
| No gastrectomy | ● | ● | ● | ● | |||
| Peritoneal metastasis | ● | ● | ● | ● | ● | ||
| Bone metastasis | ● | ● | ● | ||||
| Liver metastasis | ● | ||||||
| Lung metastasis | ● | ||||||
| Number of metastatic sites | ● | ● | |||||
| First-line TTP/PFS | ● | ● | |||||
| Laboratory test values | |||||||
| Increased ALP | ● | ● | ● | ● | ● | ||
| Increased AST | ● | ● | |||||
| Decreased albumin | ● | ● | ● | ||||
| Elevated total bilirubin | ● | ● | |||||
| Increased LDH | ● | ● | |||||
| NLR | ● | ||||||
ECOG PS: Eastern Cooperative Oncology Group performance status; TTP: time to progression; PFS: progression-free survival; ALP: alkaline phosphatase; AST: aspartate transaminase; LDH: lactate dehydrogenase; NLR: neutrophil-to-lymphocyte ratio. References [30] and [40] are reports on second-line treatment
In another report, independent prognostic factors for gastric cancer patients who underwent first-line treatment with S-1 plus cisplatin were PS ≥ 1, more than one metastatic site, and high ALP levels [30]. A report from Italy identifies PS, lactate dehydrogenase (LDH) levels, neutrophil-to-lymphocyte ratio (NLR), and PFS during second-line therapy as prognostic factors [31].
In advanced gastric cancer patients who underwent chemotherapy, high ALP levels were identified as an independent prognostic factor in multivariate analysis [32]. In a systematic review and meta-analysis of 76 clinical trials of solid cancers, high LDH levels were associated with poor prognosis [33]. In advanced gastric cancer, the albumin-globulin ratio (AGR: albumin/total protein—albumin) is an independent prognostic factor for OS and PFS [34]. In addition, a post hoc analysis of patients in the REAL-2 trial has reported on the prognostic value of the NLR [35].
In addition, LDH [36], VEGF [37], microsatellite instability (MSI) and HER2 [38], among others, have been indicated as biomarker candidates. Furthermore, there has been a report on nomograms combining HER2 status with other factors [39], as well as a report on another nomogram with seven factors [40] predicting survival.
With regard to second-line treatment, a pooled analysis of the RAINBOW and REGARD trials identified the following markers of poor prognosis: peritoneal metastases, PS 1, the presence of a primary tumor, time to progression (TTP) < 6 months, poor/unknown tumor differentiation, abnormally low blood levels of albumin, sodium, and/or lymphocytes, and abnormally high blood levels of neutrophils, aspartate aminotransferase (AST), ALP, and/or LDH [41]. Another study found PS 2, hemoglobin ≤ 11.5 g/dl, carcinoembryonic antigen (CEA) level > 50 ng/ml, the presence of three or more metastatic sites, and TTP under first-line chemotherapy of 6 months or less were poor prognostic factors [42]. Finally, PS 0–1, hemoglobin level ≥ 10 g/dl, and TTP under first-line therapy of 5 months or more were identified as favorable prognostic factors [43].
Although opinions on prognostic factors and the timing of treatment change vary, in one analysis conducted after the RAINBOW trial, it took 5.7 months to observe a reduction in PS by one grade in the ramucirumab group, but 4.3 months in the placebo group [44]. Similarly, in the TAGS trial, treatment with trifluridine/tipiracil prolonged the period to PS deterioration to > 2 compared to placebo [17]. In other words, transition to appropriate second- and subsequent lines of treatment may delay PS deterioration. On the other hand, the period from disease progression to PS deterioration is about 1 to 2 months. Therefore, if there is a decline in PS, it should be suspected that the disease may have already progressed.
If a patient has a factor related to poor prognosis, caution is warranted in regard to changes in their disease condition. If a deterioration in the parameter is observed, it is important to maintain the effectiveness of subsequent treatment by making an image-based diagnosis with PD in mind, and by monitoring the treatment effect on the whole body in a timely manner.
Tumor markers
Tumor markers are very useful in some situations for monitoring the state of disease in clinical practice. However, an increase in tumor marker levels should not be the only reason for switching a patient onto a new chemotherapy regimen. The reports discussed below focus on detecting recurrent disease and distant metastasis after curative surgery, not on patients with advanced gastric cancer undergoing chemotherapy. For example, more than 90% of patients with elevated preoperative levels of CEA also have elevated CEA levels at the time of recurrence [45]. In addition, elevated levels of markers associated with tumor growth are observed 2–3 months before the emergence of imaging abnormalities. This means that only a small change may be detected in the radiological image when the levels of tumor markers increase during treatment for advanced gastric cancer. There is an obvious correlation between the increase in tumor burden and the elevation of tumor marker values. On the other hand, clinicians should abstain from easily changing the treatment regimen if there is no symptomatic exacerbation or radiographic progression. In other words, it is of utmost importance to utilize tumor markers properly and effectively when making the decision to change treatment.
There are some clinically useful tumor markers for monitoring gastric cancer, including CEA, carbohydrate antigen 19-9 in the sialyl Lewis A group (CA19-9), sialyl Tn antigen (STN), cancer antigen 72-4 (CA72-4), cancer antigen 125 (CA125), and alpha-fetoprotein (AFP) (Table 3) [46]. Each tumor marker has its own respective characteristics. For example, CEA has been significantly associated with differentiated tumor types [47], and CEA level is an independent predictive factor for the presence of liver metastasis. Although 5–10% of patients are Lewis negative, CA19-9 is frequently associated with nodal involvement; indeed, the positive predictive value of this marker for nodal involvement is 80% or more. On the other hand, the positive predictive value of CA19-9 for peritoneal metastasis is less than 30% [48]. High serum STN is an independent factor that predicts liver metastasis and a worse outcome in gastric cancer patients [49]. The rate of CA72-4 positivity is significantly higher than that of CEA in patients with poorly differentiated adenocarcinoma, in patients with type 4 gastric cancer, and in patients with peritoneal metastases; 36% vs. 8%, 67% vs. 11%, and 69% vs. 23%, respectively [50, 51]. The level of CA125 is significantly correlated with the degree of peritoneal dissemination and patient survival [52]. CEA and CA19-9 are the most frequently measured tumor markers for the monitoring of gastric cancer. However, patients are often negative for both CEA and CA19-9 upon initial presentation. Although the levels of these markers may increase in the late phase of the disease course, patients are often still classified as negative until the terminally ill stage. Therefore, clinicians must endeavor to find other markers of disease progression beyond CEA and CA19-9.
Table 3.
Clinical relevance of serum tumor markers for gastric cancer
| T category | N category | M category | Peritoneal metastasis | Histology | Prognosis | Recurrence pattern | |
|---|---|---|---|---|---|---|---|
| CEA | Yes | Yes | Yes | No | Yes | Yes | Distant |
| CA19-9 | Yes | Yes | Yes | Yes | No | Yes | Distant |
| CA72-4 | Yes | Yes | Yes | Yes | No | Yes | Distant and/or peritoneal |
| AFP | NA | NA | Yes | NA | Yes | Yes | Liver |
| CA125 | NA | NA | NA | Yes | No | Yes | Peritoneal |
| STN | NA | NA | Yes | Yes | No | Yes | Peritoneal |
NA: not enough evidence to evaluate clinical significance was available; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9 in the sialyl Lewis A group; CA72-4: cancer antigen 72-4; AFP: alpha-fetoprotein; CA125: cancer antigen 125; STN: sialyl Tn antigen
In one example of utilizing tumor markers to inform the decision to change treatment, Hasegawa et al. [53] investigated patients with advanced gastric cancer with non-measurable peritoneal metastasis. These patients underwent treatment changes based on aggravated symptoms or elevated tumor markers, rather than on the radiological diagnosis of PD, and as a result had significantly improved OS. Peritoneal metastasis is often observed in diffuse-type adenocarcinoma, and there are many cases where no increase in tumor markers is observed in diffuse-type gastric cancer. However, Hasegawa et al. point out the utility of tumor markers with regard to making a decision to change treatment. Together, the findings of Hasegawa et al. suggest that tumor burden may already be high in patients whose treatment regimens are changed after PD is confirmed through CT scan results. As a cautionary note, that study was retrospective, and therefore, some bias may be involved; however, the overall importance of monitoring tumor markers is clearly demonstrated by that analysis.
In some exceptional cases, an initial elevation of tumor marker levels after initiation of chemotherapy doesn’t represent a sign of disease progression. This phenomenon is called a surge, which means a transient elevation of tumor marker levels despite achieving clinical benefit from chemotherapy. It has been reported that CEA and CA19-9 surges are observed in about 20% of patients, and the median time to peak is approximately 0.5–2 months after the initiation of chemotherapy [54].
Radiographic assessments
Radiographic assessment is the key method for disease evaluation, and in this section we discuss the importance of computed tomography (CT) assessment and the appropriate timing of follow-up assessments. CT assessment is needed not only to analyze tumor response but also to evaluate disease progression. In clinical practice, physicians use various procedures to monitor a patient’s disease status. These include evaluation of patients’ symptoms, laboratory data (including tumor markers) and radiographic assessment with CT or other modalities. In order to assess signs or symptoms of disease progression, clinicians must routinely conduct radiographic assessments.
In most clinical trials of first-line chemotherapy, tumor evaluation is conducted every 6 or 8 weeks [2, 3, 7, 8]. Furthermore, in most clinical trials of second- or later-line chemotherapy, CT evaluation intervals are set at 6 weeks [11, 13, 14, 55]. Since PFS during second- or third-line chemotherapy is estimated to be shorter than PFS during first-line treatment, we suggest that detailed evaluation should be carried out more often, which could be achieved through shortening the interval between CT scans.
If patients have only non-measurable lesions such as peritoneal or bone metastases with or without a primary gastric tumor, clinicians must pay close attention to signs of disease progression. It is difficult to detect signs of disease progression only from physical findings or symptoms, or laboratory data, including tumor markers. Therefore, by performing radiographic assessments routinely, clinicians will be much less likely to miss disease progression, and will detect it before clinical symptoms appear. When there is a suspicion of disease progression, even if signs of disease progression are not detected through the most recent CT evaluation, clinicians should consider the use of another radiographic assessment modality such as barium enema, or ultrasonography, to determine whether peritoneal metastasis is present. For the detection of peritoneal metastasis, CT showed a higher sensitivity (76.5%) compared with 18F-FDG PET (35.3%), although CT had a relatively lower specificity (91.6%) than did PET (98.9%) [56]. If patients have a primary gastric tumor, an upper gastrointestinal endoscopy is recommended to evaluate the disease condition directly.
Determining disease progression by symptoms
In contrast to other carcinomas, non-target lesions (including peritoneal dissemination) are common in gastric cancer. The frequency of peritoneal metastasis reported in the G-SOX [8], SPIRITS [3], and START [57] trials of first-line chemotherapy was 20%, 29% and 39%, respectively, while that reported in the WJOG4007 [11], REGARD [13], KOREA [12], and RAINBOW [14] trials of second-line chemotherapy was 26%, 31%, 45% and 47%, respectively. Since patients with severe peritoneal dissemination are excluded from such trials, its actual frequency is higher in clinical practice. In addition, because peritoneal metastasis is accompanied by various clinical symptoms that preclude an objective evaluation of disease status, the opportunity for further treatment can be missed due to rapid disease progression.
In clinical trials, tumor response is evaluated according to the revised RECIST guidelines (version 1.1). The original RECIST v1.0 clearly stated that “it is not intended that these guidelines will be used as a basis for making decisions about continued therapy”; RECIST v1.1 also takes the same position, stating that “Many oncologists in their daily clinical practice follow their patients’ malignant disease by means of repeated imaging studies and make decisions about continued therapy on the basis of both objective and symptomatic criteria. It is not intended that these RECIST guidelines play a role in that decision-making, except if determined appropriate by the treating oncologist.”
Consequently, “overall response” based on the results of response evaluation according to the RECIST guidelines “should be used to determine whether an agent or regimen shows a promising result that is worth continuing development research.” In other words, determining whether to continue the therapy or not for an individual patient should not be based on overall response (complete response/PR/SD/PD) but on comprehensive “clinical decisions” including imaging results, symptoms, physical findings, and different examinations. Accordingly, the continuation of protocol treatment may still be clinically appropriate in some patients, even when PD is determined as the overall response based on diagnostic imaging. In these cases, determining whether to continue protocol treatment should be based on clinical decisions regardless of overall response. Reciprocally, protocol treatment should be discontinued in cases that are not diagnosed as PD by response evaluation criteria based on imaging results, but are determined clinically and comprehensively by physicians to be a ‘clinical exacerbation’.
The original RECIST v1.1 publication states in special notes on assessment of progression of non-target disease that “to achieve ‘unequivocal progression’ on the basis of the non-target disease, there must be an overall level of substantial worsening in non-target disease such that, even in the presence of SD or PR in target disease, the overall tumor burden has increased sufficiently to merit discontinuation of therapy”. This means that the evaluation of PD in non-target lesions affects “decisions on whether to continue a therapy or not for each patient” and in consequence generates confusion. It should be noted that such “unequivocal progression” is an evaluation criterion limited solely to the evaluation of “PD in non-target lesions.”
Peritoneal dissemination may cause serious complications, such as intestinal obstruction, massive ascites and hydronephrosis associated with the clinical presentation of abdominal pain and feeling of fullness, vomiting, constipation, malnutrition and renal dysfunction (Table 4) [58]. The overall response rate has not been adopted as an endpoint in clinical studies because a substantial proportion of gastric cancer patients with peritoneal metastasis do not have measurable lesions according to the RECIST criteria, and clinical symptoms are more important in determining whether to discontinue treatment. Moreover, it is often difficult to differentiate treatment toxicity from disease progression in patients with complicating severe anorexia and/or nausea. In these cases, the decision to discontinue treatment (a Go/No-Go decision) should be made after a relatively short follow-up period.
Table 4.
Findings of disease progression involving peritoneal metastasis
| Progression of peritoneal metastasis |
| Aggravation of peritoneal mass, intestinal wall thickening, ascites, and intestinal stenosis |
| Aggravation of intestinal obstruction or stenosis symptoms, such as decreased food intake, abdominal pain, nausea and vomiting not attributable to chemotherapy-related adverse events |
| Aggravation of the feeling of abdominal fullness |
| Deterioration of renal function (emergence of hydronephrosis due to aggravation of peritoneal dissemination) |
| Deterioration of general condition such as a decrease in performance status determined to be caused by the original disease |
| An increase in the number of times that ascites drainage is required |
| Disease progression difficult to determine from imaging test results |
| Exacerbation of cancer pain |
| Deterioration of general condition such as a decrease in performance status determined to be caused by the original disease |
| The emergence and worsening of disseminated intravascular coagulation |
| An increase in tumor markers |
We analyzed salvage therapy trials where the active compound was compared to placebo plus best supportive care. In that type of trial, the high frequency of symptoms seen in the placebo arms as adverse events could easily be expected to suggest signs of disease progression (Table 5) [15, 17].
Table 5.
Adverse events in the placebo arms of phase 3 trials in the salvage-line setting
| REGARD (n = 115) | TAGS (n = 168) | |||
|---|---|---|---|---|
| Any grade (%) | Grade ≥ 3 (%) | Any grade (%) | Grade ≥ 3 (%) | |
| Anorexia | 23 | 3 | 30 | 6 |
| Nausea | – | – | 32 | 3 |
| Vomiting | 25 | 4 | 20 | 2 |
| Dysphagia | 10 | 4 | 4 | 2 |
| Abdominal pain | 28 | 3 | 19 | 9 |
| Diarrhea | – | – | 15 | 2 |
| Constipation | 23 | 3 | 12 | 2 |
| Fatigue | 40 | 10 | 21 | 6 |
| Dyspnea | 13 | 6 | 10 | 3 |
Therefore, if clinical symptoms or abnormal blood test data (including renal dysfunction and elevated bilirubin, or elevated tumor markers, ALP, and LDH) suggest there is an exacerbation of the disease, imaging tests should be promptly carried out. In addition to imaging test results, the clinical symptoms and blood test data must be taken into consideration and, if determined necessary, transition to a subsequent treatment should be considered.
Summary
Clinicians need to judge treatment decision based on the evaluation of tumor markers, prognostic factors, radiographic assessment and clinical symptoms (Fig. 2).
The status of tumor markers, such as CEA, CA19-9 and other markers indicative of specific sites of metastasis, should be taken into account when determining whether to change treatment in advanced gastric cancer. Clinicians should also be aware of the possibility of a transient surge in tumor marker levels just after initiation of chemotherapy despite the patient achieving clinical benefit from it.
When patients have many poor prognostic factors, such as poor PS, increased ALP levels, and peritoneal metastasis, they should be monitored more closely to avoid missing the opportunity for a timely switch in treatment regimens.
Though RECIST criteria are important in terms of response evaluation, clinical decisions should not be based solely on radiologic findings but should take into consideration other findings.
Clinicians should take into consideration symptoms, such as bowel fullness and nausea indicative of peritoneal metastasis, and specific changes in blood examination results and imaging test results. If such findings have changed, imaging tests should be carried out promptly to make a decision for Go/No-Go of the current regimen.
Fig. 2.
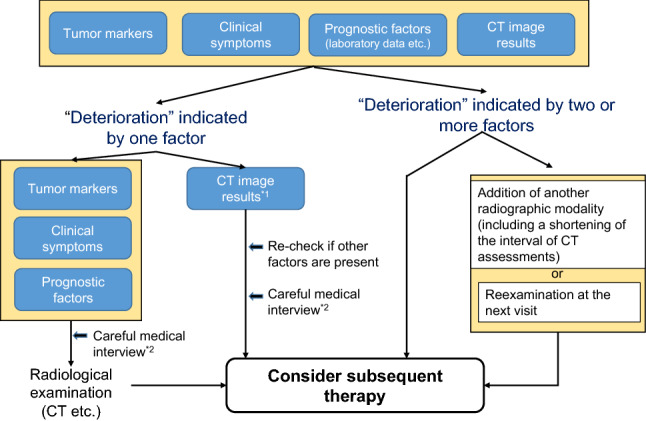
The decision to change treatment in patients with advanced gastric cancer receiving chemotherapy. *1 Deterioration indicated by CT image results includes the following; an increase in the size of the target lesion; an increase/trend for an increase in the size of non-target lesion; emergence of a new lesion; characteristic findings (bowel wall thickening, bowel dilatation, hydronephrosis, biliary dilatation, etc.). *2 A careful medical interview includes questions about; the condition of meal intake; body weight changes; symptoms such as constipation/diarrhea, feeling of fullness, relapse of previous symptoms(not only abdominal pain and nausea); other changes in daily life
Conclusion
Gastric cancer tends to progress rapidly in a short period, and without careful patient follow-up, physicians may miss the appropriate opportunity for switching to a subsequent therapy. Unlike 10 years ago, many drugs that can significantly extend survival time are now available and patients should never be deprived of the opportunity to access such effective drugs. It is of utmost importance to comprehensively capture signs that indicate exacerbation of the disease. In clinical practice, physicians sometimes face paradoxical situations such as an increase in a clinical parameter of disease worsening despite CT imaging results showing tumor shrinkage. Halting disease progression in patients is a paramount concern for clinicians. Therefore, it is critical that multiple factors are taken into account when deciding on the best clinical course of action. Needless to say, with this added level of extra care, significant improvements can be expected in the prognosis of patients with gastric cancer.
Acknowledgements
We wish to thank the help given by Dr. Hiroshi Imamura (Toyonaka Municipal Hospital, Osaka, JAPAN).
Conflict of interest
Satoru Iwasa has received research grants from Chugai Pharmaceutical, Bayer Yakuhin, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, and Merck Biopharma, and honoraria from Ono Pharmaceutical, Eli Lilly, Taiho Pharmaceutical and Chugai Pharmaceutical. Toshihiro Kudo has received donations for his department from Yakult Honsha, Chugai Pharmaceutical, and Ono Pharmaceutical, research grants from Eli Lilly, Bayer Yakuhin, and Otsuka Pharmaceutical, and honoraria from Yakult Honsha, Chugai Pharmaceutical, Ono Pharmaceutical, Eli Lilly, Bayer Yakuhin, Merck Biopharma, MSD, Takeda Pharmaceutical, Taiho Pharmaceutical and Sanofi. Daisuke Takahari has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Eli Lilly, Taiho Pharmaceutical and Chugai Pharmaceutical. Hiroki Hara has received honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Eli Lilly, Chugai Pharmaceutical, Yakult Honsha, Takeda Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo, Sanofi and MSD. Ken Kato has received research grants from Ono Pharmaceutical, MSD, Shionogi, Merck Biopharma and BeiGene. Taroh Satoh has received donations for his department from Ono Pharmaceutical, Yakult Honsha and Chugai Pharmaceutical, and research grants and honoraria from Ono Pharmaceutical, Yakult Honsha, Chugai Pharmaceutical, Eli Lilly, Bristol Myers Squibb, Merck Serono, Takeda Pharmaceutical, Taiho Pharmaceutical and MSD.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/22/2020
The article Practical guidance for the evaluation of disease progression.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 4.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148. doi: 10.1093/annonc/mdu472. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Boku N, Mizusawa J, et al. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:501–510. doi: 10.1016/S2468-1253(19)30083-4. [DOI] [PubMed] [Google Scholar]
- 10.Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 11.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 12.Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs CS, Tomasek J, Yong CJ, Trial Investigators REGARD, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 14.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 15.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled Phase III Trial of Apatinib in patients With chemotherapy-refractory advanced or metastatic aenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 17.Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–1448. doi: 10.1016/S1470-2045(18)30739-3. [DOI] [PubMed] [Google Scholar]
- 18.Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:277–287. doi: 10.1016/S2468-1253(16)30219-9. [DOI] [PubMed] [Google Scholar]
- 19.Takashima A, Boku N, Kato K, et al. Survival prolongation after treatment failure of first-line chemotherapy in patients with advanced gastric cancer: combined analysis of the Japan Clinical Oncology group trials JCOG9205 and JCOG9912. Gastric Cancer. 2014;17:522–528. doi: 10.1007/s10120-013-0309-z. [DOI] [PubMed] [Google Scholar]
- 20.Iizumi S, Takashima A, Sakamaki K, et al. Survival impact of post-progression chemotherapy in advanced gastric cancer: systematic review and meta-analysis. Cancer Chemother Pharmacol. 2018;81:981–989. doi: 10.1007/s00280-018-3569-9. [DOI] [PubMed] [Google Scholar]
- 21.Shitara K, Muro K, Shimada Y, et al. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer. 2016;19:927–938. doi: 10.1007/s10120-015-0559-z. [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa Y, Sugimoto N, Miwa H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1) Br J Cancer. 2014;110:1163–1168. doi: 10.1038/bjc.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato K, Satoh T, Muro K, et al. A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2) Gastric Cancer. 2019;22:344–354. doi: 10.1007/s10120-018-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 26.Takahari D, Boku N, Mizusawa J, et al. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan Clinical Oncology Group 9912. Oncologist. 2014;19:358–366. doi: 10.1634/theoncologist.2013-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Lim T, Uhm JE, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18:886–891. doi: 10.1093/annonc/mdl501. [DOI] [PubMed] [Google Scholar]
- 28.Kim JG, Ryoo BY, Park YH, et al. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:301–307. doi: 10.1007/s00280-007-0476-x. [DOI] [PubMed] [Google Scholar]
- 29.Koo DH, Ryoo BY, Kim HJ, et al. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: validation and comparison with previous models. Cancer Chemother Pharmacol. 2011;68:913–921. doi: 10.1007/s00280-011-1561-8. [DOI] [PubMed] [Google Scholar]
- 30.Kadowaki S, Komori A, Narita Y, et al. Long-term outcomes and prognostic factors of patients with advanced gastric cancer treated with S-1 plus cisplatin combination chemotherapy as a first-line treatment. Int J Clin Oncol. 2014;19:656–661. doi: 10.1007/s10147-013-0610-1. [DOI] [PubMed] [Google Scholar]
- 31.Fanotto V, Cordio S, Pasquini G, et al. Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer. 2017;20:825–833. doi: 10.1007/s10120-016-0681-6. [DOI] [PubMed] [Google Scholar]
- 32.Namikawa T, Ishida N, Tsuda S, et al. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer. 2018;22:684–691. doi: 10.1007/s10120-018-0897-8. [DOI] [PubMed] [Google Scholar]
- 33.Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961–970. doi: 10.3109/0284186X.2015.1043026. [DOI] [PubMed] [Google Scholar]
- 34.Bozkaya Y, Erdem GU, Demirci NS, et al. Prognostic importance of the albumin to globulin ratio in metastatic gastric cancer patients. Curr Med Res Opin. 2019;35:275–282. doi: 10.1080/03007995.2018.1479683. [DOI] [PubMed] [Google Scholar]
- 35.Grenader T, Waddell T, Peckitt C, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol. 2016;27:687–692. doi: 10.1093/annonc/mdw012. [DOI] [PubMed] [Google Scholar]
- 36.Kolev Y, Uetake H, Takagi Y, et al. Lactate Dehydrogenase-5 (LDH-5) Expression in Human Gastric Cancer: Association with Hypoxia-Inducible Factor (HIF-1a) Pathway, Angiogenic Factors Production and Poor Prognosis. Ann Surg Oncol. 2008;15:2336–2344. doi: 10.1245/s10434-008-9955-5. [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Lee HE, Yang HK, et al. High lactate dehydrogenase 5 expression correlates with high tumoral and stromal vascular endothelial growth factor expression in gastric cancer. Pathobiology. 2014;81:78–85. doi: 10.1159/000357017. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Shen H, Kapesa L, et al. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11:2959–2964. doi: 10.3892/ol.2016.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narita Y, Kadowaki S, Oze I, et al. Establishment and validation of prognostic nomograms in first-line metastatic gastric cancer patients. J Gastrointest Oncol. 2018;9:52–63. doi: 10.21037/jgo.2017.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Custodio A, Carmona-Bayonas A, Jiménez-Fonseca P, et al. Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br J Cancer. 2017;116:1526–1535. doi: 10.1038/bjc.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs CS, Muro K, Tomasek J, et al. Prognostic factor analysis of overall survival in gastric cancer from two phase III studies of second-line ramucirumab (REGARD and RAINBOW) using pooled patient data. J Gastric Cancer. 2017;17:132–144. doi: 10.5230/jgc.2017.17.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catalano V, Graziano F, Santini D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer. 2008;99:1402–1407. doi: 10.1038/sj.bjc.6604732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanagavel D, Pokataev IA, Fedyanin MY, et al. A prognostic model in patients treated for metastatic gastric cancer with second-line chemotherapy. Ann Oncol. 2010;21:1779–1785. doi: 10.1093/annonc/mdq032. [DOI] [PubMed] [Google Scholar]
- 44.Al-Batran SE, Van Cutsem E, Oh SC, et al. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol. 2016;27:673–679. doi: 10.1093/annonc/mdv625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi Y, Takeuchi T, Sakamoto J, et al. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003;6:142–145. doi: 10.1007/s10120-003-0240-9. [DOI] [PubMed] [Google Scholar]
- 46.Shimada H, Noie T, Ohashi M, et al. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33. doi: 10.1007/s10120-013-0259-5. [DOI] [PubMed] [Google Scholar]
- 47.Maehara Y, Sugimachi K, Akagi M, et al. Serum carcinoembryonic antigen level increases correlate with tumor progression in patients with differentiated gastric carcinoma following noncurative resection. Cancer Res. 1990;50:3952–3955. [PubMed] [Google Scholar]
- 48.Kodera Y, Yamamura Y, Torii A, et al. The prognostic value of preoperative serum levels of CEA and CA19-9 in patients with gastric cancer. Am J Gastroenterol. 1996;91:49–53. [PubMed] [Google Scholar]
- 49.Nakagoe T, Sawai T, Tsuji T, et al. Pre-operative serum levels of sialyl Tn antigen predict liver metastasis and poor prognosis in patients with gastric cancer. Eur J Surg Oncol. 2001;27:731–739. doi: 10.1053/ejso.2001.1199. [DOI] [PubMed] [Google Scholar]
- 50.Hamazoe R, Maeta M, Matsui T, et al. CA72-4 compared with carcinoembryonic antigen as a tumour marker for gastric cancer. Eur J Cancer. 1992;28A:1351–1354. doi: 10.1016/0959-8049(92)90517-6. [DOI] [PubMed] [Google Scholar]
- 51.Tachibana M, Takemoto Y, Nakashima Y, et al. Serum carcinoembryonic antigen as a prognostic factor in resectable gastric cancer. J Am Coll Surg. 1998;187:64–68. doi: 10.1016/s1072-7515(98)00133-1. [DOI] [PubMed] [Google Scholar]
- 52.Emoto S, Ishigami H, Yamashita H, et al. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer. 2012;15:154–161. doi: 10.1007/s10120-011-0091-8. [DOI] [PubMed] [Google Scholar]
- 53.Hasegawa H, Fujitani K, Nakazuru S, et al. Optimal treatment change criteria for advanced gastric cancer with non-measurable peritoneal metastasis: symptom/tumor marker-based versus CT-based. Anticancer Res. 2014;34:5169–5174. [PubMed] [Google Scholar]
- 54.Kim HJ, Lee KW, Kim YJ, et al. Chemotherapy-induced transient CEA and CA19-9 surges in patients with metastatic or recurrent gastric cancer. Acta Oncol. 2009;48:385–390. doi: 10.1080/02841860802446761. [DOI] [PubMed] [Google Scholar]
- 55.Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 56.Lim JS, Kim MJ, Yun MJ, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006;7:249–256. doi: 10.3348/kjr.2006.7.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START) J Cancer Res Clin Oncol. 2014;140:319–328. doi: 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamamoto Y. Complications in advanced or recurrent gastric cancer patients with peritoneal metastasis during and after palliative systemic chemotherapy. Mol Clin Oncol. 2015;3:539–542. doi: 10.3892/mco.2015.510. [DOI] [PMC free article] [PubMed] [Google Scholar]


