Abstract
Whether the clinical trial treatment effect of urate-lowering therapy (ULT) in patient with chronic kidney disease (CKD) is generalizable to real-word settings is unclear. This study aimed to compare febuxostat with allopurinol for uric acid reduction and renal protection in patients with CKD. Adult CKD patients newly treated with ULT were identified using electronic health records from 2010 to 2015 from a large healthcare delivery system in Taiwan. Patients with renal replacement therapy or undergoing ULT for <3 months were excluded. Propensity score–matched cohort study design was conducted to compare outcomes between patients initiated with febuxostat or allopurinol therapy. Cox regression analyses were employed to compare the adjusted hazards ratio (aHR) of incident event of estimated glomerular filtration rate (eGFR) ≥ 30% decrease, and the difference in longitudinal changes in serum uric acid (SUA) and eGFR between groups was analyzed using linear mixed model. Overall, 1050 CKD patients who initiated febuxostat (n = 525) or allopurinol (n = 525) treatment were observed for 2.5 years. Compared with allopurinol, febuxostat use was associated with higher rate of patients maintaining SUA target <6 mg/dL in >80% of follow-up time with a reduction in mean SUA change. There were no significant differences in the mean eGFR changes over time between the febuxostat and allopurinol groups or in the risk of eGFR decline ≥30% of baseline. Febuxostat was associated with greater reduction in SUA level than allopurinol in patients with CKD. However, febuxostat and allopurinol showed no difference in renal function changes during study follow-up. These findings require further investigation with long-term follow up in CKD patients with hyperuricemia.
Subject terms: Outcomes research, Therapeutics, Medical research, Nephrology
Introduction
Hyperuricemia has been related to the onset of chronic kidney disease (CKD)1 and increased risk of CKD progression2,3 as well as cardiovascular disease morbidity and mortality4,5. The level of serum uric acid (SUA) increases in parallel with the estimated glomerular filtration rate (eGFR) decline, which is present in 40% to 60% of patients with CKD stages 1 to 3 and in 70% of patients with CKD stage 4 or 53,6. Thus, lowering uric acid presents a critical strategy in the management and prevention of renal disease progression among patients with CKD.
For patients with gout or symptomatic hyperuricemia7–9, lowering the SUA below the target of 6 mg/dL is recommended to prevent acute flares10, quality of life deficit11, and additional medical costs12. The current urate-lowering therapy (ULT) includes xanthine oxidase inhibitors (XOIs), such as allopurinol and febuxostat, and uricosuric agents, such as benzbromarone, probenecid, and sulfinpyrazone. Because allopurinol carries a life-threatening risk of HLA-B*58:01–mediated cutaneous adverse drug reactions in some Asian populations and CKD increases additional risk for allopurinol side effects, genotyping screening before allopurinol initiation13 and starting at a lower dose then slowly titrating the dose upward to achieve the SUA target are recommended in practice. Febuxostat, a new XOI, at approved dose (80–120 mg/day), showed significantly more effectivity in lowering uric acid than allopurinol at dose commonly prescribed in practice (100–300 mg/day) and as safe as allopurinol in clinical trials14,15. Thus, febuxostat may represent an alternative treatment.
The effect of ULT on renal function is controversial16–18. A recent randomized placebo control trial suggested that febuxostat was not associated with renal protection in patients with asymptomatic hyperuricemia complicated by CKD stage 318. These discrepancies possibly can be explained by the baseline CKD stage, comorbid conditions, and magnitude of changes in eGFR from baseline on renal disease progression. Furthermore, there is still no clear agreement as to which ULT offers more effective renoprotection in CKD patients with hyperuricemia in trial settings19–22. The allopurinol-controlled Febuxostat for Cerebral and CaRdiorenovascular Event PrEvEntion StuDy (FREED) in elderly patients with CKD stage 3 suggested that stronger lowering uric acid with febuxostat was associated with indirect renal outcomes (i.e. albuminuria or proteinuria) but not the risks of eGFR decline, cardiovascular or mortality22. Thus, the aim of the present study was to study the effectiveness of febuxostat compared with allopurinol for changes in SUA, achievement of SUA goal, and eGFR decline between two groups of patients with CKD in a practice setting. As evidence directly comparing allopurinol to febuxostat among patients with CKD is limited, we hypothesized that there is no important differences in CKD progression between patients taking febuxostat and those taking allopurinol.
Methods
Study design and data source
The propensity score-matched cohort study was conducted using Chang Gung Research Database (CGRD), which is an electronic health records dataset from a group of Chang Gung Memorial Hospitals (CGMHs) in Taiwan. CGMHs provide approximately 10%–12% of health-care services in 2015 of Taiwan’s National Health Insurance (NHI) program23, which is a compulsory, single-payer health insurance program that covers over 99% of the entire population of Taiwan24. The CGRD contains detailed diagnostic, prescription, and laboratory test results from emergency department, inpatient, and outpatient settings.
Study cohort
We first identified patients aged ≥18 years and newly prescribed with febuxostat or allopurinol between January 1, 2005 and December 31, 2015, with a consistent supply for ≥90 days (with a permissible gap of ≤30 days), and set that day as the index date. Exclusion criteria were lack of any medical records 365 days before and after the index date, lack of valid laboratory test results of SUA and serum creatinine (SCr), and no CKD diagnosis during the study period. Following the 2012 KDIGO guideline25, International Classification of Diseases, Ninth Revision (ICD-9) codes for CKD were used to identify patients with CKD diagnosis at least 2 occasions more than 3 months apart within the 1-year period prior to the index date (Supplementary Table S1). To minimize potential bias introduced by advanced renal diseases, patients who had baseline eGFR ≤15 mL/min/1.73 m2, renal transplantation, and chronic dialysis (continuous ≥3 months) prior to the index date were not analyzed. Patients using any other ULT (benzbromarone, probenecid, or sulfinpyrazone) within the year prior to the index date were excluded.
Outcomes
The uric acid outcomes were based on the changes in SUA levels from baseline to the last quarter, the proportion of patients who achieved the targeted SUA level, and the proportion of patients who maintained a targeted SUA level for ≥80% (or <80%) of follow-up time. A 3-month mean SUA level <6 mg/dL was set as treatment goal for both female and male patients9, and SUA target levels of <7.7 mg/dL in male patients and <6.6 mg/dL in female patients were applied as an alternative measure26,27. Changes in mean SUA level and estimated eGFR were measured at 3-month intervals, described as mean eGFR change in the following section.
Renal outcomes were changes in eGFR from the baseline to the end of follow-up, incident episode of eGFR decline ≥30% of baseline, and renal replacement therapy (RRT) during follow-up. An eGFR decline ≥30% is strongly associated with the subsequent risk of CKD progression and has been used to assess long-term renal outcomes28. The mean eGFR change from baseline in the follow-up (eGFR) was measured to compare the effect difference between treatment groups, which was calculated as the ith 3-month interval’s mean eGFR minus mean eGFR at baseline (ΔeGFR = eGFRi−eGFRbaseline) for each individual. The baseline mean eGFR was measured based on multiple values of SCr in the latest 3 months close to the index date, and if the patients did not have sufficient SCr tests results (≥2 measures), a further period of 3 preceding months was applied. The eGFR was calculated using a Modification of Diet in Renal Disease formula, by 125I-iothalamate dilution mass spectrometry traceable serum creatinine: (175 × SCr −1.154 × age−0.203 × [0.742, if female] ×[1.212, if African American])29.
The baseline SUA level and eGFRs were obtained from ≤3 months prior to the index date, and the last follow-up SUA level and eGFRs were obtained from the record maintained before the end of follow-up (i.e., the last observation-carried-forward approach). Patients were observed from the index date until (1) the earliest date of decline in eGFR of ≥30%, chronic dialysis (i.e., ≥3 months and at least one episode in a month), or kidney transplantation; (2) loss to follow-up (defined as being 360 days without admissions before the last date in the dataset (i.e., December 31, 2015); or (3) death, whichever occurred first. Patients receiving RRT were identified by the ICD-9 code (V420) for renal transplantation and encounters for specialty care involving chronic dialysis. Death event was identified by “death code” at discharge from hospitalization. To avoid outcomes misclassification, patients who did not have admissions more than 1 years before December 31, 2015, were considered lost to follow-up and censored at the latest date in the dataset.
Confounding assessment
Demographic information, clinical condition, and medical history for the 12 months prior to the index date were retrieved for all study patients. The Quan-Charlson Comorbidity Index (CCI) score was used to categorize the degree of severity of baseline clinical conditions30. The ICD-9 codes for hypertensive diseases (401–405) and diabetes mellitus (250, 357.2, 362.0×, and 366.41) were applied to identify the presence of risk factors of renal function deterioration. Acute kidney injury (AKI) was identified by KDIGO definition of (1) increase in SCr ≥0.3 mg/dL within 48 hours or (2) increase in SCr ≥1.5 times the baseline within the prior 7 days31. Proteinuria was based on ≥2 episodes within 1 year with at least 90 days apart of (1) albumin-to-creatinine ratio ≥30 mg/g, (2) total protein in 24-hour urine ≥150 mg/day, or (3) single point of protein in urine ≥30 mg/dL (1+ to 4+) by dipstick testing.
Information on use of medicines for management of CKD complications was retrieved from the prescription-dispensing records for all study patients. Only prescriptions continuously refilled ≥90 days were assessed. They were renin-angiotensin-aldosterone system inhibitors (RASI), loop/thiazide diuretics, and lipid-lowering therapy (LLT) for lipid disorders. Prior medications used with long-term nephrotoxicity, such as immunosuppressive agents and non-steroidal anti-inflammatory drug (NSAID), were assessed.
To minimize selection bias, the propensity score derived from the allopurinol new users were matched with febuxostat new users at a ratio of 1:1 using greedy matching algorithm within the SAS software package (SAS, Cary, NC, USA)32. The covariates used to estimate patient’s propensity score including factors associated with uric acid level and renal function were age at ULT initiation, sex, baseline eGFR and SUA, proteinuria, hypertension, diabetes, AKI, use of diuretics, RASI, and individual disease conditions involved in the Quan-Charlson Comorbidity Index algorithm30. The distribution of propensity score and baseline patient characteristics between the febuxostat and allopurinol groups were examined before and after matching process to ensure balance in baseline covariates. To account for the impact of medication adherence on outcomes, the mean daily dose and measure of proportion of days covered (PDC) under ULT exposure (ULT prescribing in outpatient and inpatient settings are integrated) was employed, and selected PDC ≥ 80% was set as threshold to indicate patients who were highly adherent to chronic medication use33.
Statistical analysis
Continuous data are presented as mean±standard deviation or median (interquartile range, IQR, 25th–75th percentile), and categorical data as number and percentages. The means of the baseline characteristics were compared using unpaired or paired Student’s t-tests for continuous variables and chi-square tests for categorical variables. Differences in eGFR and SUA change between treatment groups were analyzed using unpaired t test at a 3-month interval during follow-up.
To take into account that repeated measurements (eGFR and SUA) of the same patient are correlated over time, a linear mixed model with random intercept and slope was used to estimate the longitudinal change in SUA and renal function between the febuxostat and allopurinol groups34. As the mixed model can accommodate missing data points encountered in longitudinal data without requirement of imputation of missing values34,35. To determine whether ULT is an independent prognostic factor for incident eGFR decline ≥30%, adjusted hazards ratio (aHR) was calculated using Cox proportional hazards model for the as-treated cohort. The baseline proportionality was assessed using the survival function with log-log plot in SAS package. Multivariate analyses were used to adjust for potential time-varying confounders such as presence of AKI, PDC, and mean daily doses during the follow-up time.
Lastly, to better understand the risk for CKD progression among patients underwent ULT, stratified analyses by baseline eGFR≥45 (vs < 45) mL/min/1.73 m2 were performed. Tests of significance for differences between groups were set at P < 0.05. Data processing and analyses of data were conducted using SAS Enterprise Guide version 5.1.
Compliance with ethics guidelines
The study was approved by the Institutional Review Boards (IRB) of the Chang Gung Medical Foundation at Taipei, Taiwan (201600110B0). All personal identifying information for patients was anonymous; therefore, informed consent was waived by the IRB of the Chang Gung Medical Foundation for the study. All methods were performed in accordance with the relevant guidelines and regulations of IRB of Chang Gung Medical Foundation.
Results
Patient characteristics
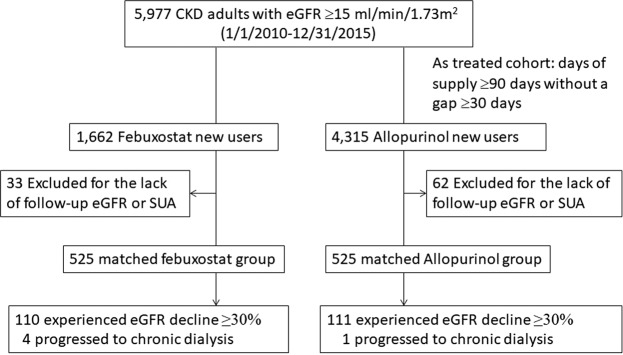
A total of 5628 new febuxostat and allopurinol users with CKD fulfilled the study’s inclusion criteria (Fig. 1). The patients’ characteristics between the febuxostat and allopurinol groups with and without matching are shown in Table 1. Compared with patients who initiated with allopurinol, those patients who initiated with febuxostat had higher eGFR <45 mL/min/1.73 m2 (71.4%); higher prevalence of proteinuria, diabetes, hypertension, and AKI; and more use of prior medications (Table 1). In the 1:1 propensity score-matched cohort, 1050 febuxostat and allopurinol matched pairs were analyzed over 2.5 years of follow-up [the mean follow-up time was 1.44 (0.67) years]. The baseline characteristics are well balanced in the matched groups and summarized in Table 1.
Figure 1.
Flowchart of patient selection.
Table 1.
Patient’s characteristics between febuxostat and allopurinol groups with and without matching.
| Characteristics | Without matching | Matched pairs2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 5628) | Allopurinol (n = 4056) | Febuxostat (n = 1572) | P value1 | Total (n = 1050) | Allopurinol (n = 525) | Febuxostat (n = 525) | P value1 | |
| Sex, n(%) | 0.1261 | 0.7713 | ||||||
| Female | 1346 (23.92%) | 992 (24.46%) | 354 (22.52%) | 248 (23.62%) | 126 (24%) | 122 (23.24%) | ||
| Male | 4282 (76.08%) | 3064 (75.54%) | 1218 (77.48%) | 802 (76.38%) | 399 (76%) | 403 (76.76%) | ||
| Age at ULT initiation | ||||||||
| mean(SD), years | 66.08 (13.83) | 66.04(13.93) | 66.19(13.56) | 0.7233 | 66.80 (14.25) | 66.10 (14.73) | 67.50 (13.73) | 0.1112 |
| Age group, n (%) | 0.3319 | 0.5274 | ||||||
| <50 | 702 (12.47%) | 514 (12.67%) | 188 (11.96%) | 137 (13.05%) | 76 (14.48%) | 61 (11.62%) | ||
| 50~60 | 985 (17.5%) | 719 (17.73%) | 266 (16.92%) | 151 (14.38%) | 77 (14.67%) | 74 (14.1%) | ||
| 60~70 | 1482 (26.33%) | 1040 (25.64%) | 442 (28.12%) | 273 (26%) | 140 (26.67%) | 133 (25.33%) | ||
| 70~80 | 1603 (28.48%) | 1172 (28.9%) | 431 (27.42%) | 299 (28.48%) | 141 (26.86%) | 158 (30.1%) | ||
| ≥80 | 856 (15.21%) | 611 (15.06%) | 245 (15.59%) | 190 (18.1%) | 91 (17.33%) | 99 (18.86%) | ||
| SUA, mean (SD) mg/dL | 8.75 (1.84) | 8.68(1.84) | 8.93(1.84) | <0.0001 | 8.92 (1.87) | 8.9(1.85) | 8.93(1.9) | 0.8201 |
| eGFR, mean (SD), ml/min/1.73m2 | ||||||||
| 45.16 (20.91) | 47.81(21.59) | 38.32(17.27) | <0.0001. | 41.11 (19.11) | 41.53(20.08) | 40.68(18.11) | 0.4741. | |
| eGFR group | <0.0001 | 0.8606 | ||||||
| 1 (≥90) | 180 (3.2%) | 154 (3.8%) | 26 (1.65%) | 31 (2.95%) | 18 (3.43%) | 13 (2.48%) | ||
| 2 (89-60) | 918 (16.31%) | 799 (19.7%) | 119 (7.57%) | 110 (10.48%) | 58 (11.05%) | 52 (9.9%) | ||
| 3a (59-45) | 1422 (25.27%) | 1117 (27.54%) | 305 (19.4%) | 230 (21.9%) | 114 (21.71%) | 116 (22.1%) | ||
| 3b (44-30) | 1679 (29.83%) | 1116 (27.51%) | 563 (35.81%) | 347 (33.05%) | 170 (32.38%) | 177 (33.71%) | ||
| 4 (29-15) | 1429 (25.39%) | 870 (21.45%) | 559 (35.56%) | 332 (31.62%) | 165 (31.43%) | 167 (31.81%) | ||
| Prior comorbid conditions | ||||||||
| CCI score group | 0.0581 | 0.9592 | ||||||
| 0 | 4470 (79.42%) | 3244 (79.98%) | 1226 (77.99%) | 804 (76.57%) | 401 (76.38%) | 403 (76.76%) | ||
| 1~3 | 576 (10.23%) | 417 (10.28%) | 159 (10.11%) | 117 (11.14%) | 58 (11.05%) | 59 (11.24%) | ||
| >=3 | 582 (10.34%) | 395 (9.74%) | 187 (11.9%) | 129 (12.29%) | 66 (12.57%) | 63 (12%) | ||
| Proteinuria | 1523 (27.06%) | 956 (23.57%) | 567 (36.07%) | <0001. | 318 (30.29%) | 158 (30.1%) | 160 (30.48%) | 0.8932. |
| Diabetes | 2254 (40.05%) | 1557 (38.39%) | 697 (44.34%) | <0001. | 457 (43.52%) | 227 (43.24%) | 230 (43.81%) | 0.8519 |
| Hypertension | 4376 (77.75%) | 3103 (76.5%) | 1273 (80.98%) | <0.001 | 842 (80.19%) | 421 (80.19%) | 421 (80.19%) | 1 |
| AKI | 746 (13.26%) | 457 (11.27%) | 289 (18.38%) | <0001 | 159 (15.14%) | 79 (15.05%) | 80 (15.24%) | 0.9314. |
| Prior medications | ||||||||
| RASI | 2562 (45.52%) | 1603 (39.52%) | 959 (61.01%) | <0001. | 552 (52.57%) | 275 (52.38%) | 277 (52.76%) | 0.9016 |
| Diuretics | 1507 (26.78%) | 985 (24.29%) | 522 (33.21%) | <0001. | 314 (29.9%) | 157 (29.9%) | 157 (29.9%) | 1 |
| LLT | 1698 (30.17%) | 1029 (25.37%) | 669 (42.56%) | <0001 | 375 (35.71%) | 192 (36.57%) | 183 (34.86%) | 0.5622 |
| Immunosuppressant | 32 (0.57%) | 22 (0.54%) | 10 (0.64%) | 0.6748. | 8 (0.76%) | 4 (0.76%) | 4 (0.76%) | 1 |
| NSAID | 319 (5.67%) | 188 (4.64%) | 131 (8.33%) | <0001. | 68 (6.48%) | 26 (4.95%) | 42 (8%) | 0.0448 |
P value indicates Student t tests or Chi-square tests between allopurinol and febuxostat groups. Matched ULT cohort was based on propensity score calculated based on baseline SUA and eGFR, age at ULT initiation, sex, prior medical history: individual disease conditions in the CCI algorithm, proteinuria, AKI, diabetes, hypertension, use of diuretics, RASI, LLT, immunosuppressant, NSAID. Abbreviations: ULT, urate-lowering therapy; eGFR, estimated glomerular filtration rate; CCI, Charlson comorbidity index; AKI, acute kidney injury; RASI, renin-angiotensin system inhibitors; LLT, lipid-lowering therapy; NSAID, non-steroidal anti-inflammatory drug.
SUA changes
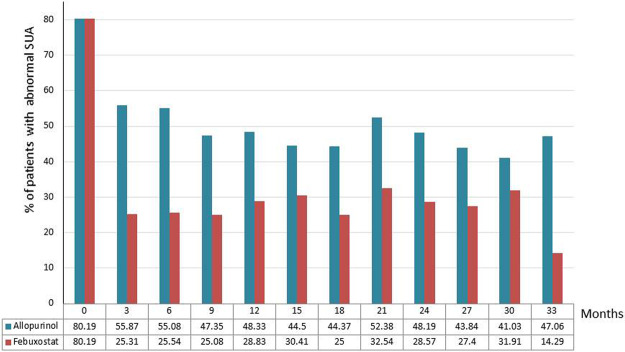
In the matched cohort, the proportion of febuxostat users achieving SUA level <6 mg/dL and sex-adjusted SUA goal were significantly higher than allopurinol users (Table 2), and the significance remained in the 3-month intervals over the study period (all P < 0.05 in Fig. 2). Patients treated with febuxostat had a greater decline in mean SUA level than those treated with allopurinol in the early phase of therapy (Supplementary Fig. S1).
Table 2.
Primary and second study outcomes.
| Allopurinol (n = 525) | Febuxostat (n = 525) | P value | |
|---|---|---|---|
| SUA changes | |||
| Δ SUA, mean(SD) | −1.48 (2.36) | −2.86 (2.59) | <0001 |
| Sex-adjusted SUA target, n (%) | 487 (92.76%) | 369 (70.29%) | <0001 |
| Sex-adjusted SUA targets maintenance ≥80% follow-up time, n(%) | 303 (57.71%) | 183 (34.86%) | <0001 |
| SUA target (<6 mg/dL), n (%) | 175 (33.33%) | 385 (73.33%) | <0001 |
| SUA targets (<6 mg/dL) maintenance ≥80% follow-up time, n(%) | 46 (8.76%) | 166 (31.62%) | <0001 |
| Renal function | |||
| Δ eGFR, mean(SD) | −0.74 (12.90) | −0.36 (10.31) | 0.6006 |
| eGFR decline ≥ 30% baseline | 111 (21.14%) | 110 (20.95%) | 0.9397 |
| Chronic dialysis ≥3 months | 1 (0.19%) | 4 (0.76%) | 0.1787 |
eGFR, estimated glomerular filtration rate; SUA, serum uric acid Δ. mean changes between the last 3-month measured level and the baseline measured level. SUA target level: <6 mg/dL; Sex-adjusted SUA target level: 7.7 mg/dL for male; 6.6 mg/dL for female patients.
Figure 2.
Patients wih abnormal mean SUA level during follow-up. Abnormal mean SUA level: ≥7.7 mg/dL (male) and ≥6.6 mg/dL (female).
Longitudinal analyses showed that febuxostat was associated with a greater mean reduction in SUA (mg/dL per 3 months) than allopurinol during follow-up (Table 3). In the adjusted model, the slope of SUA decline remained significantly greater in febuxostat than allopurinol. PDC ≥ 80% was significantly associated with a greater reduction in SUA level; meanwhile, the occurrence of AKI was associated with increased SUA level during follow-up (Table 3).
Table 3.
Factors associated longitudinal changes in SUA level.
| Coefficient estimate (β) | 95% CI | P value | ||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Unadjusted model | ||||
| Febuxostat vs Allopurinol | −0.9207 | −1.0592 | −0.7822 | <0.0001 |
| Adjusted model | ||||
| Febuxostat (vs Allopurinol) | −0.8699 | −1.0193 | −0.7206 | <0.0001 |
| PDC ≥80% (vs <80%) | −0.5423 | −0.7138 | −0.3708 | <0.0001 |
| Mean daily dose ≥50% (vs <50%) group mean | −0.0672 | −0.24 | 0.1056 | 0.446 |
| AKI occurrence during follow-up | 0.4738 | 0.2676 | 0.6801 | <0.0001 |
PDC, proportion of days covered; ≥50% mean daily doses: febuxostat: ≥40 mg/day; allopurinol: ≥100 mg/day.
Renal outcomes
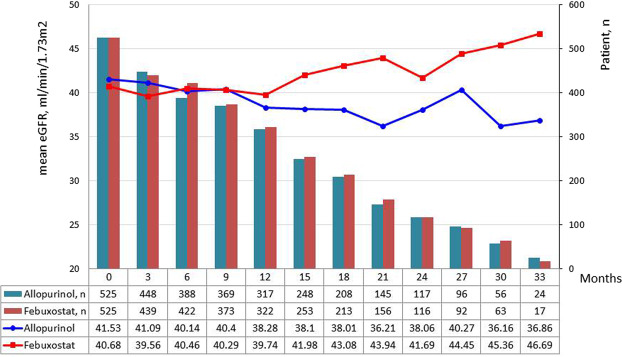
AKI development was higher in allopurinol group than febuxostat group (19.05% and 12.76%, respectively; P = 0.0054) during the study follow-up (Table 4). The mean eGFR at the end of follow-up with a 3-month interval was higher in the febuxostat group [46.69 (34.42) mL/min/1.73 m2] than that in the allopurinol group [36.86 (19.57) mL/min/1.73 m2] in Fig. 3, the mean changes in eGFR (mL/min/1.73 m2 per 3 months) between the two groups showed no significant difference over time (Table 5). In the adjusted model, febuxostat was not associated with mean eGFR changes. High mean group daily dose significantly improved eGFR, but PDC ≥ 80% and AKI were associated with worsening eGFR during follow-up (Table 5).
Table 4.
Occurrences of proteinuria and acute kidney injury during follow-up and concomitant medications uses.
| All (n = 1050) | Allopurinol (n = 525) | Febuxostat (n = 525) | P value | |
|---|---|---|---|---|
| Proteinuria during follow-up | 373 (35.52%) | 202 (38.48%) | 171 (32.57%) | 0.0456 |
| AKI during follow-up | 167 (15.9%) | 100 (19.05%) | 67 (12.76%) | 0.0054 |
| Hypertension | <0001 | |||
| persistent hypertension | 634 (60.38%) | 294 (56%) | 340 (64.76%) | |
| none | 147 (14%) | 63 (12%) | 84 (16%) | |
| baseline or follow-up | 269 (25.62%) | 168 (32%) | 101 (19.24%) | |
| RASI use | 0.0012 | |||
| persistent use | 471 (44.86%) | 229 (43.62%) | 242 (46.1%) | |
| no use | 306 (29.14%) | 135 (25.71%) | 171 (32.57%) | |
| baseline or follow-up | 273 (26%) | 161 (30.67%) | 112 (21.33%) | |
| LLT use | 0.1106 | |||
| persistent use | 331 (31.52%) | 168 (32%) | 163 (31.05%) | |
| no use | 534 (50.86%) | 253 (48.19%) | 281 (53.52%) | |
| baseline or follow-up | 185 (17.62%) | 104 (19.81%) | 81 (15.43%) | |
| Diuretics use | 0.0057 | |||
| persistent use | 251 (23.9%) | 128 (24.38%) | 123 (23.43%) | |
| no use | 597 (56.86%) | 277 (52.76%) | 320 (60.95%) | |
| baseline or follow-up | 251 (23.9%) | 128 (24.38%) | 123 (23.43%) | |
| Immunosuppressant use | 1 | |||
| persistent use | 6 (0.57%) | 3 (0.57%) | 3 (0.57%) | |
| no use | 1038 (98.86%) | 519 (98.86%) | 519 (98.86%) | |
| baseline or follow-up | 6 (0.57%) | 3 (0.57%) | 3 (0.57%) | |
| NSAIDs use | 0.006 | |||
| persistent use | 34 (3.24%) | 8 (1.52%) | 26 (5.0%) | |
| no use | 929 (88.48%) | 470 (89.5%) | 459 (87.43%) | |
| baseline or follow-up | 87 (8.29%) | 47 (7.0%) | 40 (7.62%) |
AKI, acute kidney injury; RASI, renin-angiotensin system inhibitors; LLT, lipid-lowering therapy.
Figure 3.
Changes in mean eGFR between febuxostat and allopurinol groups during follow-up.
Table 5.
Factors associated with longitudinal changes in eGFR.
| Coefficient estimate (β) | 95% CI | P value | ||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Unadjusted model | ||||
| Febuxostat vs Allopurinol | −0.6652 | −2.2895 | 0.9592 | 0.4221 |
| Adjusted model | ||||
| Febuxostat (vs Allopurinol) | −1.2869 | −2.8942 | 0.3204 | 0.1166 |
| PDC ≥ 80% (vs < 80%) | −5.1346 | −6.9697 | −3.2994 | <0.0001 |
| Mean daily dose ≥50% (vs <50%) group mean | 3.4017 | 1.5197 | 5.2838 | <0.001 |
| AKI occurrence during follow-up | −8.9936 | −11.192 | −6.7949 | <0.0001 |
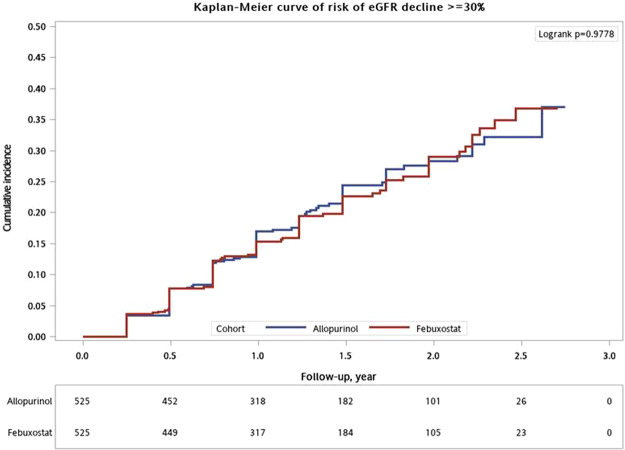
The incidence of eGFR decline ≥30% of baseline was 21% (n = 221) and 0.48% (n = 5) of the study cohort who progressed to chronic dialysis (no case of kidney transplantation) (Table 2). The cumulative probability of eGFR decline ≥30% during follow-up was not significantly different between groups (logrank test, P > 0.05) (Fig. 4). When adjusted for baseline patient characteristics and potential time-varying confounders, febuxostat appeared to have a higher risk of eGFR decline ≥30% during the entire follow-up time compared with allopurinol, but the power did not achieve statistical difference (Table 6). Baseline eGFR <30 mL/min/1.73 m2 and the occurrence of AKI were strongly associated with renal function decline; proteinuria and persistent use of diuretics were weakly associated with risk of renal function decline (Table 6).
Figure 4.
Cumulative incidence of eGFR decline ≥30% between febuxostat and allopurinol groups.
Table 6.
Factors associated with eGFR reduction >30%.
| aHR | 95% CI | P value | ||
|---|---|---|---|---|
| Febuxostat vs allopurinol | 1.294 | 0.984 | 1.7 | 0.0648 |
| Age at index date | 0.992 | 0.982 | 1.003 | 0.15 |
| Male (vs female) | 0.68 | 0.511 | 0.905 | 0.0082 |
| Baseline SUA ≥ 9 (vs < 9) mg/dL | 0.825 | 0.621 | 1.096 | 0.1845 |
| Baseline eGFR group, ml/min/1.73m2 | ||||
| ≥59 | 1 | |||
| 60-45 | 0.964 | 0.513 | 1.812 | 0.9087 |
| 44-30 | 1.598 | 0.906 | 2.818 | 0.1056 |
| <30 | 2.469 | 1.408 | 4.329 | 0.0016 |
| CCI score | 1 | 0.92 | 1.088 | 0.9914 |
| AKI occurrence during follow-up | 3.194 | 2.351 | 4.34 | <0.0001 |
| Proteinuria occurrence during follow-up | 1.517 | 1.147 | 2.006 | 0.0035 |
| Persistent hypertension | 1.557 | 0.851 | 2.85 | 0.1506 |
| Persistent use of RASI | 1.015 | 0.736 | 1.399 | 0.93 |
| Persistent use of diuretics | 1.409 | 1.028 | 1.931 | 0.0332 |
| Persistent use of NSAID | 0.85 | 0.517 | 1.398 | 0.5211 |
| PDC ≥80% (vs <80%) | 0.777 | 0.585 | 1.032 | 0.081 |
| Mean daily dose ≥50% (vs <50%) group mean | 0.912 | 0.683 | 1.218 | 0.5346 |
Use of concomitant medications (Table 3). CCI, Charlson comorbid index; AKI, acute kidney injury; RASI, renin-angiotensin system inhibitors; NSAIDs,Non-Steroidal Anti-Inflammatory Drug; PDC, proportion of days covered; Unadjusted HR: 0.996 (95% CI, 0.765, 1.297, P = 0.9781); Stratified analysis by baseline eGFR <45 and ≥45 mL/min/1.73m2 (Supplementary Table S3).
When restricted to a group of patients with baseline eGFR ≥45 mL/min/1.73 m2, new users of febuxostat had a higher hazard of eGFR progression than new users of allopurinol (aHR, 1.6; 95% CI, 0.83–3.083); a higher hazard, but not statistically significant, was also found in patients with eGFR <45 mL/min/1.73 m2 (aHR, 1.254; 95% CI, 0.927–1.695) after adjustment for multiple risk factors (Table S3).
Discussion
This study is one of few that conducted head-to-head comparisons of febuxostat and allopurinol for SUA reduction and renal outcomes among patients with CKD. The present study supports that febuxostat was associated with superior effects on SUA reduction in CKD patients during the follow-up (−0.9207 mg/dL per 3-month interval). Febuxostat appeared to have no difference with allopurinol on eGFR changes or risk of developing eGFR decline ≥30% during the study period. The study results also suggest that the presence of AKI following the ULT therapy was associated with an increase in SUA level and a risk of CKD progression.
Hyperuricemia per se is not an indication for specific ULT. Evidence to date derived from two randomized controlled trials with 28 weeks use of ULT show that febuxostat was more effective in achieving SUA target (<6 mg/dL) than allopurinol in patients with CKD for gout14,20. Febuxostat has been rapidly adopted (increased 22.9% of users in 3 years) since 2013 in the practice setting as a major alternative to allopurinol36. The present CKD cohort study comparing febuxostat to allopurinol as treatment (≥3 months) with over 2.5 years of follow-up supports that febuxostat new users lead to a more rapid reduction in SUA level and likely sustain targeted SUA treatment goal over time than allopurinol new users.
Taiwan’s Food and Drug Administration approved usual dosage for gout and hyperuricemia is 40–80 mg/day of febuxostat and 100–300 mg/day of allopurinol. The initial dose of ULT for patients with renal insufficiency usually is 50% usual dosage and can be titrated to maintain the SUA target goal. As lowering of uric acid is dose-dependent, it is worth to note that the initial doses and exposure length of ULT may be attributable to different results between clinical trial and practice setting. We recognize that the mean doses during study follow-up time in both febuxostat (46.31 ± 16.9 mg/day) and allopurinol (105.26 ± 44.78 mg/day) groups (Supplementary Table S2) are lower than those of clinical guideline. The higher mean dose effect revealed a decrease in SUA level, but it was not statistically different in eGFR decline between febuxostat and allopurinol treatments. The SUA reduction effects of febuxostat and allopurinol are considered conservative in this CKD cohort compared with that of trial settings. The dosage of ULT in the present study was considered similar to the FREED trial. There were 67.4% of patients received 40 mg in the febuxostat group (32.7 ± 11.3 mg/day during 36-month follow-up time) and 27.2% of patients received 100 mg allopurinol, the changes in SUA was significantly higher in the febuxostat group than in allopurinol, whereas no difference in eGFR changes between comparison groups22.
Current evidence suggested that the cause of kidney function progression in patients with hyperuricemia is multifactorial. Most studies evaluating the effects of urate lowering on renal outcomes have been in non-gout populations with varying severity of baseline renal function, and most of these studies were compared to placebo16–18,37. For instance, in CKD stage 3–4, randomized, placebo-controlled trials have showed either significantly increased eGFR of 3.3 ± 1.2 mL/min/1.73 m2 due to allopurinol during a 12-month of follow-up37 or that febuxostat was not significantly associated with eGFR increase (from 31.5 ± 13.6 to 34.7 ± 18.1 mL/min/1.73 m2, P = 0.3) over 6 months of therapy17. It is worth noting that a recent meta-analysis of nine randomized placebo-controlled trials (2,141 patients) showed a higher eGFR at 6 months follow-up (weighted mean difference [WMD], 2.69 ml/min/1.73m2; 95%CI, 1.52–3.87) in febuxostat than placebo in patients with CKD (eGFR < 60 ml/min/1.73m2)38.
Head-to-heard comparison of XOIs in the FREED study, the mean change in eGFR from baseline per year revealed no significant differences between febuxostat and allopurinol groups [−0.37 (−2.32 to 1.44) vs. −0.69 (−2.63 to 1.39) mL/min/1.73m2, P = 0.606] over 36-month follow-up22. A review of observational studies also showed febuxostat had no difference in yearly eGFR change (WMD, 0.01 ml/min/1.73m2/year) comparing with allopurinol in a small group of kidney transplant patients (n = 79) with asymptomatic hyperuricemia39. The present study supported these head-to-head comparative studies results by assessed the mean changes in eGFR over time and showed no difference between febuxostat and allopurinol in CKD patients.
The role of uric acid reduction on CKD progression could be complicated by CKD comorbid diseases, which might have potential associations of uric acid with established hypertension, dyslipidemia, insulin resistance, proteinuria, and cardiovascular disease22,40–42. For instance, the dose-dependent association between SUA level and increased prevalence of CKD was demonstrated in Thai patients with hypertension42. Another important finding in the study is that ULT effects on renal function changes may be mediated by the presence of AKI during follow-up. In the adjusted models, patients with AKI were associated with increased SUA level and increased risk of eGFR decline ≥30%. AKI is a well-recognized risk of CKD progression. Hyperuricemia, defined as >6.5 mg/dL in women and >7 mg/dL in men, has also been recognized as an independent predictor for AKI28. Elevated SUA (>9.4 mg/dL) associated with an increased risk for AKI development within 7 days of hospital admission as well as a need for dialysis were demonstrated in a single inpatient setting43. AKI is prevalent in patients with CKD in the present study (15.9%) and patients with SUA > 9.4 mg/dL at hospital admission (36.7%)43. Although the causes of AKI and its association with ULT are beyond the scope of the current study, these results shed light to further investigations between the associations of lowering SUA, AKI development, and renal progression in CKD population.
Other comorbid conditions proteinuria occurrence during study follow-up and persistent use of diuretics, indicating worsened renal function, were associated with additional risk of eGFR decline ≥30%. Because the use of gout diagnosis codes is likely limited in electronic health records to confirm urate crystal information, the present study was unable to assess the interaction association between ULT and gout presence on renal outcomes.
The present study applied new user design with propensity score matching technique in a newly-diagnosed CKD cohort offered an opportunity to minimize biased estimate of comparative effects on SUA reduction and renal function progression between febuxostat and allopurinol treatments. However, there are limitations in the present study. First, as with any other observational studies, residual confounders, such as body mass index, dietary intake, obesity, and healthy life style might have biased the study results. These residual confounders are not likely going to change the observed difference between febuxostat and allopurinol due to imbalanced residual confounders in the CKD population. In addition, more than one SUA reference ranges were applied to ensure the robustness of the treatment effect in the present study. Limitations of the present study include the relatively short follow-up period for detecting differences between comparison groups in renal function changes. Lastly, the study results may be not generalizable for the entire CKD patient populations because sampling and ULT prescribing patterns were performed in a healthcare delivery system in Taiwan. For instance, allopurinol use has substantially declined due to the concern of allopurinol-related severe cutaneous adverse reaction, and a gradual increase in overall ULT use due to allopurinol switching and introduction of febuxostat in the study setting36.
Conclusion
These results suggest that febuxostat was superior to allopurinol on sustained reduction in SUA in patients with CKD, but patients who received either febuxostat or allopurinol have no difference in renal function changes in a routine clinical setting. Closely monitor serum creatinine and uric acid for patients with XOI therapy is equally important to early identify AKI and prevent renal function deterioration. Further long-term follow up studies are needed to assess the difference in renal outcomes between febuxostat and allopurinol in CKD patients with hyperuricemia to determine cost-effective practice.
Supplementary information
Acknowledgements
We thank Ms. Pei-Chi Lu for setting up the dataset for analysis and helping with presentation of results in the tables and figures. We are grateful to Mrs. Ying-Jen Hsu and Jiun-Hua Liao in the Department of Management of Information Systems for electronic medical records acquisition. We are also grateful to Ms. Dai-Hua Liu in the Medical Record Services for disease coding assistance, and Ms. Tsai-Ling Chao and Ms. Yu-Shao Chiang in the Division of Laboratory Medicine for help with laboratory results validation in Kaohsiung Chang Gung Memorial Hospital. This study not supported by any external funding.
Author contributions
Concept and design: Peng, Hsu. Acquisition, analysis of data: Peng, Hsu. Validation and Interpretation of data: All authors. Drafting of the manuscript: Peng, Tain, Hsu. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Peng, Yang, Hsu.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67026-1.
References
- 1.Li L, et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iseki K, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642–50. doi: 10.1016/S0272-6386(04)00934-5. [DOI] [PubMed] [Google Scholar]
- 3.Chonchol M, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50(2):239–47. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283(18):2404–10. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 6.Madero M, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53(5):796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna D, et al. American College of R. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken) 2012;64(10):1447–61.. doi: 10.1002/acr.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan KM, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford) 2007;46(8):1372–4. doi: 10.1093/rheumatology/kem056a. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, et al. Therapeutics ESCfICSI. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65(10):1312–24. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51(3):321–5. doi: 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- 11.Khanna PP, Perez-Ruiz F, Maranian P, Khanna D. Long-term therapy for chronic gout results in clinically important improvements in the health-related quality of life: short form-36 is responsive to change in chronic gout. Rheumatology (Oxford) 2011;50(4):740–5. doi: 10.1093/rheumatology/keq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halpern R, Fuldeore MJ, Mody RR, Patel PA, Mikuls TR. The effect of serum urate on gout flares and their associated costs: an administrative claims analysis. J Clin Rheumatol. 2009;15(1):3–7. doi: 10.1097/RHU.0b013e3181945d2c. [DOI] [PubMed] [Google Scholar]
- 13.Ke CH, et al. Cost-effectiveness Analysis for Genotyping before Allopurinol Treatment to Prevent Severe Cutaneous Adverse Drug Reactions. J Rheumatol. 2017;44(6):835–843. doi: 10.3899/jrheum.151476. [DOI] [PubMed] [Google Scholar]
- 14.Becker MA, et al. Febuxostat Compared with Allopurinol in Patients with Hyperuricemia and Gout. N Engl J Med. 2005;353(23):2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 15.Singh JA, Akhras KS, Shiozawa A. Comparative effectiveness of urate lowering with febuxostat versus allopurinol in gout: analyses from large U.S. managed care cohort. Arthritis Res Ther. 2015;17:120. doi: 10.1186/s13075-015-0624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–9. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Sircar D, et al. Efficacy of Febuxostat for Slowing the GFR Decline in Patients With CKD and Asymptomatic Hyperuricemia: A 6-Month, Double-Blind, Randomized, Placebo-Controlled Trial. Am J Kidney Dis. 2015;66(6):945–50. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, et al. Febuxostat Therapy for Patients With Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am J Kidney Dis. 2018;72(6):798–810. doi: 10.1053/j.ajkd.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Sezai A, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU-FLASH trial for CKD) J Cardiol. 2015;66(4):298–303. doi: 10.1016/j.jjcc.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, et al. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: a parallel-group, randomized, controlled trial. Clin Exp Nephrol. 2015;19(6):1044–53. doi: 10.1007/s10157-015-1095-1. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, et al. An allopurinol‐controlled, multicenter, randomized, double‐blind, parallel between‐group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. International Journal of Rheumatic Diseases. 2014;17(6):679–686. doi: 10.1111/1756-185X.12266. [DOI] [PubMed] [Google Scholar]
- 22.Kojima, S. et al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur Heart J. (2019). [DOI] [PMC free article] [PubMed]
- 23.Chang Gung Memorial Hospital (CGMH). About CGMH: medical service overview: Chang Gung Medical Foundation, https://www.cgmh.org.tw/cgmh/about/about_04.htm (2015).
- 24.Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood) 2003;22((3)):61–76. doi: 10.1377/hlthaff.22.3.61. [DOI] [PubMed] [Google Scholar]
- 25.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 26.Chuang S-Y, Lee S-C, Hsieh Y-T, Pan W-H. Trends in hyperuricemia and gout prevalence: Nutrition and Health Survey in Taiwan from 1993-1996 to 2005-2008. Asia Pacific journal of clinical nutrition. 2011;20(2):301. [PubMed] [Google Scholar]
- 27.Lee M-S, et al. High prevalence of hyperuricemia in elderly Taiwanese. Asia Pac J Clin Nutr. 2005;14(3):285–292. [PubMed] [Google Scholar]
- 28.Ejaz AA, et al. Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol. 2007;2(1):16–21. doi: 10.2215/CJN.00350106. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42(1):125–32. doi: 10.1016/S0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 31.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl. 2, 1–138 (2012).
- 32.Parsons, L. S. Performing a 1:N Case-Control Match on Propensity Score. 165–29.
- 33.Benner JS, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 34.Shou, H. et al. Analytic Considerations for Repeated Measures of eGFR in Cohort Studies of CKD. Clin J Am Soc Nephrol. 12(8) (2017). [DOI] [PMC free article] [PubMed]
- 35.Twisk J, de Boer M, de Vente W Heymans MJJoce. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. 66(9), 1022–1028 (2013). [DOI] [PubMed]
- 36.Peng, Y. L. et al. The impact of adoption of a new urate-lowering agent on trends in utilization and cost in practice. PloS one. 14(8) (2019). [DOI] [PMC free article] [PubMed]
- 37.Sezer S, Karakan S, Atesagaoglu B, Acar FN. Allopurinol reduces cardiovascular risks and improves renal function in pre-dialysis chronic kidney disease patients with hyperuricemia. Saudi J Kidney Dis Transpl. 2014;25(2):316–20. doi: 10.4103/1319-2442.128520. [DOI] [PubMed] [Google Scholar]
- 38.Chewcharat, A. et al. Febuxostat as a Renoprotective Agent for Treatment of Hyperuricemia: A Meta‐analysis of Randomized Controlled Trials. Internal Medicine Journal (2020). [DOI] [PubMed]
- 39.Chewcharat, A. et al. Efficacy and safety of Febuxostat for Treatment of Asymptomatic Hyperuricemia among Kidney Transplant Patients: A Meta‐analysis of Observational Studies. Clinical Transplantation (2020). [DOI] [PubMed]
- 40.Puig JG, Ruilope LM. Uric acid as a cardiovascular risk factor in arterial hypertension. J Hypertens. 1999;17(7):869–72. doi: 10.1097/00004872-199917070-00001. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Sparrow D, Vokonas PS, Landsberg L, Weiss ST. Uric acid and coronary heart disease risk: evidence for a role of uric acid in the obesity-insulin resistance syndrome. The Normative Aging Study. Am J Epidemiol. 1995;142(3):288–94. doi: 10.1093/oxfordjournals.aje.a117634. [DOI] [PubMed] [Google Scholar]
- 42.Kaewput W, et al. Association between serum uric acid and chronic kidney disease in patients with hypertension: A multicenter nationwide cross-sectional study. J Evid Based Med. 2019;12(4):235–242. doi: 10.1111/jebm.12364. [DOI] [PubMed] [Google Scholar]
- 43.Cheungpasitporn W, Thongprayoon C, Harrison AM, Erickson SB. Admission hyperuricemia increases the risk of acute kidney injury in hospitalized patients. Clinical kidney journal. 2016;9(1):51–56. doi: 10.1093/ckj/sfv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.