Abstract
Various failure mechanisms have been identified in total knee arthroplasty (TKA). We hereby present one case of failure, which stands out because of its rapid and destructive progression. We report the case of a 72-year-old Caucasian female patient who developed a large bone osteolytic lesion of the femur after TKA. The patient presented to our hospital 7 years after the initial surgery, complaining of persistent knee pain. The lesion affected the distal half of the femur and, after a diagnostic workup, required a resection of 20 cm and reconstruction with a tumor prosthesis. Subsequent pathological analysis revealed a reaction to cement and prosthesis components. Periprosthetic osteolysis continues to be a major problem, and a reaction to cement and prosthesis components can be an elusive cause of TKA failure.
Keywords: Total knee arthroplasty, Osteolytic femoral lesion, Limb preservation prosthesis
Introduction
The leading causes of total knee arthroplasty (TKA) failure are aseptic loosening of components, infection, instability, and polyethylene wear [1]. Periprosthetic osteolysis results from wear debris, which leads to activation of macrophages and a foreign body tissue reaction [1]. This reaction results in an increased osteoclasts’ activity, with resorption of bone matrix and/or soft-tissue reactions [1]. Although this process is well documented in the literature, some authors report extensive cyst-like bone inflammatory reactions that stand out by their location or size, sometimes mimicking tumor lesions [[2], [3], [4], [5], [6]]. On the other hand, culture-negative cases of periprosthetic joint infection can be difficult to diagnose and go unnoticed [7]. Therefore, extensive bone osteolytic lesions pose diagnostic difficulties and, owing to the concomitant risk of fracture, require careful clinical evaluation and treatment. We hereby present a patient who developed a large bone osteolytic lesion of the distal femur, which needed a revision TKA with a tumor reconstruction prosthesis.
Case history
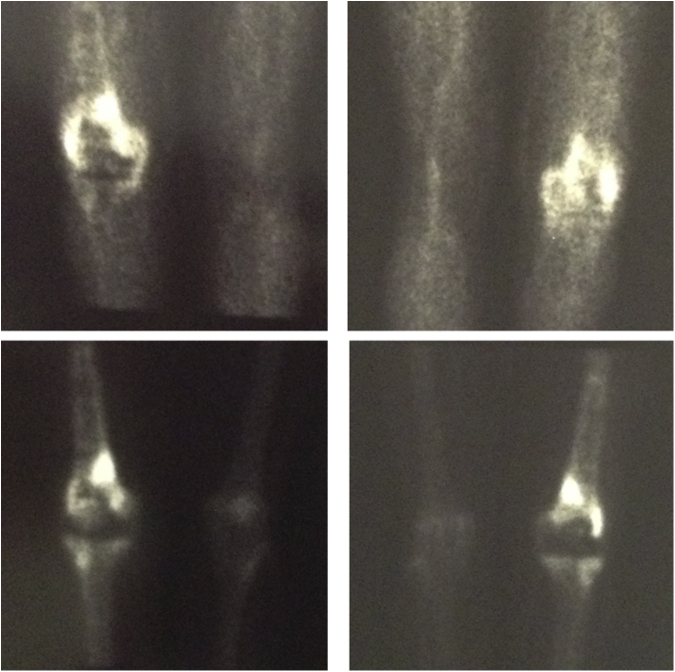
A 72-year-old Caucasian female, who consented to participate in this case report, suffered from osteoarthritis of her right knee joint and underwent joint replacement surgery in another institution (September 2005). A cemented total knee prosthesis (Performance, Biomet®, Warsaw, IN) was placed. According to the patient, the initial postoperative course was uneventful. However, she reports knee edema since the surgery, and therefore, 6 months after, a revision surgery was performed because of suspected infection that was apparently ruled out. Meanwhile, she developed a lesion in the distal femur and was observed by an orthopaedic tumor specialist. A workup was performed with magnetic resonance imaging (MRI) and a 99mTc scintigraphy. The MRI showed no invasive characteristics of the lesion. The scintigraphy showed hypervascularity in the femur’s distal third and pharmacologic heterogenic hyperfixation, suggesting a lytic lesion and inflammatory activity in the same area (Fig. 1). A biopsy of the lesion was subsequently carried out, which identified a benign reactional lesion, thus excluding a malignant lesion. No further clinical or imagiologic data were available.
Figure 1.
Five-year postoperative scintigraphy. Five-year postoperative anterior and posterior images of a 99mTc scintigraphy showing pharmacologic heterogenic hyperfixation mainly in the right distal femur. Some hyperfixation is also seen extending to the femoral diaphysis and proximal tibia (early-phase scan above and late-phase scan below).
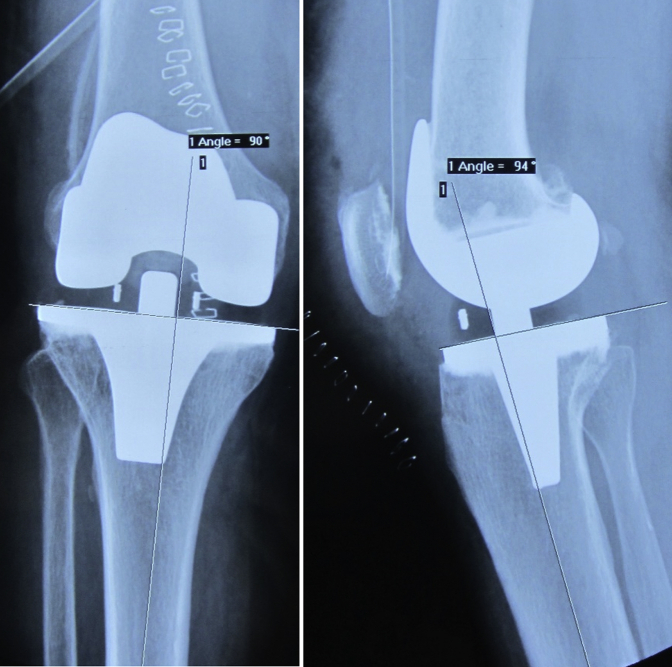
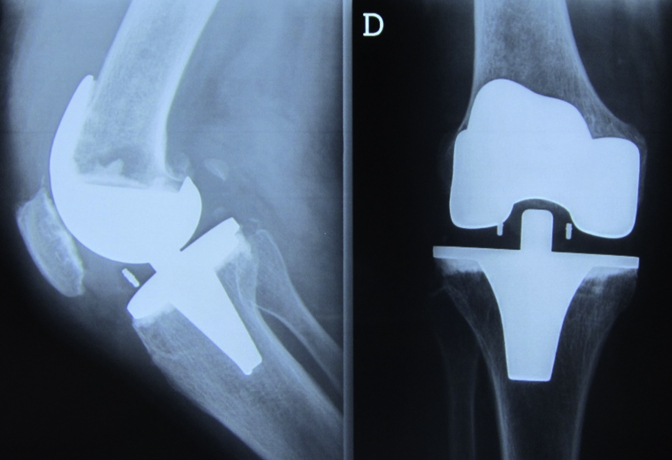
The patient presented to our hospital 7 years after because of persistent knee pain. There was no history of trauma or constitutional symptoms. She was submitted to a thyroidectomy in 2012 because of a benign multinodular goiter. On physical examination, the knees were aligned, and she had a moderate knee effusion on the right, without tension or other inflammatory signs. The knee had an arc of mobility of 125° with full extension, and it was slightly unstable to varus-valgus stress. Review of previous radiographs showed evidence of an intramedullary lytic lesion, 7 months after the initial surgery, progressing to affect the distal femur in just 3 years (Figure 2, Figure 3, Figure 4). When she came to our hospital, almost half of the femur was affected. The lytic lesion progressed to the femoral diaphysis with cortical thinning and no significant reactive sclerosis (Fig. 5). At this point, after the initial appointment, we deemed important to exclude an infection and a hypersensitivity reaction to the prosthesis material or bone cement. The blood workup showed no leukocytosis, a C-reactive protein of 5 mg/dL, and an erythrocyte sedimentation rate of 67 mm/h. Joint liquid (1) and blood culture (2) studies were negative (joint liquid cytochemical analysis unavailable). A hypersensitivity reaction to metals and polymethylmethacrylate (PMMA) was evaluated by a dermatologic specialist, with cutaneous patch tests, which were negative.
Figure 2.
Immediate postoperative knee radiographs. Anteroposterior and lateral immediate postoperative knee radiographs showing the tibial component in an apparently slight varus position.
Figure 3.
Seven-month postoperative radiographs. Seven-month postoperative anteroposterior and lateral radiographs showing a lytic lesion behind the femur implant, adjacent to the central part.
Figure 4.
Three-year postoperative radiographs. Three-year postoperative anteroposterior and lateral radiographs showing progression of the lesion and femoral notching. Apparent osteolysis adjacent to the anterolateral part of the tibial component is also seen.
Figure 5.
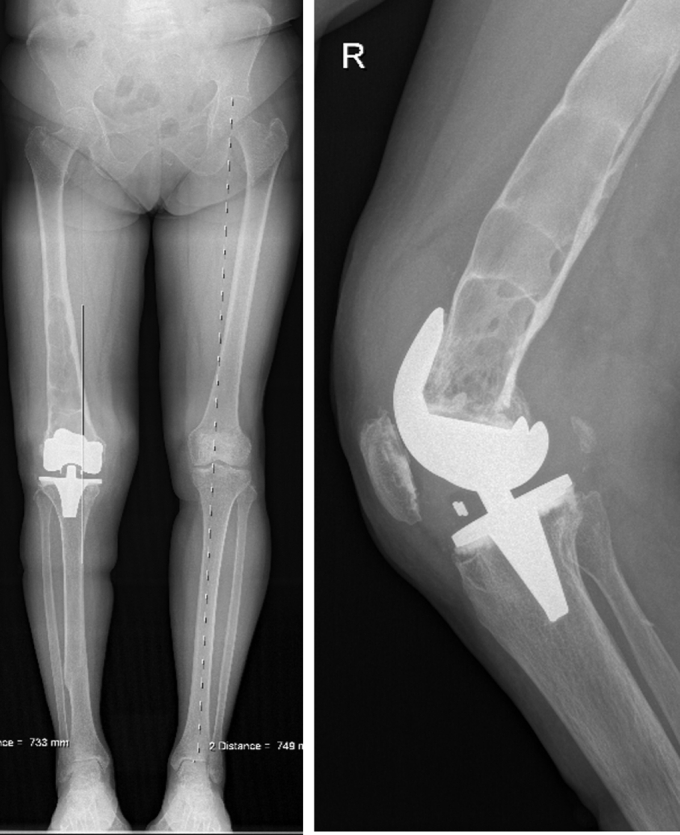
Seven-years postoperative radiographs. Seven-year postoperative long-axis and lateral knee radiographs showing progression of the previous lesions.
The patient was submitted to surgery with a working differential diagnosis of prosthetic joint infection vs a pseudotumor lesion secondary to PMMA cement or ultra-high-molecular-weight polyethylene (UHMWPE) wear debris. The synovium was unremarkable, and there was no macroscopic evidence of debris. The femoral component was loose, whereas the tibial and patellar components were stable. The UHMWPE liner was macroscopically grossly preserved. Synovial, femoral canal, and interface membrane tissue (which resembled a brownish cystic membrane) were collected, and an intraoperative extemporaneous analysis was undertaken. That showed chronic active inflammation rich in plasmocytes around necrosis, and therefore, infection could not be ruled out with certainty (Fig. 6). We proceeded with removal of the prosthesis and placement of a spacer. Further analysis of collected tissue showed osteolysis, chronic synovitis, and a foreign-body reaction. This reaction was located around spaces where deposits of a black material and vacuoles (probably due to cement) could be seen (Fig. 6). Surgical specimens were cultured for 2 weeks, as well as new blood samples, which were all negative. Sonification of the explanted prosthesis was unavailable. The patient completed an antibiotic course with flucloxacillin.
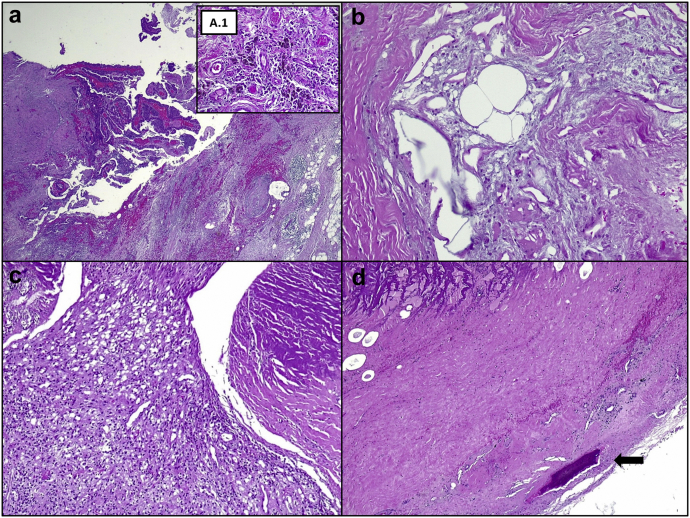
Figure 6.
Histological analysis of surgical specimens. Synovial and macrophagic reaction (a) to the metal prosthesis material (A.1 – dark pigment). Vacuoles left by partially dissolved cement material and squeezed multinucleated giant cells (b). Xanthomatosis-like reaction (c). The reabsorbed bone (arrow), organized fibrous tissue, and vacuoles left by partially dissolved cement (d). Hematoxylin and eosin 10×, 20×, 40×, 40×, and 10×.
Three weeks later, the patient underwent revision surgery, with removal of the spacer and distal resection of the femur, 20 cm above the joint line (Figure 7, Figure 8). A revision with a tumor reconstruction prosthesis was performed (type LPS, DePuy Synthes, West Chester, PA). The pathological analysis of the femur yielded no further information.
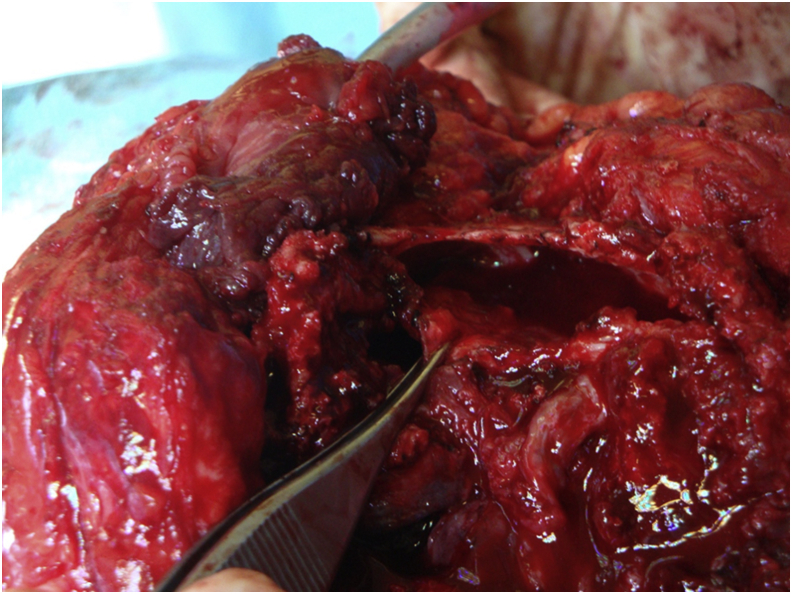
Figure 7.
Femur’s intraosseous cystic lesion. An intraoperative photograph of the femur’s intraosseous cystic lesion.
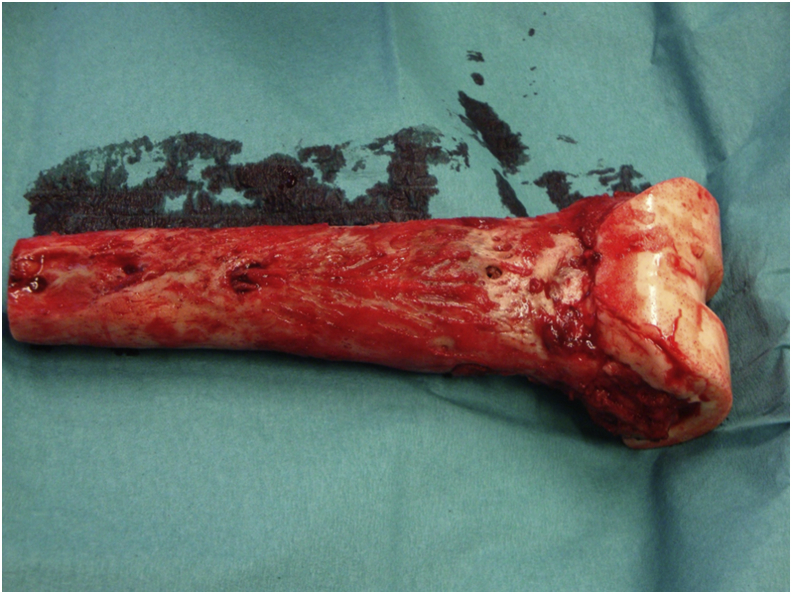
Figure 8.
Resected femur. An intraoperative photograph of the resected femur with a spacer in place.
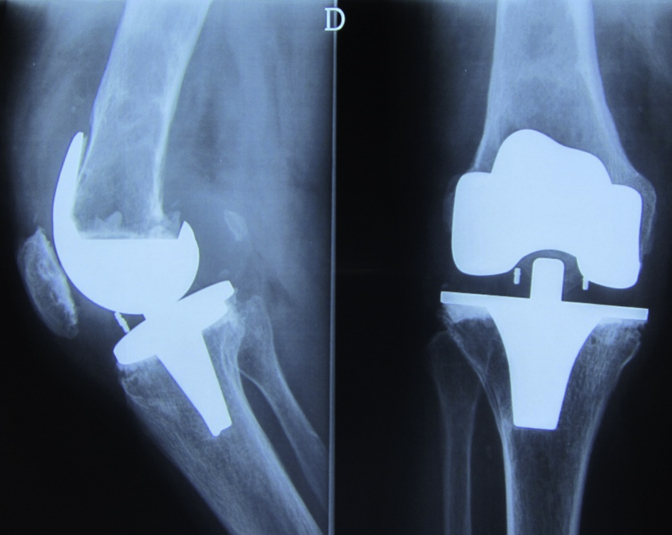
Six years after surgery, the patient is well, satisfied with the surgery, with a mobility arc of 120°, without any need of walking aid (Fig. 9).
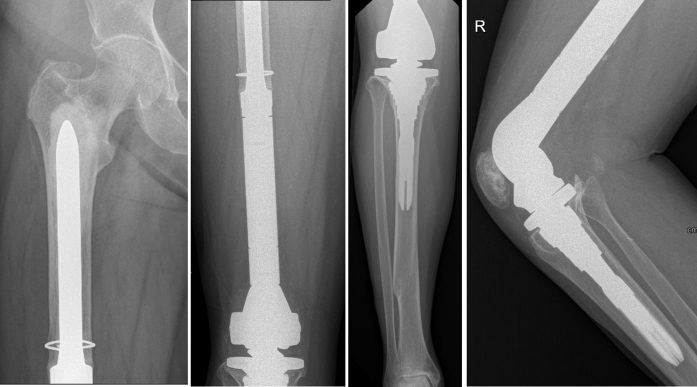
Figure 9.
Four-year postreconstruction radiographs. Anteroposterior and lateral radiographs 4 y after reconstruction.
Discussion
Osteolytic pseudotumor lesions occur after TKA because of generation of wear debris that trigger an osteoclastic process, driven by activated macrophages [1]. Although there have been extensive reports of osteolytic pseudotumor lesions around the knee, there is no report, to our knowledge, of such a rapid and exuberant expansible lesion. The importance of the current report lies in the recognition that such osteolytic processes can happen fast, compromising the stability of the arthroplasty and causing impending fractures.
Having in mind the presented clinical data, the initial differential diagnosis of this lesion was as follows: foreign-body inflammation and osteolytic pseudotumor lesion secondary to PMMA cement, UHMWPE wear debris, or prosthesis components; TKA infection with consequent osteomyelitis; benign neoplasia and both primary and metastatic malignancy.
The differential diagnosis of malignant lesions is long, but history, tumor characteristics, location, and radiographic features can frequently lead to a diagnosis (as is the case with giant cell tumor, which can be diagnosed and differentiated from the shown pseudotumor with MRI). The patient had a history of a thyroid hyperplasia but with no evidence of malignancy. The previous workup performed by an orthopaedic tumor specialist, as detailed previously (imaging and histology), deemed this diagnosis unlikely [8].
Infection had to be carefully ruled out, as it can masquerade foreign-body reaction and neoplasia [9]. Specifically, subacute osteomyelitis can present insidiously with pain, with or without swelling, and laboratory markers of inflammation can be normal. This led us to perform an intraoperative extemporaneous analysis of collected tissue and to delay definitive surgery, as infection could not be ruled out in a timely manner. We found this entity unlikely as several blood cultures (drawn at different timings) and surgical specimens’ cultures were negative. Pathological analysis also revealed a predominance of chronic inflammation, which does not support infection [7,10]. At that time, we decided to perform the second surgery 3 weeks after the first. Another option would be to prolong antibiotic therapy and to wait 3 to 6 months, with serial clinical, analytical, and culture follow-up, to definitively exclude infection. However, as this was an active patient, we felt there was a huge fracture risk with minimal trauma. As the mentioned workup revealed a low probability of infection, and to avoid a fracture and/or patient immobilization for a long time, we decided to proceed with the revision’s second stage. Nonetheless, some cases of prosthetic joint infection are, in fact, culture negative, so infection cannot be definitively excluded in this case. If that were the case, it could explain the topography of the lesion and the fact that it has not relapsed since.
The continuum of periprosthetic osteolysis and aseptic loosening continues to be a relatively poorly understood phenomenon, with different proposed pathogenic mechanisms [11]. There are certainly both mechanical and biological contributing factors, patient, implant, or surgeon related. Foreign-body giant-cell reaction has initially been described in association with PMMA cement, although it most commonly occurs with UHMWPE or prosthesis components [12]. Different levels of inflammation and consequent osteolysis can occur, depending on the size of particles [1]. This granulomatous reaction can lead to soft-tissue masses or, if intraosseous, can lead to extensive osteolytic pseudotumor areas within the bone. The histological analysis of the surgical tissue suggested that a reaction to prosthesis components and PMMA cement was the culprit of the extensive patient’s reaction, although cutaneous tests were negative (although these tests have been shown to be unreliable for determining true metal allergy in TKA) [13]. This suggests that the osteolytic reaction was not secondary to UHMWPE wear, as occurs in most cases, but probably due to an early debonding leading to osteolysis and generation of PMMA and metal debris [14]. Cheng et al. [14] report the failure of a tibial implant due to metal and PMMA debris that may also have resulted from an early debonding and consequent micromotion. Their histological findings are identical to ours, with no evidence of UHMWPE debris or macroscopic wear. Their patient did not present, however, with such an osteolytic lesion, although the case was treated 2 years after the index procedure. Although we do not have further information regarding the early course of our case, we believe the original pain and edema were due to the patient’s initial reaction to PMMA and prosthesis’ components. We cannot fully explain why this happened only on the femur side and why it has not relapsed since (the final histological diagnosis was only made after surgery and review of the case). We believe that the design of the prosthesis’ femoral component, which allowed communication to the femoral canal, could have allowed cement to migrate in an unusual amount to the femoral canal, leading to such reaction. The tibial component did not allow such communication.
Interestingly, there have been various reports in the literature regarding isolated tibial aseptic loosening, which can shed some light on the different modes of aseptic loosening failure [[14], [15], [16]]. Arsoy et al. [15] found a particular type of tibial implant to have a disproportional high percentage of aseptic loosening as the cause of failure. The patients in their study were mostly asymptomatic and diagnosed in routine surveillance radiographs, which showed tibial debonding with minimal amounts of osteolysis, contrasting to our case. They did not, however, establish the failure mechanism of that device. To our knowledge, there are no reports of failure related specifically to the implant used in the initial arthroplasty of our patient. On the other hand, Hazelwood et al. identified high-viscosity cement as a common factor among patients with isolated tibial aseptic loosening, which is known to have diminished bone penetration (we have no information regarding the type of cement used in the initial arthroplasty in our case) [16].
This is a case of a TKA failure, with a radical progression and treatment, in part due to the large observation period that the patient was exposed to (the reason of which we can only speculate about). If she had been submitted to surgery earlier, the outcome could have been different. Although we believe that the etiology of this lesion was a foreign-body reaction, it is important to note that a culture-negative periprosthetic joint infection is another possibility. Regarding surgical treatment options, this tumor reconstruction prosthesis conferred a great degree of stability and the possibility to remove the entire lesion. On the other hand, this option implied long stems and cement use, with limited options in case of a prosthetic failure. The stems, however, as they occupy the femoral canal can also serve as a mechanical block to an unusual cement migration and a new possible reaction. The extended muscular detachments could also have led to a low functional result. Nonetheless, the patient is well, with no signs of relapse, and with a good functional result. Another option would be to use a hinged prosthesis complemented with an autograft or allograft, but we felt the choice made would be a more stable construct.
Summary
Even with every care, periprosthetic osteolysis continues to be a major problem. A reaction to cement and prosthesis’ components can be an elusive cause and lead, as illustrated, to a dramatic outcome. As the number of TKAs continues to increase, it is possible that reactions such as this will appear more often and surgeons must be ready to deal with them.
Conflict of interest
The authors declare there are no conflicts of interest.
Acknowledgments
The authors would like to thank Prof. Dr. João Gamelas for his assistance in reviewing the case.
Footnotes
The work was performed in Hospital Beatriz Ângelo.
Appendix A. Supplementary data
References
- 1.Dalling J.G., Math K., Scuderi G.R. Evaluating the progression of osteolysis after total knee arthroplasty. J Am Acad Orthop Surg. 2015;23:173. doi: 10.5435/JAAOS-D-13-00189. [DOI] [PubMed] [Google Scholar]
- 2.Sanchis-Alfonso V., Alcacer-García J. Extensive osteolytic cystlike area associated with polyethylene wear debris adjacent to an aseptic, stable, uncemented unicompartmental knee prosthesis: case report. Knee Surg Sport Traumatol Arthrosc. 2001;9:173. doi: 10.1007/s001670000178. [DOI] [PubMed] [Google Scholar]
- 3.Vernon B.A., Bollinger A.J., Garvin K.L., McGarry S.V. Osteolytic lesion of the tibial diaphysis after cementless TKA. Orthopedics. 2011;34:224. doi: 10.3928/01477447-20110124-30. [DOI] [PubMed] [Google Scholar]
- 4.Maheshwari A.V., Muro-Cacho C.A., Temple H.T. Aggressive proximal tibio-fibular joint ganglion cyst after a total knee arthroplasty — a case report. Knee. 2008;15:411. doi: 10.1016/j.knee.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Niimi R., Hasegawa M., Sudo A., Uchida A. A large metallic cyst caused by wear particles after total knee arthroplasty. Arch Orthop Trauma Surg. 2006;127:51. doi: 10.1007/s00402-006-0214-6. [DOI] [PubMed] [Google Scholar]
- 6.Kenan S., Kahn L., Haramati N., Kenan S. A rare case of pseudotumor formation associated with methyl methacrylate hypersensitivity in a patient following cemented total knee arthroplasty. Skeletal Radiol. 2016;45:1115. doi: 10.1007/s00256-016-2372-0. [DOI] [PubMed] [Google Scholar]
- 7.Yoon H.-K., Cho S.-H., Lee D.-Y. A review of the literature on culture-negative periprosthetic joint infection: epidemiology, diagnosis and treatment. Knee Surg Relat Res. 2017;29:155. doi: 10.5792/ksrr.16.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphey M.D., Robbin M.R., McRae G.A., Flemming D.J., Temple H.T., Kransdorf M.J. The many faces of osteosarcoma. Radiographics. 1997;17:1205. doi: 10.1148/radiographics.17.5.9308111. [DOI] [PubMed] [Google Scholar]
- 9.Cottias P., Tomeno B., Anract P., Vinh T.S., Forest M. Subacute osteomyelitis presenting as a bone tumour. Int Orthop. 1997;21:243. doi: 10.1007/s002640050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bori G., McNally M.A., Athanasou N. Histopathology in periprosthetic joint infection: when will the Morphomolecular diagnosis be a reality? Biomed Res Int. 2018;2018:1412701. doi: 10.1155/2018/1412701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo J., Goodman S.B., Konttinen Y.T., Wimmer M.A., Holinka M. Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms. Acta Biomater. 2013;9:8046. doi: 10.1016/j.actbio.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman S.B., Fornasier V.L., Kei J. The effects of bulk versus particulate ultra-high-molecular-weight polyethylene on bone. J Arthroplasty. 1988;3:S41. doi: 10.1016/s0883-5403(88)80007-x. [DOI] [PubMed] [Google Scholar]
- 13.Teo W.Z.W., Schalock P.C. Metal hypersensitivity reactions to orthopedic implants. Dermatol Ther (Heidelb) 2017;7:53. doi: 10.1007/s13555-016-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng K., Pruitt L., Zaloudek C., Ries M.D. Osteolysis caused by tibial component debonding in total knee arthroplasty. Clin Orthop Relat Res. 2006;443:333. doi: 10.1097/01.blo.0000196044.42413.c7. [DOI] [PubMed] [Google Scholar]
- 15.Arsoy D., Pagnano M.W., Lewallen D.G., Hanssen A.D., Sierra R.J. Aseptic tibial debonding as a cause of early failure in a modern total knee arthroplasty design knee. Clin Orthop Relat Res. 2013;471:94. doi: 10.1007/s11999-012-2467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazelwood K.J., O’Rourke M., Stamos V.P., McMillan R.D., Beigler D., Robb W.J. Case series report: early cement-implant interface fixation failure in total knee replacement. Knee. 2015;22:424. doi: 10.1016/j.knee.2015.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.