Abstract
We report a novel application of targeted sclerotherapy to eradicate high-output chylothorax. The patient underwent thoracic duct embolization; however, cannulation of the thoracic duct failed, and thoracic duct disruption was performed. Leakage continued; therefore, the leakage site in the mediastinum was punctured directly under fluoroscopic guidance and a drainage catheter was inserted, followed by sclerotherapy using OK-432. Finally, leakage stopped and chylothorax improved. This technique may be useful for refractory chylothorax in patients where thoracic duct embolization fails.
Keywords: Chylothorax, Direct puncture, OK-432, Sclerotherapy
Introduction
Chylothorax is a rare but potentially life-threatening complication of surgical procedures. Because the thoracic duct transports large amounts of lymph from the gastrointestinal tract, liver, and iliac regions, chylothorax can compromise the immune system and lead to nutritional deficiency. Its mortality rate is reported to be 25%-50% [1,2]. Treatment for chylothorax is initially conservative, such as total parenteral nutrition or intravenous administration of octreotide [2]. Chylothorax for which conservative treatment is ineffective requires other treatment. Traditionally, surgical thoracic duct ligation has been the standard treatment for refractory chylothorax. Morbidity and mortality rates are 38% and 2.1%, respectively [1].
Percutaneous thoracic duct embolization (TDE) has recently emerged as an alternative therapy to surgical thoracic duct ligation for chylothorax [3]. It is a minimally invasive treatment with good outcomes, but it is not always an easy procedure for any operator [4]. We herein report a case in which percutaneous TDE for high-output chylothorax failed but was successfully treated with targeted sclerotherapy at the site of lymphatic leakage.
Case report
A 70-year-old man with esophageal cancer underwent esophagectomy, and posterior mediastinal route gastric tube reconstruction was performed. A chylothorax appeared on postoperative day (POD) 2. Conservative treatment with complete parenteral nutrition and octreotide administration was ineffective. An average of 2000 mL of pleural effusion was sustained per day and the serum albumin level decreased to 1.5 g/dL at POD 12. Since conservative therapy was ineffective and surgical ligation was judged to be difficult due to poor general patient condition, we decided to perform lymphangiography for TDE.
Intranodal lymphangiography was performed on POD 16. Both inguinal lymph nodes were punctured under ultrasound guidance, and iodized oil (Lipiodol; Guerbet Japan, Tokyo, Japan) was injected. The cisterna chyli connected to the thoracic duct was visualized at the Th12/L1 intervertebral level (Fig. 1a). Extravasation of iodized oil was visualized on the left side of the eighth thoracic vertebra (Fig. 1b). We attempted to access the thoracic duct by transabdominal puncture of the cisterna chyli. We punctured the cisterna chyli with a 21-gauge Chiba needle under fluoroscopy but could not advance the guide wire into the thoracic duct (Fig. 1c). Therefore, we abandoned the TDE and performed needle disruption of the cisterna chyli, completing the procedure.
Fig. 1.
First lymphangiography revealing the cisterna chyli (a) and a concentration of Lipiodol (b) on the left side of the thoracic vertebra. The cisterna chyli is punctured with a 21-gauge Chiba needle (c); however, it is not possible to advance the guide wire into the thoracic duct.
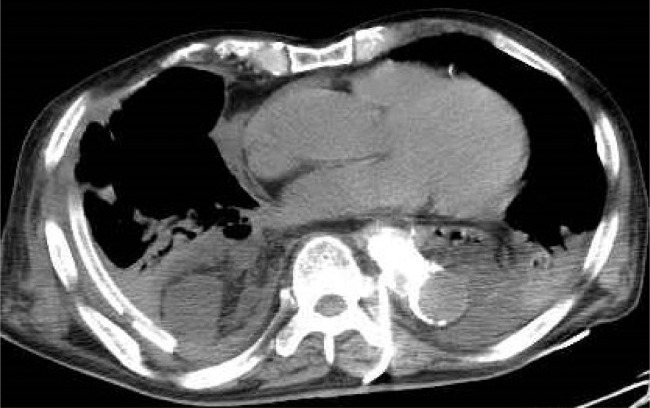
Pleural effusion decreased after the procedure, but chyle pleural effusion increased again considerably after increasing enteral nutrition. Pleural effusion reached an average of 2000 mL per day, and lymphangiography was performed again at POD 50. On the second lymphangiography, the cisterna chyli was not visible and may have been occluded by the previous procedure. However, an exceptionally thin lymphatic pathway ascending to the left side of the vertebral body was visualized, through which extravasation was confirmed at the same location as in the previous lymphangiography (Fig. 2a). The thoracic duct distal to the extravasation was not visualized. Therefore, it was impossible to access the thoracic duct through the cisterna chyli transabdominally or retrogradely; consequently, we decided to perform sclerotherapy by directly puncturing the lymphatic leakage site. We placed the patient in the prone position, and percutaneously punctured the lymphatic leakage site with a 19-gauge coaxial needle from the left margin of the eighth thoracic vertebra (Fig. 2b), and inserted an 8.5-French drainage catheter (Dawson-Mueller Multipurpose Drainage Catheter; Cook Japan Inc, Tokyo, Japan). By injecting iodine contrast agent from the drain, a semiencapsulated cavity around the lymphatic leakage site was visualized and confirmed to communicate with the thoracic cavity (Fig. 3a, b). We dissolved 8.4 mg of OK-432 (Picibanil; Chugai Pharmaceutical, Tokyo, Japan) in 12 mL of saline, which was injected via the drainage catheter; the drainage catheter was then clamped. Computed tomography after second lymphangiography confirmed the drainage catheter in the lymphatic leakage site (Fig. 4). We then administered three additional doses of OK-432 (14 mg) via the drainage catheter at the bedside. The chylothorax had almost disappeared after approximately 1 week. Even after the restart of enteral nutrition, the chylothorax did not recur and the patient was discharged.
Fig. 2.
On the second lymphangiography, the lymphatic leakage site is confirmed at the same location as the previous examination (a). The cisterna chyli is not visible and may have been be occluded by the previous procedure. The patient is placed in the prone position, and the lymphatic leakage site is percutaneously punctured with a 19-gauge coaxial needle from the left margin of the eighth thoracic vertebra (b). Following this, a drainage catheter is inserted.
Fig. 3.
Injection of iodine contrast media through the drainage catheter reveals a partially encapsulated fluid collection (a), which is connected to the thoracic cavity (b).
Fig. 4.
Computed tomography after second lymphangiography showing a drainage catheter in the lymphatic leakage site.
Discussion
We have presented a case in which percutaneous TDE for refractory chylothorax failed but was successfully treated with targeted sclerotherapy by direct puncture of the lymphatic leakage site. Percutaneous TDE has recently become a minimally invasive alternative to surgical thoracic duct ligation because of its effectiveness and feasibility [3,4].
To perform TDE, it is necessary to access the thoracic duct. Percutaneous transabdominal antegrade access is popular and frequently selected [3,4]. The retrograde transvenous approach is another option, while the translumbar approach is rarely used [2,7,8]. However, the thoracic duct cannulation success rate is reported to be 57%-67% [4,5,9], which is not high enough, even for operators in high-volume centers. In a general hospital, the chances of encountering a chylothorax requiring intervention may not be higher than that of in a high-volume center, and it may be difficult to become familiar with the procedure, and therefore difficult to master the technique sufficiently. Transabdominal TDE is a minimally invasive treatment with few complications, but the thoracic duct is often inaccessible [3], [4], [5], [9]. This may be the most troublesome part of the procedure. There are several possible factors why the thoracic duct may not be accessible. First, anatomical factors: the lymphatic vessels have many anatomical variations. The cisterna chyli may not be visible on lymphography and may be thin. The thoracic duct may be duplicated or plexiform, or located behind the aorta [6]. Second, patient factors such as difficulty in holding breath or severe obesity are also important. Third, operator experience: it is difficult to gain experience, as previously mentioned [4]. If the thoracic duct is not accessible via the transabdominal approach, alternatives include a translumbar approach, a retrograde approach from the left venous angle, or a direct puncture of the thoracic duct under ultrasound guidance [2,7,8].
In our case, the thoracic duct distal to the extravasation was not visualized on either lymphangiography. Since the thoracic duct was ligated and resected at the time of surgery for esophageal cancer, the retrograde approach to the thoracic duct was difficult. Needle disruption of the thoracic duct or cisterna chyli is another common option in cases of failed thoracic duct cannulation [3,4]. This is a technique that uses a puncture needle to crush the thoracic duct or cisterna chyli, referred to by Itkin et al. as “the last resort” [4]. The rate at which chylothorax improves with needle disruption of the thoracic duct or cisterna chyli has been reported as 34%-72% [4,5,9], but this too low. Cope et al. reported that 15 of 58 chylothorax patients were not cured by TDE or needle disruption of the thoracic duct or cisterna chyli. Seven of these were improved by surgical ligation, but 8 died either because of inoperability or due to surgical treatment complications [1]. One possible cause of needle disruption failure may be the anatomical variation of the lymphatic vessels. Furthermore, since there are inflows of intestinal lymph and hepatic lymph into the cisterna chyli, which cannot be visualized by intranodal lymphangiography [2], it is presumed that there might be some cases in which it is difficult to completely interrupt the lymphatic flow by needle disruption. On the second lymphangiography in our case, the cisterna chyli was not visible and may have been occluded by the first lymphangiography and needle disruption. However, a thin lymphatic pathway ascending to the left side of the vertebral body was visualized, and connecting extravasation was confirmed. Development of the collaterals of the lymphatic vessels may have caused chylothorax recurrence after needle disruption.
If both TDE and needle disruption are unsuccessful, what are the alternatives to surgical treatment? Several reports have described the successful use of OK-432 for the treatment of chylothorax by pleurodesis. Huan et al. reported that chylothorax after pneumothorax surgery persisted for 30 days without responding to conservative treatment but resolved immediately after pleurodesis using OK-432 [9]. Matsukuma et al. reported that the congenital chylothorax of the newborn infants whose conservative treatment was ineffective resolved after administration of OK-432 from the chest tube [10]. OK-432 stimulates an inflammatory response that causes local inflammation, resulting in regression of the lesion [11]. The advantages of OK-432 over other sclerosants, such as ethanol and anticancer drugs, in the treatment of lymphangioma are low tissue damage and low peri-lesional fibrosis. This allows for subsequent surgery in case of failure [12]. However, treatment with pleurodesis alone is often ineffective for high-output chylothorax [13]. We believed that rather than nonselectively administering OK-432 into the thoracic cavity, which has a large space for adhesion, it is more efficient to inject it into the lymphatic leakage site itself. In our case, the lymphatic leakage site was connected to the thoracic cavity after forming a cyst-like fluid collection. In other words, the lymphatic leakage site had an incomplete lymphocele-like morphology; therefore, injection of a sclerosant into this cavity could close the cyst wall and complete the lymphocele, thereby stopping lymphatic leakage into the chest cavity.
In conclusion, targeted sclerotherapy using OK-432 by direct puncture of the lymphatic leakage site may be an alternative treatment option for high-output chylothorax when TDE or needle disruption is ineffective.
Footnotes
Competing interest: None.
References
- 1.Cope C. Vol. 10. Lippincott Williams and Wilkins; 2004. Management of chylothorax via percutaneous embolization; pp. 311–314. (Current opinion in pulmonary medicine). [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto T., Yamagami T., Kato T., Hirota T., Yoshimatsu R., Masunami T. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. 2009;82(976):286–290. doi: 10.1259/bjr/64849421. [DOI] [PubMed] [Google Scholar]
- 3.Inoue M., Nakatsuka S., Yashiro H., Tamura M., Suyama Y., Tsukada J. Lymphatic intervention for various types of lymphorrhea: access and treatment. Radiographics. 2016;36(7):2199–2211. doi: 10.1148/rg.2016160053. [DOI] [PubMed] [Google Scholar]
- 4.Itkin M., Kucharczuk J.C., Kwak A., Trerotola S.O., Kaiser L.R. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139(3):584–590. doi: 10.1016/j.jtcvs.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Boffa D.J., Sands M.J., Rice T.W., Murthy S.C., Mason D.P., Geisinger M.A. A critical evaluation of a percutaneous diagnostic and treatment strategy for chylothorax after thoracic surgery. Eur J Cardiothorac Surg. 2008;33(3):435–439. doi: 10.1016/j.ejcts.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Johnson O.W. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016;26(8):2482–2493. doi: 10.1007/s00330-015-4112-6. [DOI] [PubMed] [Google Scholar]
- 7.Pamarthi V., Stecker M.S., Schenker M.P., Baum R.A., Killoran T.P., Han A.S. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol. 2014;25(9):1398–1404. doi: 10.1016/j.jvir.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Guevara C.J., Rialon K.L., Ramaswamy R.S., Kim S.K., Darcy M.D. US–guided, direct puncture retrograde thoracic duct access, lymphangiography, and embolization: feasibility and efficacy. J Vasc Interv Radiol. 2016;27(12):1890–1896. doi: 10.1016/j.jvir.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Huang S.Y., Yeh C.M., Chou C.M., Chen H.C. Chylothorax after left side pneumothorax surgery managed by OK-432 pleurodesis: an effective alternative. J Chinese Med Assoc. 2014;77(12):653–655. doi: 10.1016/j.jcma.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Matsukuma E., Aoki Y., Sakai M., Kawamoto N., Watanabe H., Iwagaki S. Treatment with OK-432 for persistent congenital chylothorax in newborn infants resistant to octreotide. J Pediatr Surg. 2009;44(3):e37–e39. doi: 10.1016/j.jpedsurg.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Ogita S., Tsuto T., Nakamura K., Deguchi E., Tokiwa K., Iwai N. OK-432 therapy for lymphangioma in children: why and how does it work? J Pediatr Surg. 1996;31(4):477–480. doi: 10.1016/S0022-3468(96)90478-9. [DOI] [PubMed] [Google Scholar]
- 12.Sichel J.-Y., Udassin R., Gozal D., Koplewitz B.Z., Dano I., Eliashar R. OK-432 therapy for cervical lymphangioma. Laryngoscope. 2004;114(10):1805–1809. doi: 10.1097/00005537-200410000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Cope C., Kaiser L.R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13(11):1139–1148. doi: 10.1016/S1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]