Abstract
Background.
To improve our understanding of urea cycle disorders (UCDs) prospectively followed by two North American (NA) and European (EU) patient cohorts.
Aims.
Description of the NA and EU patient samples and investigation of the prospects of combined and comparative analyses for individuals with UCDs.
Methods.
Retrieval and comparison of the data from 1095 individuals (NA: 620, EU: 475) from two electronic data bases.
Results.
The proportion of females with ornithine transcarbamylase deficiency (fOTC-D), particularly those being asymptomatic (asfOTC-D), was higher in the NA than in the EU sample. Exclusion of asfOTC-D resulted in similar distributions in both samples. Mean age at first symptoms was higher in NA than in EU patients with late onset (LO), but similar for those with early (≤ 28 days) onset (EO) of symptoms. Also mean age at diagnosis and diagnostic delay for EO and LO patients were similar in NA and EU. In most patients (including fOTC-D), diagnosis was made after the onset of symptoms (59.9%) or by high-risk family screening (24.7%), and less often by newborn screening (8.9%) and prenatal testing (3.7%). Analysis of clinical phenotypes revealed that EO patients presented with more symptoms than LO individuals but that numbers of symptoms correlated with plasma ammonium concentrations in EO patients only. Liver transplantation was reported for 90 NA and 25 EU patients.
Conclusions.
Combined analysis of databases drawn from distinct populations opens the possibility to increase sample size for natural history questions, while comparative analysis utilising differences in approach to treatment can evaluate therapeutic options and enhance long-term outcome studies.
Introduction
The Urea Cycle Disorders Consortium (UCDC) was founded by the National Institutes of Health in 2003 in the United States as part of the Rare Diseases Clinical Research Network (http://www.rarediseasesnetwork.org/cms/UCDC). The European registry and network for intoxication type metabolic diseases (E-IMD) was established in 2011 (http://www.e-imd.org), funded by the European Union from 1st January, 2011 to 30th April, 2014 in the framework of the Health Programme 2008 – 2013 and later on by mixed funding sources (for details see acknowledgements). Descriptions of the research networks have been published previously (Seminara et al 2010; Summar et al 2014; Kolker et al 2015). The objective of both projects is to improve knowledge on the natural history, treatment and outcome of urea cycle disorders (UCDs) by collecting longitudinal data on clinical, biochemical and treatment parameters, to foster training for families and professionals, and to develop evidence-based recommendations for six enzymopathies, i.e. deficiencies of N-acetylglutamate synthase (NAGS-D; OMIM #237310), carbamylphosphate synthetase 1 (CPS1-D; OMIM #237300), ornithine transcarbamylase (OTC-D; OMIM #311250), argininosuccinate synthetase (ASS-D; OMIM #215700), argininosuccinate lyase (ASL-D; OMIM #207900), arginase 1 (ARG1-D; OMIM #207800), and two transporter defects, i.e. deficiencies of the citrin or aspartate/glutamate carrier (CITR-D; OMIM #603471 and #605814; UCDC only) and the mitochondrial ornithine transporter 1 causing hyperornithinemia-hyperammonemia-homocitrullinuria syndrome (HHH syndr.; OMIM #238970). The estimated cumulative prevalence of UCDs has been reported as 1 in 52,000–35,000 newborns (Dionisi-Vici et al 2002; Summar et al 2013; Nettesheim et al 2017). Based on large data samples, several UCDC and E-IMD research projects have been published previously (Morgan et al 2011; Nagamani et al 2012; Ah Mew et al 2013; McGuire et al 2013; Summar et al 2013; Batshaw et al 2014; Burrage et al 2014; Gallagher et al 2014; Kolker et al 2015; Lee et al 2015; Heringer et al 2016; Jamiolkowski et al 2016; Posset et al 2016).
The aim of the present study is to investigate similarities and differences in North American (NA) and European (EU) UCD samples from both registries, and to demonstrate how data can be combined to answer specific research questions. In particular, we analysed age at onset of symptoms and age at diagnosis, changes in modes of diagnoses over time, computed risks for early (EO) and late disease onset (LO) in individuals diagnosed early and treated pre-symptomatically, and used data from the E-IMD registry to describe the phenotype of signs and symptoms at time of diagnosis.
Patients and Methods
Written informed consent was obtained from all participants before enrolment in the study and the baseline visit in countries where this was legally required. Patients who died before the starting date of E-IMD (1st January 2011) are not recorded in the E-IMD registry. Data were retrieved from the respective electronic data bases with cut-off dates for the E-IMD sample at 16th March, 2015 to allow for comparability with a previous publication (Posset et al 2016), and 16th January, 2016 for the UCDC sample. The UCDC data base includes data from European research sites in Zurich and Heidelberg. For a transatlantic comparison, subjects from Heidelberg and Zurich enrolled in both registries or only in UCDC were assigned to the EU sample.
Commonalities and differences of the UCDC and E-IMD registries
Both registries use remote data entry via electronic forms comprising clinical and biochemical data regarding diagnosis and treatment from baseline, regular follow-up and emergency visits. The UCDC data model comprises about 570, the E-IMD registry 490 variables, with an overlapping set of 258 variables. The disjoint sets mostly contain specifications of variables in the overlapping set. For example, UCDC collects medical history data prior to study enrolment in more detail than E-IMD, whereas metabolic emergencies are recorded in greater detail by the E-IMD. Furthermore, some common variables were measured on different levels (e.g. medication with or without dosage information). Both studies collect data about intellectual development and quality of life, with the UCDC dataset containing more formal neurocognitive testing details. Variables and values analysed in the present study are summarised in Table 1.
Table 1.
Variables, definitions and values
| Variable | Values (abbreviation) |
|---|---|
| Metabolic defect | • ARG1-D, Arginase 1 deficiency • ASL-D, Argininosuccinate lyase deficiency • ASS-D, Argininosuccinate synthetase deficiency • fOTC-D, female ornithine transcarbamylase deficiency • mOTC-D, male ornithine transcarbamylase deficiency • CITR-D, Citrin deficiency • CPS1-D, Carbamylphosphate synthetase 1 deficiency ASx, asymptomatic • HHH syndr., Hyperornithinemia-hyperammonemia-homocitrullinuria syndrome • NAGS-D, N-Acetylglutamate synthase deficiency |
| Sex | male/female |
| Mode of diagnosis | • Prenatal testing (PT) • Newborn screening (NBS) • High-risk family screening (FH) • Metabolic investigation after onset of symptoms (SX) |
| Ages and times | • Age at diagnosis, if detected by PT set as zero (no prenatal UCD treatment) • Age at first symptoms • Age at last visit • Diagnostic delay (age at diagnosis minus age at first symptoms; negative when diagnosed pre-symptomatically, positive when symptomatic before diagnosis) • Observation period (time last visit minus time study inclusion) |
| Survival | Yes/no |
| Changing from asymptomatic to symptomatic state | Yes/no |
| Mode of confirmation of diagnosis | • Enzyme analysis • Metabolite analysis including amino acids • Mutation analysis • Pedigree analysis |
| Onset | • Early onset (EO; ≤ 28 days) • Late onset (LO; > 28 days) • Asymptomatic (AS) |
| Symptomatic state | Yes/no E-IMD definition: showing clinical symptoms associated with the respective diagnosis UCDC definition: symptoms associated with plasma ammonium concentrations > 100 μmol/L and/or liver transplant and/or ≥ 2 signs and symptoms: recurrent vomiting, protein intolerance, episodic lethargy, developmental and/or intellectual disabilities, abnormal neurological examination, brain edema on cranial MRI or CT scan, chronic migraine, and/or (episodic) psychosis |
| Signs and symptoms leading to the diagnosis unique to the E-IMD registry | • Blood abnormalities • Hyperexcitability • Impaired consciousness • Muscular hypertonia • Muscular hypotonia • Neurology other than seizures • Seizures • Sepsis-like appearance • Vomiting and feeding problems |
The following variables were used in UCDC and E-IMD for combined and comparative analysis between NA and EU.
Statistical analysis
The R environment for statistical computing (2016) was used for statistical and graphical computations. Frequencies were analysed with log-linear models using the vcd-package for R (Zeileis et al 2007). Contingency data of two or more independent variables are visualised as mosaic plots and differences between observed and expected frequencies were computed as standardised Pearson residuals by the vcd-package. Positive residuals indicate observed frequencies higher than expected and negative residuals observed frequencies lower than expected under the null-hypothesis that frequency distributions will not be associated with the independent variables. Pearson residuals are calculated as (observed-expected)/SQRT (expected) and can be interpreted as z-scores with residuals greater than 2 and less than −2 to be statistically significant at the 95% level. Examples are explained in the results paragraph. Frequency distributions were compared with chi-square tests, and two- and three- way analyses of frequencies were tested with log-linear models. Ages at first symptoms and at diagnosis, diagnostic delays (age at diagnosis minus age at first symptoms) for onset types (EO versus LO), geographic regions (NA versus EU) and diseases were analysed with 3-way analysis of variance (ANOVA). Differences between means were tested with t-tests, and differences between medians with the Wilcoxon rank sum test.
Results
Description of the NA and EU study population
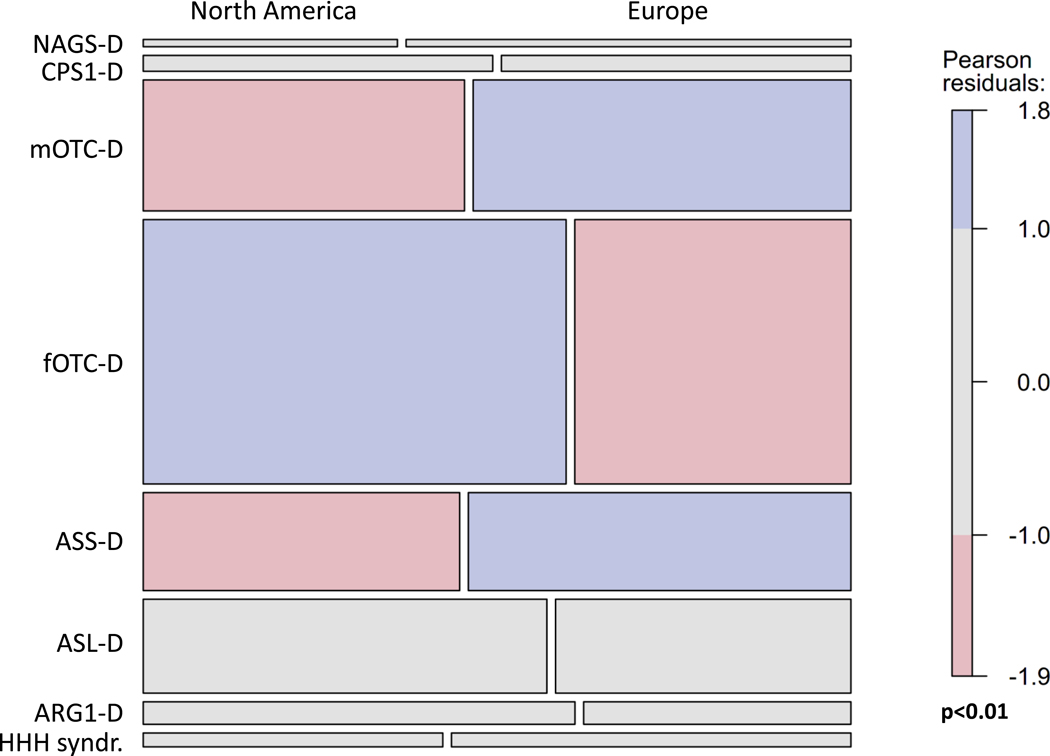
Overall, 980 UCD patients (NA, EU: 530, 450) from 18 countries and 115 (NA, EU: 90, 25) liver-transplanted individuals with UCDs were enrolled in the two registries (Table 2, Suppl. Table 1). The most frequent diagnoses were female (fOTC-D) and male OTC-D (mOTC-D), followed by ASS-D, and ASL-D as well as NAGS-D, CPS1-D, ARG1-D, CITR-D and HHH syndr., with each of these corresponding to less than 5% of reported patients. Figure 1 shows that compared with the EU sample, in the NA sample proportions of mOTC-D and ASS-D were lower, whereas that of fOTC-D was higher (p<0.01). Case mixes in both samples became similar after exclusion of individuals with fOTC-D (p=0.17), particularly of asymptomatic fOTC-D (asfOTC-D; p=0.23). Liver transplantation was most frequent in mOTC-D (Table 2). Due to the higher proportion of fOTC-D, in the NA sample two-thirds are female and one-third is male, whereas the distribution of sexes is more balanced in the EU sample (Table 2). The median observation time (i.e. time from study inclusion until last visit) was 4.0 years [interquartile range (IQR): 1.4 – 6.4] in NA and 0.6 years (IQR: 0 – 1.4) in the EU.
Table 2.
Case mix, onset type, and mode of diagnosis in the North American and European samples
| All Diagnoses n (%) | NAGS-D n (%) | CPS1-D n (%) | mOTC-D n (%) | fOTC-D n (%) | ASS-D n (%) | ASL-D n (%) | ARG1-D n (%) | HHH syndr. n (%) | CITR-D n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total without Liver Transplant (LTx) | NA EU |
530 (100) 450 (100) |
4 (0.8) 7 (1.6) |
12 (2.3) 12 (2.7) |
91 (17.2) 107 (23.8) |
242 (45.7) 158 (35.1) |
66 (12.5) 81 (18.0) |
82 (15.5) 60 (13.3) |
21 (4.0) 13 (2.9) |
9 (1.7) 12 (2.7) |
3 (0.6) n/a |
| Male | NA EU |
184 (34.7) 217 (48.2) |
1 (25.0) 3 (42.9) |
6 (50.0) 8 (66.7) |
91 (100) 107 (100) |
n/a n/a |
32 (48.5) 44 (54.3) |
39 (47.6) 36 (60.0) |
8 (38.1) 8 (61.5) |
5 (55.6) 11 (91.7) |
2 (66.7) n/a |
| Female | NA EU |
346 (65.3) 233 (51.8) |
3 (75.0) 4 (57.1) |
6 (50.0) 4 (33.3) |
n/a n/a |
242 (100) 158 (100) |
34 (51.5) 37 (45.7) |
43 (52.4) 24 (40.0) |
13 (61.9) 5 (38.5) |
4 (44.4) 1 (8.3) |
1 (33.3) n/a |
| LTx patients | NA EU |
90 (100) 25 (100) |
n.i. n.i. |
12 (13.3) 6 (24.0) |
32 (35.6) 13 (52.0) |
7 (7.8) 3 (12.0) |
19 (21.1) 3 (12.0) |
17 (18.9) n.i. |
3 (3.3) n.i. |
n.i. n.i. |
n.i. n/a |
| Age of onset | |||||||||||
| Early onset (EO) | NA EU |
123 (23.2) 119 (26.4) |
2 (50.0) 4 (57.1) |
6 (50.0) 7 (58.3) |
34 (37.4) 21 (19.6) |
8 (3.3) 5 (3.2) |
38 (57.6) 48 (59.3) |
31 (37.8) 31 (51.7) |
3 (14.3) 1 (7.7) |
1 (11.1) 2 (16.7) |
n.i. n/a |
| Late onset (LO) | NA EU |
226 (42.6) 195 (43.3) |
2 (50.0) 1 (14.3) |
5 (41.7) 4 (33.3) |
39 (42.9) 52 (48.6) |
119 (49.2) 91 (57.6) |
9 (13.6) 15 (18.5) |
30 (36.6) 18 (30.0) |
15 (71.4) 7 (53.8) |
5 (55.6) 7 (58.3) |
2 (66.7) n/a |
| Asymptomatic (ASx) | NA EU |
157 (29.6) 74 (16.4) |
n.i. n.i. |
1 (8.3) 1 (8.3) |
14 (15.4) 17 (15.9) |
99 (40.9) 40 (25.3) |
19 (28.4) 8 (9.9) |
21 (25.6) 3 (5.0) |
1 (4.8) 4 (30.8) |
1 (11.1) 1 (8.3) |
1 (33.3) n/a |
| Presentation leading to diagnosis | |||||||||||
| Prenatal testing (PT) | NA EU |
29 (5.5) 7 (1.6) |
n.i. n.i. |
1 (8.3) n.i. |
7 (7.7) 1 (0.9) |
13 (5.4) 4 (2.6) |
3 (4.5) 1 (1.2) |
5 (6.1) 1 (1.7) |
n.i. n.i. |
n.i. n.i. |
n.i. n/a |
| Newborn screening (NBS) | NA EU |
64 (12.1) 22 (4.8) |
n.i. n.i. |
1 (8.3) 1 (8.3) |
1 (1.1) 1 (0.9) |
1 (0.4) 0 (0.0) |
25 (37.9) 7 (8.6) |
30 (36.6) 8 (13.3) |
4 (19.0) 4 (30.8) |
n.i. 1 (8.3) |
2 (66.7) n/a |
| Symptomatic (SX) | NA EU |
277 (52.3) 310 (68.9) |
3 (75.0) 7 (100.0) |
10 (83.3) 10 (83.3) |
65 (71.4) 77 (72.0) |
105 (43.4) 91 (57.6) |
33 (50.0) 65 (80.2) |
39 (47.6) 45 (75.0) |
14 (66.7) 7 (53.8) |
7 (77.8) 8 (66.7) |
1 (33.3) n/a |
| Family History (FH) | NA EU |
146 (27.5) 97 (21.5) |
n.i. n.i. |
n.i. 1 (8.3) |
13 (14.3) 21 (19.6) |
118 (48.8) 59 (37.3) |
4 (6.1) 6 (7.4) |
7 (8.5) 5 (8.3) |
2 (9.5) 2 (15.4) |
2 (22.2) 3 (25.0) |
n.i. n/a |
ARG1-D, arginase 1 deficiency; ASx, asymptomatic; ASL-D, argininosuccinate lyase deficiency; ASS-D, argininosuccinate synthetase deficiency; CITR-D, citrin deficiency; CPS1-D, carbamylphosphate synthetase 1 deficiency; EU, Europe; fOTC-D, female ornithine transcarbamylase deficiency; HHH syndr., Hyperornithinemia-hyperammonemia-homocitrullinuria syndrome; LTx, liver transplantation; mOTC-D, male ornithine transcarbamylase deficiency; NA, North America; n/a, not applicable (since disease was not included in the European study sample); NAGS-D, N-acetylglutamate synthase deficiency; NBS, newborn screening; n.i., no information in the databases; SX, Selective metabolic investigation after onset of symptoms; UCD, urea cycle disorder. Patients in NA and EU without information on particular variables are counted as missing data and are not included in the table.
Fig. 1.
Mosaic plot of the association of diagnoses with geographical region. The height of the boxes in a row represents the proportion of a particular diagnosis. The widths of two boxes in a row represent the proportions of individuals with a particular diagnosis in each of the two geographic areas; i.e. the North American (left) and the European sample (right). Significance disappeared after exclusion of fOTC-D, particularly asfOTC-D (for details see text).
Age at last visit was different for specific diagnoses (p < 0.001) with CPS1-D, mOTC-D, ASS-D, ASL-D, and ARG1-D representing predominately paediatric populations, while fOTC-D represented an adult population in both datasets. In NA most individuals with NAGS-D and HHH syndr. were in the adult age groups, while those in EU were in the paediatric age group, recognizing that the samples are relatively small (Table 3). Accordingly, the median age at last visit was higher in NA than in EU (p < 0.001). Fatal disease was observed in 59 patients (6% from the NA and 4.6% from the EU sample) most of whom were mOTC-D (NA: 62.2 %; EU: 63.3%) and died in the neonatal period (Table 3). Both registries included subjects who were enrolled post-mortem, as well as those who were alive at enrolment but died during the study (NA=7, EU=2). Taking into account the different time spans of the two studies (UCDC=14 years, E-IMD=5 years), the annual death rate in both datasets was about equal (7 patients in 14 years versus 2 patients in 5 years).
Table 3.
Times at disease manifestation and diagnosis in the North American and European samples
| Total Median [Q1, Q3]; n | NAGS-D Median [Q1, Q3]; n | CPS1-D Median [Q1, Q3]; n | mOTC-D Median [Q1, Q3]; n | fOTC-D Median [Q1, Q3]; n | ASS-D Median [Q1, Q3]; n | ASL-D Median [Q1, Q3]; n | ARG1-D Median [Q1, Q3]; n | HHH syndr. Median [Q1, Q3]; n | CITR-D Median [Q1, Q3]; n | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at last visit [years] | NA EU |
17.7 [7.2, 33.4]; 530 11.8 [5.6, 21.5]; 450 |
26.6 [24.4, 48.5]; 4 8.5 [0.9, 16.6]; 7 |
8.2 [1.5, 33.9]; 12 9.8 [7.0, 13.3]; 12 |
15.1 [5.2, 28.4]; 91 10.9 [4.1, 18.0]; 107 |
29.3 [13.8, 41.0]; 242 19.9 [9.9, 33.4]; 158 |
9.2 [4.2, 15.8]; 66 8.3 [3.9, 15.0]; 81 |
8.9 [4.0, 20.1]; 82 9.3 [5.2, 15.4]; 60 |
16.9 [5.8, 23.2]; 21 8.1 [5.6, 10.7]; 13 |
28.8 [10.5, 53.5]; 9 11.9 [9.2, 24.6]; 12 |
5.8 [0.6, 10.9]; 3 n/a |
| Age at first symptoms EO [days] | NA EU |
2 [1, 4]; 113 3 [2, 4]; 113 |
1 [1, 1]; 1 2 [2, 4]; 4 |
2 [1, 2]; 6 3 [2, 3]; 6 |
2 [1, 2]; 31 3 [2, 5]; 20 |
4 [2, 10]; 8 3 [3, 4]; 5 |
2 [1, 4]; 37 2 [2, 3; 47 |
3 [2, 7]; 27 3 [2, 4]; 29 |
11 [7, 14]; 2 n.i. |
1 [1, 1]; 1 4 [2, 6]; 2 |
n.i. n/a |
| Age at first symptoms LO [days] | NA EU |
731 [335, 2557]; 197 585 [300, 1813]; 180 |
731 [731, 731]; 2 3650 [3650, 3650] 1 |
5844 [3287, 11323]; 5 795 [495, 1140]; 4 |
913 [396, 3287]; 38 630 [300, 2190]; 50 |
731 [365, 1826]; 102 720 [365, 2160]; 83 |
304 [244, 411]; 8 390 [150, 540]; 14 |
365 [122, 700]; 21 210 [150, 1080]; 15 |
761 [183, 1461]; 14 815 [34, 1440]; 6 |
1461 [883, 17897]; 5 450 [274, 630]; 7 |
106 [29, 183]; 2 n/a |
| Age at diagnosis EO [days] | NA EU |
4 [3, 8]; 109 4 [3, 9]; 116 |
3653 [3653, 3653]; 1 5 [2, 34]; 4 |
3 [2, 6]; 5 10 [4, 540]; 6 |
3 [2, 4]; 30 4 [3, 9]; 20 |
472 [64, 1141]; 8 9 [3, 13]; 5 |
4 [3, 6]; 34 5 [3, 7]; 48 |
5 [3, 7]; 27 4 [3, 9]; 30 |
396 [8, 1826]; 3 270 [270, 270]; 1 |
2 [2, 2]; 1 48 [6, 90]; 2 |
n.i. n/a |
| Age at diagnosis LO [days] | NA EU |
1424 [417, 4018]; 221 730 [360, 2190]; 185 |
10775 [731, 20819]; 2 3650 [3650, 3650]; 1 |
5844 [4383, 11323]; 5 1650 [870, 2100]; 4 |
1461 [548, 4018]; 39 725 [330, 2400]; 50 |
1826 [700, 4383]; 119 945 [510, 2555]; 84 |
533 [320, 2845]; 8 330 [240, 570]; 15 |
731 [17, 2192]; 29 210 [61, 1080]; 18 |
1096 [731, 2922]; 13 1308 [35, 2190]; 6 |
1461 [883, 17897]; 5 639 [450, 780]; 7 |
365 [365, 365]; 1 n/a |
| Age at diagnosis ASx [days] | NA EU |
8766 [30, 12419]; 143 3650 [21, 12045]; 69 |
n.i. n.i. |
n.i. 90 [90, 90]; 1 |
4383 [852, 11323]; 14 2190 [15, 13140]; 15 |
10592 [7488, 13332]; 96 10768 [3650, 12775]; 38 |
6 [4, 13]; 14 3 [3, 10]; 7 |
26 [5, 594]; 16 19 [5, 2190]; 3 |
30 [30, 30]; 1 21 [9, 36]; 4 |
15341 [15341, 15341]; 1 25 [25, 25]; 1 |
7 [7, 7]; 1 n/a |
| Diagnostic delay EO [days] | NA EU |
1 [0, 4]; 103 1 [0, 3]; 113 |
3652 [3652, 3652]; 1 1 [0, 30]; 4 |
1 [1, 2]; 5 7 [2, 535]; 6 |
1 [0, 2]; 29 0 [0, 3]; 20 |
460 [58, 1134]; 8 5 [0, 10]; 5 |
0 [0, 3]; 33 1 [0, 3]; 47 |
1 [0, 4]; 24 1 [0, 2]; 29 |
1100 [389, 1812]; 2 n.i. |
1 [1, 1]; 1 44 [0, 88]; 2 |
n.i. n/a |
| Diagnostic delay LO [days] | NA EU |
61 [0; 731]; 195 0 [0, 300]; 173 |
10044 [0, 20089]; 2 0 [0, 0]; 1 |
0 [0, 0]; 5 570 [30, 1305]; 4 |
0 [0, 274]; 38 0 [0, 98]; 48 |
228 [0, 731]; 102 30 [0, 365]; 79 |
15 [0, 527]; 8 8 [0, 90]; 14 |
0 [0, 761]; 21 0 [0, 330]; 15 |
0 [0; 122]; 13 1 [0, 986]; 5 |
0 [0, 0]; 5 244 [0, 330]; 7 |
183 [183, 183]; 1 n/a |
| Age of death [days] | NA EU |
7 [4, 481]; 37 13 [4, 35]; 22 |
n.i. n.i. |
60 [4, 229]; 5 4 [4, 4]; 2 |
5 [4, 17]; 23 13 [8, 29]; 14 |
4923 [2590, 10243]; 4 2245 [1960, 2530]; 2 |
5 [5, 5]; 1 902 [7, 1797]; 2 |
7 [4, 10]; 2 3787 [4, 7570]; 2 |
10240 [3842, 16638]; 2 n.i. |
n.i. n.i. |
n.i. n/a |
ARG1-D, arginase 1 deficiency; ASx, asymptomatic; ASL-D, argininosuccinate lyase deficiency; ASS-D, argininosuccinate synthetase deficiency; CITR-D, citrin deficiency; CPS1-D, carbamylphosphate synthetase 1 deficiency; EO, early onset; EU, Europe; fOTC-D, female ornithine transcarbamylase deficiency; HHH syndr., Hyperornithinemia-hyperammonemia-homocitrullinuria syndrome; LO, late onset; mOTC-D, male ornithine transcarbamylase deficiency; NA, North America; NAGS-D, N-acetylglutamate synthase deficiency; n.i., no information on this variable entered in the databases; UCD, urea cycle disorder; n/a, not applicable since disease was not included in the European study sample. Patients in NA and EU without information on particular variables are counted as missing data and are not included in the table.
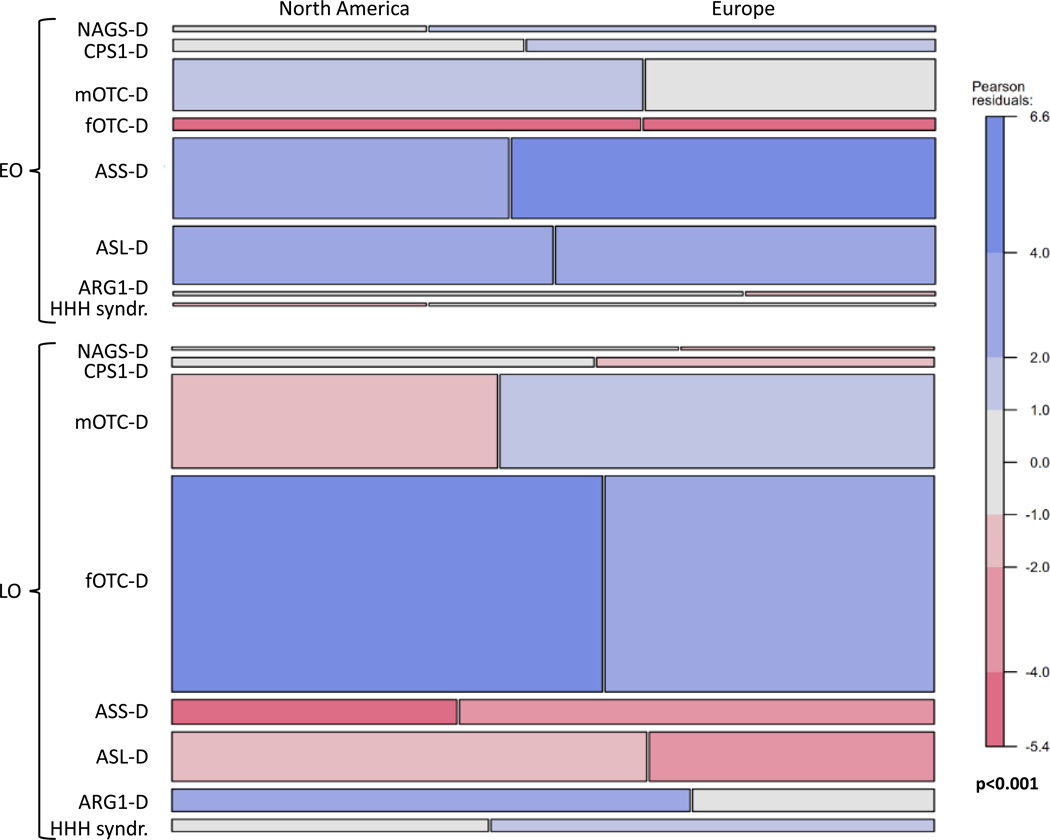
Proportions of patients with EO (NA, EU: 23.2%, 26.4%) and LO for first symptoms (NA, EU: 42.6%, 43.3%) were similar in both registries (Table 2). Asymptomatic individuals were 1.8 times more frequent in the UCDC than in the E-IMD database (NA, EU: 29.6%, 16.4%); however onset information was not available for 24 individuals (4.5%) in the NA and 62 (13.9%) in the EU sample. Figure 2 shows the mosaic plot of the association of diagnoses, onset, and geographical region. Time at onset showed a significant disease-specific pattern (p< 0.001, log-linear model); in both datasets the most frequent disorders presenting with EO were ASS-D, ASL-D and mOTC-D, while fOTC-D and mOTC-D most frequently presented as LO. A major difference was that EU mOTC-D individuals presented mainly as LO while NA patients as EO. Out of 263 asymptomatic individuals (NA, EU: 181, 82) at study inclusion, during the observation period 32 (12.2%) became symptomatic, 24 in the NA sample (13.2%) and eight in the EU sample (9.8%). These included fOTC-D (NA, EU: 14, 1), mOTCD-D (1, 1) ASL-D (7, 2), ASS-D (1, 2), ARG-D (1, 1), and NAGS-D (0, 1). For all diagnoses except ARG1-D, age at diagnosis was similar for EO subjects in both datasets. Age at diagnosis was similar for all diagnoses in LO subjects, but not for asymptomatic individuals, for whom, except for OTC-D, ASS-D, and ASL-D, numbers were very small (for details see Supplementary Figure 1, Table 3). The triple interaction of the ANOVA of mean age at diagnosis by geographic region, onset type, and disease was not significant (p=0.87, ANOVA) indicating similar patterns in both data bases.
Fig. 2.
Mosaic plot of the association of diagnoses, type of onset, and geographical region. The upper part illustrates early onset (EO), the lower part late onset (LO). The height of the boxes in a row represents the proportion of a particular diagnosis. The widths of two boxes in a row represent the proportions of individuals with a particular diagnosis in each of the two geographic areas; i.e. the North American (left) and the European sample (right) (for details see text).
Age at first symptoms was homogeneous in EO patients across diseases within a very short time window during the first ten days of life and in LO patients by the end of the second year of life. Due to large differences in age at first symptoms between NA and EU in patients with LO NAGS-D, CPS1-D or HHH syndr. (Supplementary Figure 2, Table 3), the interaction of geographic region with disease and onset type showed a trend (p=0.064, ANOVA).
For diagnostic delay, the interaction of age of onset with mode of diagnosis was significant (p=0.03). Delay was more similar in EO patients (PT: mean=−1.13 days, SD=0.7; NBS: 1.41, 3.9; SX: 78, 332; FH: 174, 544) than in LO patients (PT: mean=−897, SD=1542; NBS: −589, 771; SX: 597, 1813; FH: 1889, 4244). The triple interaction of geographic region, onset type and mode of diagnosis was not significant (p=0.68).
Mode of diagnosis and its change over time
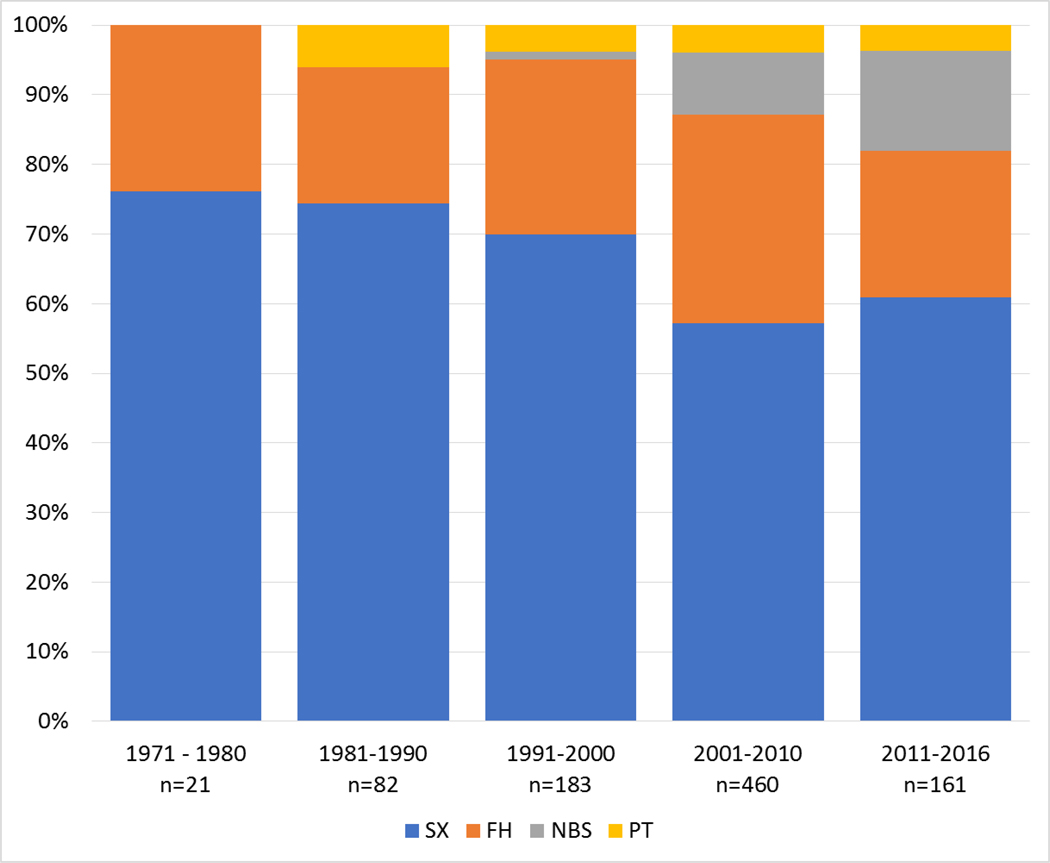
Ranking modes of diagnosis resulted in the same order in both samples from SX being the most frequent to FH, NBS and PT (Table 2, Figure 3). Starting with the year 1971, dividing the observation period into intervals of 10 years revealed that PT began in the eighties and NBS in the mid-nineties. While PT exceeded five per cent only once (between 1981 and 1990), the proportion of NBS increased continuously. SX and FH remained the dominant modes of diagnosis in all decades.
Fig. 3.
Modes of diagnosis and their distributions over five decades from 1971 until 2016. SX, Selective metabolic investigation after onset of symptoms; FH, Family history triggered investigation; NBS, Newborn screening; PT, Prenatal testing.
Confirmation of suspected UCDs in North America and Europe
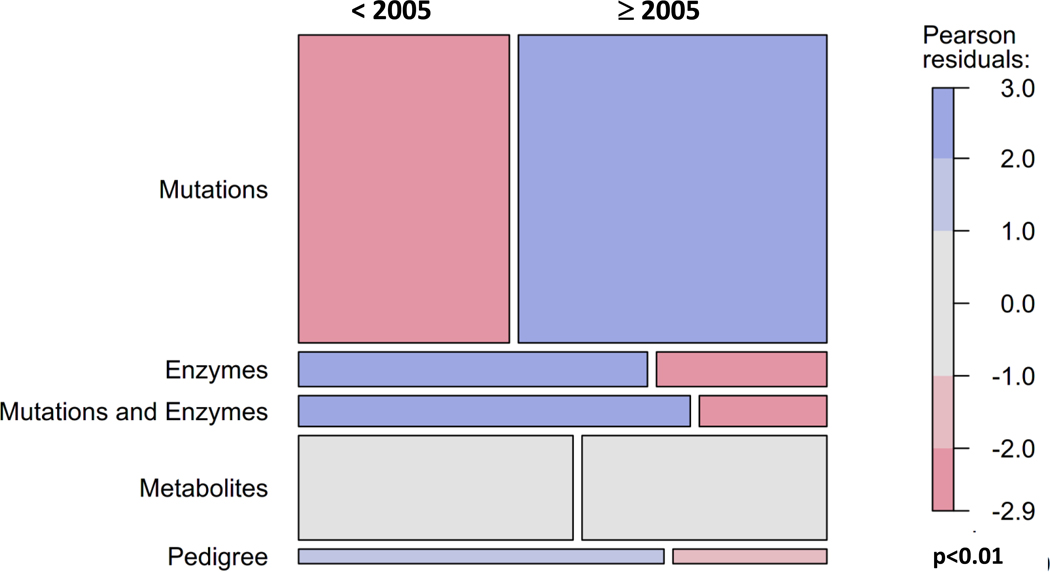
Suspected diagnoses were confirmed by analysis of metabolites, enzyme activity, or mutations, by combinations of these methods, or by pedigree analysis. (Suppl. Table 2). Methods were classified into five groups: (1) mutation analysis alone or with metabolites (NA: 326 patients, EU: 279 patients), (2) enzyme analysis alone or with metabolites (NA, EU: 43, 28); (3) mutation plus enzyme analysis or mutation plus enzyme plus metabolite analysis (NA, EU: 30, 30), (4) metabolites only (NA, EU: 103, 113), and (5) pedigree analysis (NA, EU: 28, 0), the latter only for the X-linked OTC-D. Since the precise dates of confirmatory diagnostic measures are unknown in both samples, the age at diagnosis was used as a surrogate, and both samples were divided in approximately equal moieties before 2005 (NA, EU: 295, 186) and after 2005 (NA, EU: 280, 259). Frequency distributions of diagnostic groups (1) to (4) did not differ in NA and EU (χ2=4.46, df=3; p=0.22), but before 2005 mutation analysis was performed less frequently and enzymatic as well as pedigree analysis more often than afterwards, when this pattern reversed (Figure 4).
Fig. 4.
Mosaic plot of the association of methods used for confirmation before 2005 and afterwards in the combined sample of UCD and E-IMD patients. In both geographic regions there is a clear shift from using metabolites and enzymes to mutation analysis.
Early diagnosis, pre-symptomatic diagnosis, and onset type
Table 4 shows data from 353 individuals diagnosed as newborns; 315 (89%) within the first ten days of life, 254 (81%) having an EO. To model the potential effect of NBS, we focused the analysis on 82 individuals who were diagnosed pre-symptomatically within the first 10 days. 43 of them (52,4%) remained asymptomatic. Together with 18 individuals with LO, 61 remained asymptomatic during the neonatal period. From all individuals diagnosed pre-symptomatically in the neonatal period, 53 remained asymptomatic (ASS-D: n=18, 33.9%; fOTC-D: n=13, 24.5%; ASL-D: n=10, 18.9%; mOTC-D: n=8, 15.1%; ARG1-D: n=2, 3.8%; each HHH syndr. and CITR-D: n=1, 1.9%), while 21 developed EO and 26 LO symptoms.
Table 4.
Time at diagnosis in the neonatal period (0 – 28 days), frequencies of pre-symptomatic diagnosis and onset of symptoms in UCD patients (including 83 patients with LTx)
| Diagnosis in the neonatal period | Pre-symptomatic diagnosis in the neonatal period | |||||||
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (days) | EO n (%column) |
LO n (%column) |
ASx n (%column) |
n (%column) | EO n (%column) |
LO n (%column) |
ASx n (%column) |
|
| 0 – 3, n=153 | 119 (43.4) | 10 (38.5) | 24 (45.3) | 54 (54.0) | 20 (95.2) | 10 (38.5) | 24 (45.3) | |
| 4 – 10, n=162 | 135 (49.3) | 8 (30.8) | 19 (35.8) | 28 (28.0) | 1 (4.8) | 8 (30.8) | 19 (35.8) | |
| 11 – 15, n=17 | 8 (2.9) | 5 (19.2) | 4 (7.5) | 9 (9.0) | 0 (0.0) | 5 (19.2) | 4 (7.5) | |
| 16 – 28, n=21 | 12 (4.4) | 3 (11.5) | 6 (11.3) | 9 (9.0) | 0 (0.0) | 3 (11.5) | 6 (11.3) | |
| 0 – 28, n=353 | 274 (100) | 26 (100) | 53 (100) | 100 (100) | 21 (100) | 26 (100) | 53 (100) | |
EO, early onset; LO, late onset; ASx, asymptomatic; LTx, liver transplantation
Clinical phenotype of EO and LO patients at time of diagnosis
Data from 111 EO patients and 130 LO patients revealed nine signs and symptoms at the time of their first clinical presentation (Suppl. Table 3). The number of signs and symptoms in EO (mean 3.0, SD=1.4, range 1–8) versus LO individuals (mean=2.1, SD=1.1, range 0–6) were significantly different (t=5.3, df=204.2, p<.001). For 88 EO and 100 LO patients, peak plasma ammonium concentrations at onset were available. Mean values were significantly higher (t=8.2, df=98.8, p<.001) in the EO group (mean=1009 μmol/L, median=807 μmol/L, SD=814, range: 18–4645 μmol/L) than in the LO group (mean=271 μmol/L, median=221 μmol/L, SD=226, range: 27–1800 μmol/L). The correlation between numbers of signs and symptoms and plasma ammonium concentrations was significant in EO (r=0.37, t=3.8, df=86, p<.001) but not in LO patients (r=0.08, t=.84, df=98, p<.4). In both onset groups for each sign or symptom t-tests were computed for differences of plasma ammonium concentrations in patients showing versus not showing a particular sign or symptom. Differences were significant for sepsis-like appearance (t = −3.03, df = 84.6, p-value = 0.003) and seizures (t = −3.35, df = 48.4, p-value = 0.002) only for EO subjects. Mean plasma ammonium concentration for 24 individuals with both signs was 1520 μmol/L (SD=1085), however, even patients without both signs still had a mean ammonium concentration of 577 μmol/L (SD=423). In LO only impaired consciousness showed a trend for patients with this sign having slightly higher plasma ammonium concentrations (mean 290 μmol/L, SD=246, n=73) than those without this sign (mean 218 μmol/L, SD=2157, n=27, t = −1.7, df = 73.4, p < 0.09).
Discussion
Analysis of 1095 individuals with a UCD diagnosis from the UCDC and E-IMD registry revealed six results: 1.) As published previously (Summar et al 2014), OTC-D was the most frequent diagnosis in both samples. The case mixes in NA and EU differed due to the higher frequency of fOTC-D, particularly asfOTC-D patients in NA. Exclusion of asfOTC-D allows to combine both registries into a common analysis. The higher rate of asfOTC-D in the NA sample is due to the explicit aim of the UCDC to investigate asymptomatic females. 2.) Proportions of patients with LO were higher than of those with EO in both samples highlighting either that LO is more and EO is less frequent than previously assumed, or that patients with EO, the more severe phenotype, may have been less likely to be enrolled due to early death or even may have died without a diagnosis. Overall, death seems to be underreported when compared to the literature (Burgard et al 2016; Nettesheim et al 2017). Nine individuals died during the study, however the annual death rates were similar. 3.) Disease-specific age at diagnosis and diagnostic delay were similar in both samples. Delay was particularly long for ultra-rare UCDs (NAGS-D, ARG1-D) and rare clinical presentations (EO fOTC-D). 4.) Modes of diagnosis changed over time following technological development. 5.) Although only 26% of patients diagnosed during the first 10 days were asymptomatic at the time of diagnosis, 52% of them remained asymptomatic until the last visit. 6.) At the time of diagnosis EO and LO patients presented with a variety of nonspecific signs and symptoms. In EO patients only sepsis-like appearance and seizures were associated with peak plasma ammonium concentrations, whereas in LO patients no association was found.
Disease onset
Despite strong efforts have been made by both consortia to increase the proportion of patients with EO (Batshaw et al 2014), in both samples, EO was less frequently reported than LO. This is in agreement with previous results from the UCDC and E-IMD registries (Tuchman et al 2008; Seminara et al 2010; Kolker et al 2015), but in contrast to other studies showing a predominance of EO patients (Nassogne et al 2005; Nettesheim et al 2017). As both registries are based on voluntary participation, it might be more arduous for individuals with EO and thus a more severe clinical phenotype to participate. Moreover, UCD patients with EO have a high risk of neonatal mortality (Burgard et al 2016; Nettesheim et al 2017). Therefore, many of these patients may have died without having an appropriate diagnosis or in hospitals not participating in the E-IMD and UCDC registries. Finally, the study design of the E-IMD registry excludes UCD patients who died before the year 2011.
Modes and confirmation of diagnosis, diagnostic delay, and effect of early diagnosis
Particularly, after the turn of the millennium (2001–2016), use of methods suitable for early diagnosis (NBS, FH, PT) increased to approximately 40% of newly diagnosed UCD patients. The majority of individuals, however, still received their diagnosis after clinical presentation, requiring an astute clinician. It should be kept in mind that identification of individuals with mitochondrial or proximal UCDs (NAGS-D, CPS-D and OTC-D) – unlike those having cytosolic or distal UCDs (ASS-D, ASL-D) – is unreliable by standard NBS programmes using citrulline as a biomarker, because low citrulline is less sensitive than high citrulline.
Confirmation of diagnosis changed from analyses of enzymes and metabolites before 2005 to genetic analysis afterwards. Diagnostic delays for SX and FH were shorter in EO than in LO patients. EO, associated with more severe symptoms, particularly sepsis-like appearance and seizures as well as higher plasma ammonium concentrations than LO, may have triggered a more rapid diagnostic work-up. LO patients may benefit from NBS and PT, whereas the impact on patients with EO remains to be elucidated, since only a small proportion can be diagnosed before the appearance of first symptoms (in our dataset symptoms preceded NBS diagnosis by 1.41 days). This is in line with a recent E-IMD study (Posset et al 2016). Importantly, 81% of patients diagnosed during the first 10 days of life had an EO. This should be considered for the establishment of NBS programmes. A higher proportion of EO patients can only be identified pre-symptomatically when NBS samples will be taken very early and processed very fast. But even then, some EO patients will show first symptoms before the NBS result is available. About half of patients diagnosed before the onset of first symptoms during the neonatal period remained asymptomatic during the study time, and a substantial number of LO patients would also have benefited from pre-symptomatic identification by NBS.
Clinical phenotypes of EO and LO patients at the time of diagnosis
The initial clinical phenotype of UCD patients is important for early diagnosis and was previously described (Nassogne et al 2005; Summar et al 2008; Haberle et al 2012; Kolker et al 2015). The most common initial symptoms of UCD patients are: 1.) neurological (decreased level of consciousness, abnormal motor function, seizures), 2.) gastrointestinal (vomiting, poor feeding), 3.) psychiatric (hallucinations, personality changes) in older individuals, and 4.) sepsis-like picture particularly in neonates. These signs and symptoms are potentially triggered by catabolism (e.g. infections) or high protein load. Most patients presented with combinations of two (LO) or three (EO) symptoms. In LO subjects plasma ammonium concentrations were comparatively low. They were neither correlated with the number of signs and symptoms, nor with any particular sign, indicating a non-specific phenotype. In contrast, plasma ammonium concentrations in EO subjects were significantly higher and correlated with the number of signs and symptoms. A sepsis-like appearance in combination with seizures was associated with a particularly severe metabolic phenotype. Specificities of single symptoms are low, but it remains to be tested whether combinations of symptoms are more predictive.
Conclusion
Combined and comparative analysis of observational registries for patient with rare disorders such as UCDs can be an important research strategy. We described differences and similarities of the UCDC and the E-IMD databases, the two largest studies of UDC patients. Differences, such as ages of patients, can be used to increase age ranges in cross-sectional developmental studies, and different cohorts allow investigation of historical trends in clinical practice. Similarities allow increased sample sizes and statistical power or even the design of new studies. The example of fOTC-D has shown that careful attention must be paid to the case mixes of different datasets to ensure that comparisons reflect true differences, and not ascertainment bias. We have shown how this issue can be managed. The analysis of early and pre-symptomatically diagnosed subjects as a model for newborn screening also has demonstrated that targeted evaluation of subsets of large combined datasets can be a promising strategy.
Outlook
Based on this data analysis of the combination of two large UCD datasets, in-depth studies on specific questions regarding practice and outcome in UCDs are underway, including evaluation of treatment modalities, feasibility and efficacy of NBS programmes, effects of liver transplantation and cognitive function in UCDs.
Supplementary Material
Suppl. Fig. 1 Age at diagnosis on a logarithmic scale for early and late onset as well as asymptomatic individuals across the seven UCD diagnoses (for detailed numeric information see Table 3).
Suppl. Fig. 2 Age at first symptoms on a logarithmic scale for early and late onset individuals across the seven UCD diagnoses (for detailed numeric information see Table 3).
Acknowledgements
The UCDC longitudinal study has received funding from National Institute for Child Health and Development (NICHD U54 HD061221), the O’Malley Family Foundation, the Kettering Fund, the Dietmar Hopp Foundation, and the Rothenberg Family Foundation.
The E-IMD patient registry has been received funding by the European Union (E-IMD; EAHC no 2010 12 01; coordinator: Stefan Kölker), in the framework of the Health Programme. After the end of the EU funding period the E-IMD patient registry has been sustained by funding from the Kindness-for-Kids Foundation (Munich, Germany), the Kettering Foundation (Dayton, Ohio, USA), and Dietmar Hopp Foundation (St. Leon-Rot, Germany). M. Baumgartner and J. Häberle (Zurich, Switzerland) are supported by radiz – Rare Disease Initiative Zurich, a clinical research priority programme of the University of Zurich. C. Dionisi-Vici (Rome, Italy) is supported by the association “La vita è un dono”.
All Urea Cycle Disorders Consortium (UCDC) sites contributed to the Longitudinal Study dataset. Principal investigators have been listed in the individual contributors section. Study coordinators include: Sondra Bloxam, Linnea Brody, Liora Caspi, Sara Elsbecker, Luca Fierro, Audrey Lynn, Mary Mullins, Ulrike Mütze, Cassandra Papaleo, Irma Payan, Jennifer Seminara, Kara Simpson, Rebecca Singer, and Kimberly Wallis. Study neuropsychologists include: Fabienne Dietrich Alber, Talin Babikian, Heidi Bender, Christopher Boys, David Breiger, Corinna Buerger, Susan E. Caudle, Mina Nguyen-Driver, Elizabeth Kerr, Eva Mamak, Jacqueline H. Sanz, Rachel Tangen, and Greta Wilkening. We would also like to acknowledge the contributions of former longitudinal study PIs: Stephen Cederbaum, Annette Feigenbaum, Douglas S. Kerr, Uta Lichter-Konecki, and Margretta R. Seashore.
Abbreviations
- ARG1-D
Arginase 1 deficiency
- ASx
Asymptomatic
- (as)fOTC-D
(Asymptomatic) female ornithine transcarbamylase deficiency
- ASL-D
Argininosuccinate lyase deficiency
- ASS-D
Argininosuccinate synthetase deficiency
- CITR-D
Citrin deficiency
- CPS1-D
Carbamylphosphate synthetase 1 deficiency
- E-IMD
European registry and network for intoxication type metabolic diseases
- EU
Europe(an)
- EO
Early onset
- FH
Family history triggered investigation
- fOTC-D
Female ornithine transcarbamylase deficiency
- HHH syndr
Hyperornithinemia-hyperammonemia-homocitrullinuria syndrome
- IQR
Interquartile range
- LO
Late onset
- LTx
Liver transplantation
- mOTC-D
Male ornithine transcarbamylase deficiency
- NA
North America(n)
- NAGS-D
N-Acetylglutamate synthase deficiency
- NBS
Newborn screening
- PT
Prenatal testing
- SQRT
Square root
- SX
Selective metabolic investigation after onset of symptoms
- UCDC
Urea Cycle Disorders Consortium
- UCD(s)
Urea cycle disorder(s)
Additional individual contributors (to be listed in the PubMed, in alphabetical order)
From the UCDC
Susan A. Berry
University of Minnesota, Minneapolis, Minnesota, USA
Lindsay Burrage
Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA
Curtis Coughlin
Children’s Hospital Colorado and University of Colorado School of Medicine, Aurora, Colorado, USA
George A. Diaz
Icahn School of Medicine at Mount Sinai, New York, New York, USA
Renata C. Gallagher
University of California San Francisco, San Francisco, California, USA
Andrea Gropman
Children’s National Health System and The George Washington School of Medicine, Washington, District of Columbia, USA
Cary O. Harding
Oregon Health and Science University, Portland, Oregon, USA
Brendan Lee
Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA
Cynthia Le Mons
National Urea Cycle Disorders Foundation, Pasadena, California, USA
J. Lawrence Merritt II
University of Washington and Seattle Children’s Hospital, Seattle, Washington, USA
Sandesh CS Nagamani
Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA
Andreas Schulze
The Hospital for Sick Children and University of Toronto, Toronto, Ontario, Canada
Tamar Stricker
University Children’s Hospital Zurich, Zurich, Switzerland
Mendel Tuchman
Children’s National Health System and The George Washington School of Medicine, Washington, District of Columbia, USA
Susan Waisbren
Harvard Medical School and Boston Children’s Hospital, Boston, Massachusetts, USA
Derek Wong
David Geffen School of Medicine at UCLA, Los Angeles, California, USA
Marc Yudkoff
University of Pennsylvania School of Medicine and Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA
James Weisfeld-Adams
Children’s Hospital Colorado and University of Colorado School of Medicine, Aurora, Colorado, USA
From the E-IMD
Jean-Baptiste Arnoux
Hôpital Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris, Service de Maladies Métaboliques, Paris, France
Ivo Barić
University Hospital Center Zagreb and University of Zagreb, School of Medicine, Zagreb, Croatia
Annet M. Bosch
Academisch Medisch Centrum, Department of Pediatrics, Amsterdam, Netherlands
Brigitte Chabrol
Centre de Référence des Maladies Héréditaires du Métabolisme, Service de Neurologie, Hôpital d’Enfants, CHU Timone, Marseille, France
Anupam Chakrapani
Metabolic Unit Great Ormond Street Hospital and Institute for Child Health, University College London, London, United Kingdom
Elisenda Cortès-Saladelafont
Hospital San Joan de Deu, Servicio de Neurologia and CIBERER, ISCIII, Barcelona, Spain
Maria L. Couce
Hospital Clinico Universitario de Santiago de Compostela, Metabolic Unit, Department of Pediatrics, Santiago de Compostela, Spain
Francois Eyskens
Universitair Ziekenhuis Antwerpen, Antwerpen, Belgium
Peter Freisinger
Klinik für Kinder- und Jugendmedizin, Klinikum am Steinenberg, Reutlingen, Germany
Florian Gleich
Centre for Pediatric and Adolescent Medicine, Division of Neuropediatrics and Inherited Metabolic Diseases, University Hospital Heidelberg, Im Neuenheimer Feld 430, 69120 Heidelberg, Germany
Stephanie Grünewald
Metabolic Unit Great Ormond Street Hospital and Institute for Child Health, University College London, London, United Kingdom
Johannes Häberle
University Children’s Hospital Zurich, Division of Metabolism and Children’s Research Centre, Steinwiesstraße 75, CH-8032 Zurich, Switzerland
Wuh-Liang Hwu
Department of Medical Genetics, National Taiwan University, Hospital, Taipei City, Taiwan
Anil Jalan
N.I.R.M.A.N., Om Rachna Society, Vashi, Navi Mumbai, Mumbai, India
Daniela Karall
Medical University of Innsbruck, Clinic for Pediatrics I, Inherited Metabolic Disorders, Innsbruck, Austria
Corine de Laet
Hôpital Universitaire des Enfants Reine Fabiola, Brussels, Belgium
Martin Lindner
University Children’s Hospital Frankfurt, Frankfurt, Germany
Allan M. Lund
Centre for Inherited Metabolic Diseases, Departments of Pediatrics and Clinical Genetics, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
Diego Martinelli
Ospedale Pediatrico Bambino Gésu, U.O.C. Patologia Metabolica, Rome, Italy
Linda de Meirleir
University Hospital Vrije Universiteit Brussel, Bruxelles, Belgium
Elaine Murphy
National Hospital for Neurology and Neurosurgery, Charles Dent Metabolic Unit, London, United Kingdom
Chris Mühlhausen
University Childreńs Hospital, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Giorgia Olivieri
Ospedale Pediatrico Bambino Gésu, U.O.C. Patologia Metabolica, Rome, Italy
Chris Ottolenghi
Hôpital Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris, Service de Maladies Métaboliques, Paris, France
Esmeralda Rodrigues
Unidade de Doenças Metabólicas, Serviço de Pediatria, Hospital de S. João, EPE, Porto, Portugal
Laura Rubert
Azienda Ospedaliera di Padova, U.O.C. Malattie Metaboliche Ereditarie, Padova, Italy
Manuel Schiff
Hôpital Robert Debré, APHP and Université Paris-Diderot, Reference Centre for Inborn Errors of Metabolism, Paris, France
Adrijan Sarajlija
Belgrade University, School of Medicine, Belgrade, Republic of Serbia
Etienne Sokal
Cliniques Universitaires St Luc, Université Catholique de Louvain, Service Gastroentérologie and Hépatologie Pédiatrique, Bruxelles, Belgium
Jolanta Sykut-Cegielska
Institute of Mother and Child, Screening Department, Warsaw, Poland
John H. Walter
Manchester Academic Health Science Centre, University of Manchester, Willink Biochemical Genetics Unit, Genetic Medicine, Manchester, United Kingdom
Monique Williams
Erasmus MC-Sophia Kinderziekenhuis, Erasmus Universiteit Rotterdam, Rotterdam, Netherlands
Jiri Zeman
Department of Paediatrics, First Faculty of Medicine and General Faculty Hospital, Prague, Czech Republic
Footnotes
Conflict of Interest
Stefan Kölker receives funding from Horizon Pharma Ireland Limited for the European Post-Authorization Registry for Ravicti® (glycerol phenylbutyrate) oral liquid in partnership with the E-IMD (RRPE) (EU PAS Register no. EUPAS17267; http://www.encepp.eu/). Georg F. Hoffmann, Peter Burgard and Stefan Kölker receive funding from the Dietmar Hopp Foundation (St. Leon-Rot, Germany) for coordinating the study “Newborn Screening and Metabolic Medicine 2020 (NBS2020)” including individuals with urea cycle disorders. The sponsors have in no way influenced the design, conductance, analysis and report of the present study.All other authors declare that they have no conflict of interest.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human studies (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from all patients or their legal guardians prior to study inclusion in countries where this was needed by law.
Details of the contributions of individual authors
Design and conduct of the study: All authors.
Collection of patient data: All authors.
Statistical analysis: Peter Burgard, Sven Garbade, Florian Gleich, Roland Posset, and Stefan Kölker.
Manuscript writing: Roland Posset, Peter Burgard, Sven Garbade, Shawn McCandless, and Stefan Kölker.
Manuscript revision and correction: All authors.
Animal rights
This article does not contain animal subjects.
References
- Ah Mew N, Krivitzky L, McCarter R, Batshaw M, Tuchman M, Urea Cycle Disorders Consortium of the Rare Diseases Clinical Research N (2013) Clinical outcomes of neonatal onset proximal versus distal urea cycle disorders do not differ. J Pediatr 162: 324–329 e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batshaw ML, Tuchman M, Summar M, Seminara J, Members of the Urea Cycle Disorders C (2014) A longitudinal study of urea cycle disorders. Mol Genet Metab 113: 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard P, Kolker S, Haege G, Lindner M, Hoffmann GF (2016) Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders--review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis 39: 219–229. [DOI] [PubMed] [Google Scholar]
- Burrage LC, Jain M, Gandolfo L, Lee BH, Members of the Urea Cycle Disorders C, Nagamani SC (2014) Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol Genet Metab 113: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisi-Vici C, Rizzo C, Burlina AB, et al. (2002) Inborn errors of metabolism in the Italian pediatric population: a national retrospective survey. J Pediatr 140: 321–327. [DOI] [PubMed] [Google Scholar]
- Gallagher RC, Lam C, Wong D, Cederbaum S, Sokol RJ (2014) Significant hepatic involvement in patients with ornithine transcarbamylase deficiency. J Pediatr 164: 720–725 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberle J, Boddaert N, Burlina A, et al. (2012) Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringer J, Valayannopoulos V, Lund AM, et al. (2016) Impact of age at onset and newborn screening on outcome in organic acidurias. J Inherit Metab Dis 39: 341–353. [DOI] [PubMed] [Google Scholar]
- Jamiolkowski D, Kolker S, Glahn EM, et al. (2016) Behavioural and emotional problems, intellectual impairment and health-related quality of life in patients with organic acidurias and urea cycle disorders. J Inherit Metab Dis 39: 231–241. [DOI] [PubMed] [Google Scholar]
- Kolker S, Dobbelaere D, Haberle J, et al. (2015) Networking Across Borders for Individuals with Organic Acidurias and Urea Cycle Disorders: The E-IMD Consortium. JIMD Rep 22: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolker S, Garcia-Cazorla A, Valayannopoulos V, et al. (2015) The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis 38: 1041–1057. [DOI] [PubMed] [Google Scholar]
- Lee B, Diaz GA, Rhead W, et al. (2015) Blood ammonia and glutamine as predictors of hyperammonemic crises in patients with urea cycle disorder. Genet Med 17: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PJ, Lee HS, members of the Urea Cycle Disorders C, Summar ML (2013) Infectious precipitants of acute hyperammonemia are associated with indicators of increased morbidity in patients with urea cycle disorders. J Pediatr 163: 1705–1710 e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TM, Schlegel C, Edwards KM, et al. (2011) Vaccines are not associated with metabolic events in children with urea cycle disorders. Pediatrics 127: e1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamani SC, Shchelochkov OA, Mullins MA, et al. (2012) A randomized controlled trial to evaluate the effects of high-dose versus low-dose of arginine therapy on hepatic function tests in argininosuccinic aciduria. Mol Genet Metab 107: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassogne MC, Heron B, Touati G, Rabier D, Saudubray JM (2005) Urea cycle defects: management and outcome. J Inherit Metab Dis 28: 407–414. [DOI] [PubMed] [Google Scholar]
- Nettesheim S, Kolker S, Karall D, et al. (2017) Incidence, disease onset and short-term outcome in urea cycle disorders -cross-border surveillance in Germany, Austria and Switzerland. Orphanet J Rare Dis 12: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posset R, Garcia-Cazorla A, Valayannopoulos V, et al. (2016) Age at disease onset and peak ammonium level rather than interventional variables predict the neurological outcome in urea cycle disorders. J Inherit Metab Dis 39: 661–672. [DOI] [PubMed] [Google Scholar]
- Seminara J, Tuchman M, Krivitzky L, et al. (2010) Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab 100 Suppl 1: S97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summar ML, Dobbelaere D, Brusilow S, Lee B (2008) Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr 97: 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summar ML, Endo F, Kolker S (2014) On the Creation, Utility and Sustaining of Rare Diseases Research Networks: Lessons learned from the Urea Cycle Disorders Consortium, the Japanese Urea Cycle Disorders Consortium and the European Registry and Network for Intoxication Type Metabolic Diseases. Mol Genet Metab 113: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summar ML, Koelker S, Freedenberg D, et al. (2013) The incidence of urea cycle disorders. Mol Genet Metab 110: 179–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman M, Lee B, Lichter-Konecki U, et al. (2008) Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab 94: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeileis A, Meyer D, Hornik K (2007) Residual-based shadings for visualizing (conditional) independence. Journal of Computational and Graphical Statistics 16: 507–525. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1 Age at diagnosis on a logarithmic scale for early and late onset as well as asymptomatic individuals across the seven UCD diagnoses (for detailed numeric information see Table 3).
Suppl. Fig. 2 Age at first symptoms on a logarithmic scale for early and late onset individuals across the seven UCD diagnoses (for detailed numeric information see Table 3).