Abstract
Purpose
To report asymptomatic progressive fundus depigmentation and choroidal thinning in the absence of intraocular inflammation in a patient treated with checkpoint inhibitors.
Observations
A 69-year-old woman with metastatic cutaneous melanoma, treated with checkpoint inhibition (nivolumab, ipilimumab and pembrolizumab), developed asymptomatic progressive fundus depigmentation associated with choroidal thinning in both eyes over 26 months. Serial multimodal imaging was obtained over the study period including fundus photography, fundus autofluorescence and optical coherence tomography (OCT). Over 26 months, the central choroidal thickness decreased by 34% (from 270μm to 92μm, mean between both eyes). Concurrently, central retinal thickness remained stable (206μm to 214μm, mean between both eyes). There were no findings of intraocular inflammation, subretinal fluid or retinal pigment epithelium disturbance. The patient reported no visual symptoms and maintained a visual acuity of 20/25+ in the right eye and 20/30 in the left eye throughout the observation period. Concurrently, cutaneous vitiligo and poliosis, inclusive of her periorbital dermis and eyelashes also developed.
Conclusions and importance
Progressive fundus depigmentation and choroidal thinning can be observed with checkpoint inhibition in the absence of intraocular inflammation.
Keywords: Cancer, Checkpoint inhibitor, Fundus vitiligo, Leptochoroid, Poliosis, Progressive choroidal thinning
1. Introduction
Cancer cells can elude the immune system by hijacking natural regulatory systems known as immune checkpoints and inhibit tumor specific T-cells, thereby protecting themselves from immune attack.1 There are now a number of FDA approved drugs known as checkpoint inhibitors (CPIs) that alter this immune response and have resulted in improved patient survival in metastatic cutaneous melanoma and other tumors.1
The three classes of CPIs (cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), programmed cell death (PD-1) and programmed cell death protein 1 (PD-L1) inhibitors) prevent tumor cells from evading the immune system. CPIs are intended to potentiate the T-lymphocyte response against tumor cells,1 but these T-lymphocytes can also target normal tissue resulting in inflammatory adverse events. This includes ocular inflammation which occurs in approximately 1% of patients and involves various anatomical parts of the eye including the orbit/adnexa, ocular surface, uveal tract, retina, extraocular muscles, cranial nerves and optic nerve.2, 3 These ocular side effects are thought to represent inflammation in the context of a heightened off-target immune response to healthy ocular tissue. However, the following case demonstrates progressive choroidal depigmentation and choroidal thinning in a patient on long-term treatment of ipilimumab, nivolumab and pembrolizumab for metastatic melanoma, and in the absence of intraocular inflammation or visual symptoms.
2. Case report
A 69-year-old Caucasian female with a history of metastatic cutaneous melanoma that originated in the fifth metatarsal of her right foot, developed metastases to the brain, liver and lung. Biopsy of the liver metastasis revealed HRAS and SPRED1 genetic mutations. She was seen for a baseline ophthalmic examination 6 weeks after starting ipilimumab and nivolumab for her metastatic disease. Her ocular history was negative for previous surgeries or injury and she was taking atorvastatin for elevated cholesterol and escitalopram for anxiety.
Best corrected visual acuities were 20/25 in the right eye and 20/30 in the left eye, attributed to nuclear sclerosis in both eyes. Intraocular pressures were 11 and 12 mmHg in each eye with no intra-ocular inflammation. External and fundus examination was unremarkable with no adverse effects noted from her immunotherapy and the patient was advised to return in 3 months for follow-up.
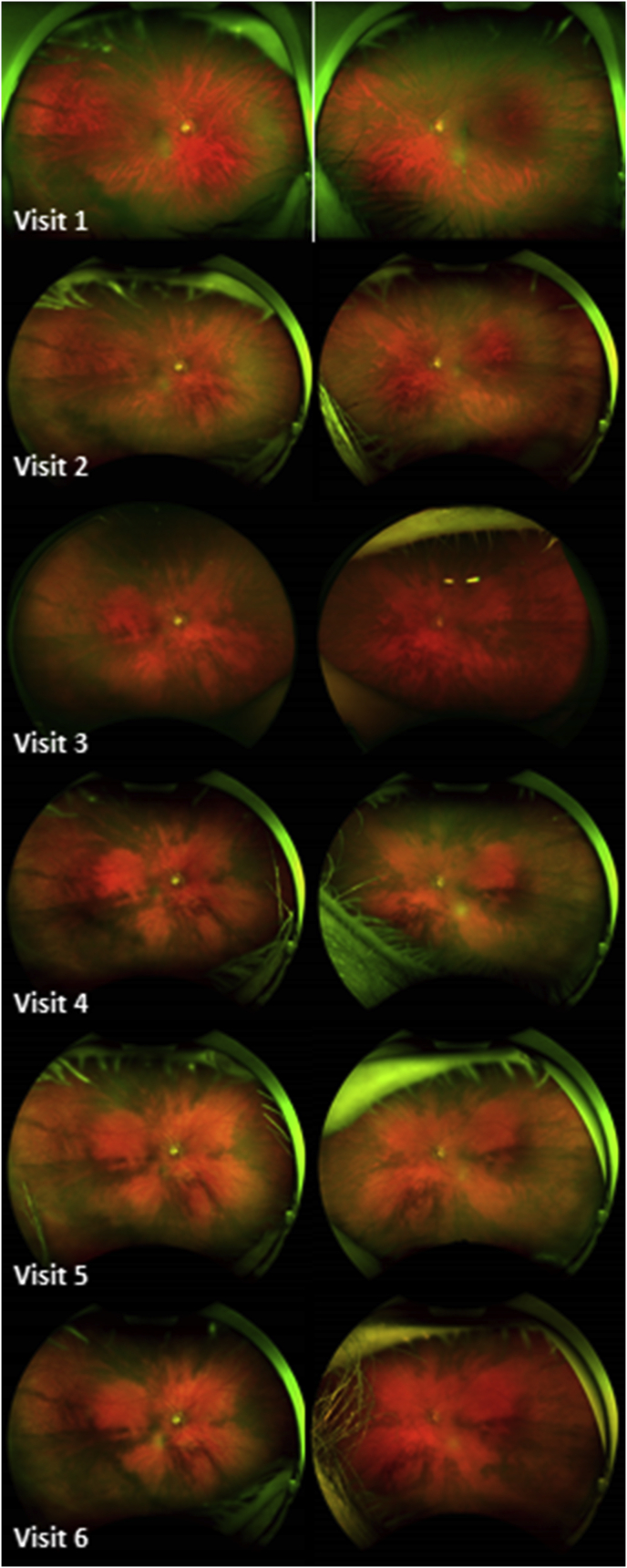
Over the subsequent 26 months of follow up, she completed 2 months of ipilimumab and nivolumab, followed by 12 months of pembrolizumab and finally switched to her current maintenance of nivolumab monotherapy. During this treatment course, her vision was stable, her intraocular pressures remained normotensive and there was no evidence of intraocular inflammation in either the anterior or posterior segments; nor evidence of past or present subretinal fluid. However, there was a noticeable difference in the fundus exam compared to baseline, with progressive areas of depigmentation and small geographic areas of unchanged pigmentation (Fig. 1). Furthermore, she developed poliosis of her eyelid cilia and the patient reported cutaneous vitiligo. Approximately 7 months into treatment, she was treated with a two-week course of topical fluorometholone ointment for left lower eyelid blepharitis which promptly resolved. She denied auditory and neurological symptoms.
Fig. 1.
Change in choroidal pigmentation in both eyes (right eye on the left and left eye on the right) over a period of 26 months: note the progressive fundus depigmentation in an almost petaloid configuration around the optic nerve and involving the macula.
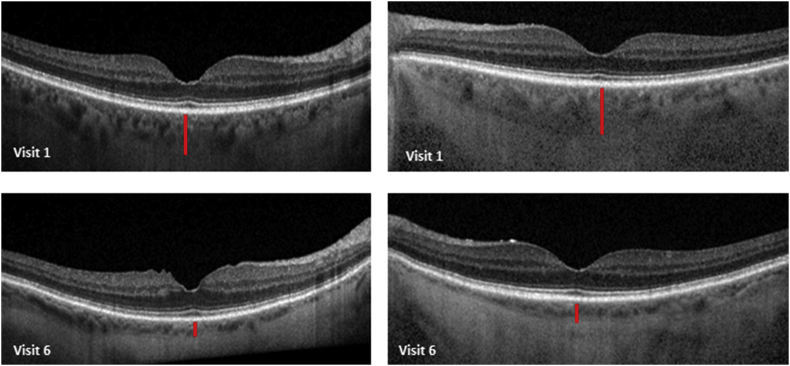
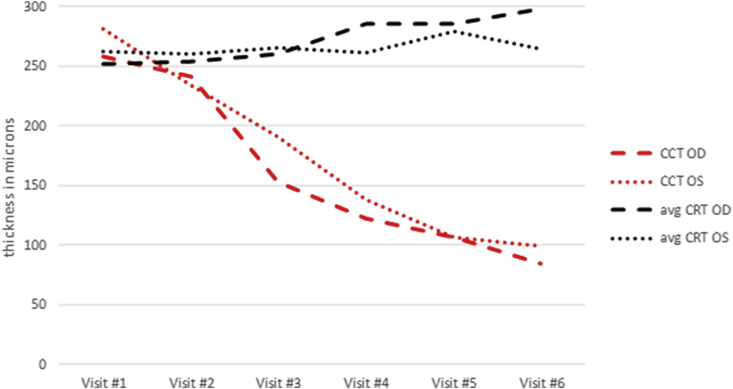
In an attempt to better understand her fundus depigmentation, the optical coherence tomography (OCT) of her choroidal and retinal thickness as well as caliber of the retinal pigment epithelium (RPE) were retrospectively reviewed. Choroidal thickness was measured as the vertical distance from Bruch's membrane to the choroidal-scleral border using the caliper tool on the Heidelberg Spectralis OCT. Likewise, the average central retinal thickness (CRT) was defined by the distance between the internal limiting membrane (ILM) and the outer RPE at the border of Bruch's membrane. Over the course of 6 serial exams in a 26-month period, the central choroidal thickness decreased by 34% (from 270μm to 92μm, mean between both eyes) (Fig. 2). Conversely, the average CRT remained stable (206μm to 214μm, mean between both eyes) (Fig. 3); in addition to no observable change in the optical coherence tomography of the RPE.
Fig. 2.
Change in central choroidal thickness in both eyes (right eye on the left and left eye on the right) over a period of 26 months. The red vertical line highlights the approximate choroidal thickness. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Graph demonstrating the gradual decrease in central choroidal thickness (CCT) in microns in the right eye (red dashed line) and left eye (red dotted line). While choroidal thickness declines, the mean central retinal thickness in the right eye (CRT) (black dashed line) and left eye (black dotted line) remains relatively stable throughout the visits. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
CPIs are associated with adverse ocular events in 1% of patients and believed to be inflammatory in etiology. We describe a case of progressive fundus depigmentation and choroidal thinning (leptochoroid) over 26 months of checkpoint inhibition, and in the absence of overt intraocular inflammation. The stability in retinal thickness and absence of abnormalities in the RPE suggests an association between the fundus depigmentation and choroidal thinning.
Many conditions are now recognized to be associated with choroidal thinning. These include: macular degeneration, diurnal (evening) measurements, glaucoma, retinal angiomatous proliferation, retinoblastoma after intra-arterial chemotherapy, Best's vitelliform stage 5, idiopathic subfoveal neovascularization, hypertension, nicotine, migraine, retinitis pigmentosa, increasing age and increasing axial length.4 Our patient did not fit any of the criteria aforementioned, except perhaps increasing age. However, age related thinning does not develop over this short a time span. Previous studies of normal patients found that central choroidal thickness typically changes about 1.1–3.14 microns per year.5 Our patient had an average change in choroidal thickness of 177 microns in both eyes over the span of 26 months highlighting that this degree of change in choroidal thickness is not related to aging.
One case of choroidal depigmentation has been reported with checkpoint inhibition, but in the context of Vogt-Harada-Koyanagi (VKH)-like disease (aka the “sunset glow” fundus) and with the associated inflammatory findings,6 not found in our patient. The case previously described reports fundus findings at a single time point and made no comment on the condition of the choroid, nor its thickness. It is interesting to note that other cases of CPI-associated VKH-like findings did not report fundus depigmentation, and the choroid was reportedly thickened (or normal) as would be expected with choroiditis.7, 8, 9 Clearly, our case of progressive fundus depigmentation and leptochoroid in the absence of past or present inflammation is distinct from these previously published cases.
We were intrigued by our patients’ integumentary (skin and hair) depigmentation in the context of concomitant fundus depigmentation. The literature makes an association between cutaneous vitiligo and “choroidal” hypopigmentation in healthy patients without inflammation or RPE changes (and without the use of CPIs).10 In these two cases, choroidal thickness was not measured, nor were there changes noted over time, but it offers an explanation for a possible link between cutaneous vitiligo and choroidal hypopigmentation. Both choroidal and skin melanocytes are derived from neural crest cells, unlike the RPE which is derived from the neuroectoderm.11 It would follow that the use of CPIs to eradicate melanoma cells would have similar effects on similarly-derived choroidal and skin melanocytes giving rise to fundus vitiligo and poliosis with stable retinal and RPE findings, as noted in our patient.
Our case uniquely demonstrates that CPIs can be associated with an adverse ocular event that does not appear to be overtly inflammatory or symptomatic in nature. Furthermore, this case suggests an association between progressive fundus depigmentation and choroidal thinning, the implications of which warrant further investigation.
Patient consent
The patient consented to publication of the case orally.
Funding
The Fund for Ophthalmic Knowledge and the New York Community Trust had no role in the design or conduct of this research.
This research was funded in part through the NIH/NCI Cancer Center Support Grant (P30 CA008748), Ludwig Collaborative and Swim Across America Laboratory, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA. Parker Institute for Cancer Immunotherapy, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA. Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA, Weill Cornell Medicine, New York, NY 10065, USA.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
Dr. Wolchok:
Consultant for: Adaptive Biotech; Advaxis; Amgen; Apricity; Array BioPharma; Ascentage Pharma; Astellas; Bayer; Beigene; Bristol Myers Squibb; Celgene; Chugai; Elucida; Eli Lilly; F Star; Genentech; Imvaq; Janssen; Kleo Pharma; Kyowa Hakko Kirin; Linneaus; MedImmune; Merck; Neon Therapuetics; Northern Biologics; Ono; Polaris Pharma; Polynoma; Psioxus; Puretech; Recepta; Takara Bio; Trieza; Sellas Life Sciences; Serametrix; Surface Oncology; Syndax,; Syntalogic.
Research support: Bristol Myers Squibb; Medimmune; Merck Pharmaceuticals; Genentech; Sephora.
Equity in: Potenza Therapeutics; Tizona Pharmaceuticals; Adaptive Biotechnologies; Elucida; Imvaq; Beigene; Trieza; Linneaus; Honoraium: Esanex
The following authors have no financial disclosures: JC, KAJ, DHA, JHF.
Acknowledgements
None.
References
- 1.Villasboas J.C. The Basics of Cancer Immunotherapy. Springer; Rochester, NY: 2018. Next generation immunotherapy in lymphoma: checkpoint blockade, chimeric antigen receptor T cells, and beyond; pp. 95–114. [Google Scholar]
- 2.Dalvin L.A., Shields C.L., Orloff M. Checkpoint inhibitor immune therapy, systemic indications and ophthalmic side effects. Retina. 2018;38:1063–1078. doi: 10.1097/IAE.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 3.Liu Catherine Y., Francis Jasmine H., Pulido Jose S., Abramson David H. Ocular side effects of systemically administered chemotherapy. https://www.uptodate.com/contents/ocular-side-effects-of-systemically-administered-chemotherapy Available at:
- 4.Francis J.H., Habib L.A., Abramson D.H. Peripheral leptochoroid: clinical and anatomical findings. Br J Ophthalmol. 2017:1–6. doi: 10.1136/bjophthalmol-2017-310406. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuncer I., Karahan E., Zengin M.O. Choroidal thickness in relation to sex, age, refractive error, and axial length in healthy Turkish subjects. Int Ophthalmol. 2015;35:403–410. doi: 10.1007/s10792-014-9962-4. [DOI] [PubMed] [Google Scholar]
- 6.Crosson J.N., Laird P.W., Debiec M. Vogt-Koyanagi-Harada-like syndrome after CTLA-4 inhibition with ipilimumab for metastatic melanoma. J Immunother. 2015;38:80–84. doi: 10.1097/CJI.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantopoulos D., Kendra K., Letson A., Cebulla C. Bilateral choroidopathy and serous retinal detachments during ipilimumab treatment for cutaneous melanoma. JAMA Ophthalmology. 2015;133(8):965–966. doi: 10.1001/jamaophthalmol.2015.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong R.K., Lee J.K., Huang J.J. Bilateral drug (Ipilimumab)-Induced vitritis, choroiditis, and serous retinal detachments suggestive of vogt-koyanagi-harada syndrome. Retin Cases Brief Rep. 2012;6(4):423–426. doi: 10.1097/ICB.0b013e31824f7130. [DOI] [PubMed] [Google Scholar]
- 9.Bricout M., Petre A., Amini-Adle M. Vogt-Koyanagi-Harada-like syndrome complicating pembrolizumab treatment for metastatic melanoma. J Immunother. 2017;40:77–82. doi: 10.1097/CJI.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 10.Vingerling J.R., Owens S., Van der Meijden W.I. Cutaneous vitiligo associated with choroidal hypopigmentation. Eye. 2004;18:939–940. doi: 10.1038/sj.eye.6701351. [DOI] [PubMed] [Google Scholar]
- 11.Cook C.S., Ozanis V., Jakobiec F.A. Duane's Ophthalmology. Lippincot-Raven; Philadelphia, PA: 1991. Prenatal development of the eye and its adnexa; pp. 1–93. [Google Scholar]