Abstract
An endo-1,4-β-xylanase, XynA, from Thermomyces lanuginosus VAPS-24, was purified to homogeneity and exhibited a molecular mass of approximately 20 kDa. The protein sequence of XynA was found to be similar to those of other Thermomyces lanuginosus derived xylanases and, as a result, could be used as a model enzyme for understanding the protein structure–activity relationship and facilitating protein engineering to design enzyme variants with desirable properties. Therefore, this xylanase will be an attractive candidate for applications in the biofuel and fine chemical industries for the degradation of xylans in steam pre-treated biomass.
Keywords: Endo-1,4-β-xylanase; Tryptic mapping; Thermomyces lanuginosus; Protein sequence; Xylanase structure
The application of microbial enzymes in industry has increased due to the global demand for green technology [1, 2]. Thermomyces lanuginosus xylanases are excellent examples of microbial enzymes that have found widespread applications in industry [3]. The thermophilic fungus, T. lanuginosus, is well-known to produce excellent levels of cellulase-free thermostable xylanases [3]. Although generally categorized into numerous glycoside hydrolase (GH) families (5, 7, 8, 10, 11, 30, 43, 62 and 98), most industrially relevant xylanases generally belong to GH families 10 and 11 [4]. The structure of the xylanase from T. lanuginosus has been reported to closely resemble those of other GH11 xylanases [5]. GH11 enzymes have been found to have a β-jelly-roll fold and an extended N-terminal region consisting of 11 amino acids. A number of protein engineering approaches have been employed to improve the thermostability of enzymes [6].
A crude xylanase (XynA) concentrate sample from T. lanuginosus VAPS-24 (Gene Accession Number: KU366607) was kindly provided by the Enzyme Technology and Protein Bioinformatics Laboratory, Department of Microbiology, Maharshi Dayanand University, Rohtak, Haryana, India. Details regarding the isolation and identification of the VAPS24 strain, and production of the xylanase, XynA, are detailed in Kumar et al. 2017 [7]. XynA was then purified via ultra-filtration using Amicon® Ultra filters as described previously [7]. The specific activity of the purified XynA was 49.96 IU/mg protein, where one IU was defined as the release of 1 µmol of reducing sugar xylose equivalent from wheat-flour arabinoxylan per min at 50 °C. An ultra-filtration purified XynA sample was run on 12% SDS-PAGE as described previously [8] and the corresponding protein band was digested with trypsin to identify XynA xylanase as per the method proposed by Shevchenko and co-workers [9]. Digestion of the XynA enzyme was performed using 5–50 µl of trypsin (10 ng/µl) (depending on the size of the protein band) overnight at 37 °C. Digests were identified using a Dionex Ultimate 3000 RSLC system attached to an AB Sciex6600 TipleTOF mass spectrometer after re-suspension in 20 µl of 2% (v/v) acetonitrile/0.2% (v/v) formic acid solution. Initially, de-salting of the peptides was performed, followed by separation on an Acclain PepMap C18 RSLC column. Elution of the peptide was performed using a 4–60% gradient of B (A: 0.1% (v/v) formic acid; B: 80% (v/v) acetonitrile/0.1% (v/v) formic acid) at a flow-rate of 8 μl/min over 15 min. An electrospray voltage of 5.5 kV was standardized for the emitter.
Peaks (Bioinformatics Solutions) were used for evaluation of the obtained MS/MS spectra with a conventional database comprising sequences of Eurotiales (Uniprot Swissprot), in addition to several sequences from commonly contaminating proteins as described previously [10]. The identification of proteins was permitted if they could be formed at larger than 99.0% probability and contained a minimum of 1 identified peptide (at 95% and greater). The peptide and proteins false discovery rate (FDR) cut-offs were set to 0.1 and 1%, respectively.
The T. lanuginosus VAPS-24 xylanase, XynA, could be purified to a 98.84% yield with a purification fold of 3.92 and a specific activity of 49.96 IU/mg on wheat-flour arabinoxylan (see Table 1). The ultrafiltration fraction after purification constituted the enzyme solution used in all subsequent enzyme assays. SDS-PAGE analysis of the purified enzyme displayed a single band with an estimated molecular mass of 20 kDa (Fig. 1). The single band of the XynA enzyme on the SDS-PAGE was then excised and subjected to tryptic digestion and evaluated using a Dionex Ultimate 3000 RSLC system attached to an AB Sciex6600 TipleTOF mass spectrometer.
Table 1.
Protein purification table for the purification of T. lanuginosus VAPS-24 xylanase, XynA
| Fraction | Volume (ml) | Total protein (mg) | Activity (U/ml) | Total activity (U) | Specific activity (U/mg) | Fold purification | Yield (%) |
|---|---|---|---|---|---|---|---|
| Crude | 12 | 16.65 | 17.69 | 212.28 | 12.73 | 1 | 100 |
| Ultra-filtrate | 14 | 4.24 | 14.99 | 209.83 | 49.96 | 3.92 | 98.84 |
The Ultra-filtrate fraction was composed of the > 10 kDa and < 30 kDa cut-off fractions
Fig. 1.
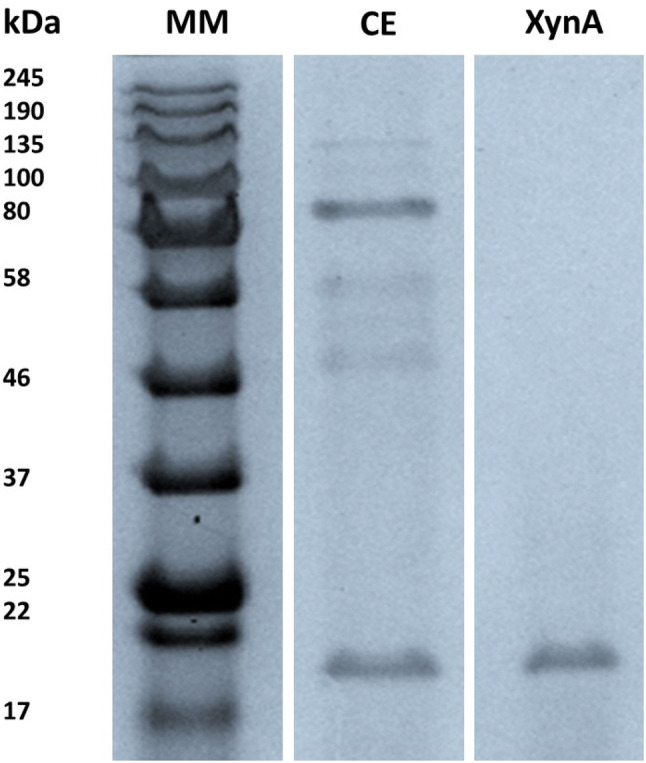
SDS-PAGE analysis of the ultra-filtration purified XynA from T. lanuginosus VAPS-24. Lane 1: (MM) molecular weight standard, lane 2: (CE) crude extract of XynA and lane 3: XynA purified by ultra-filtration
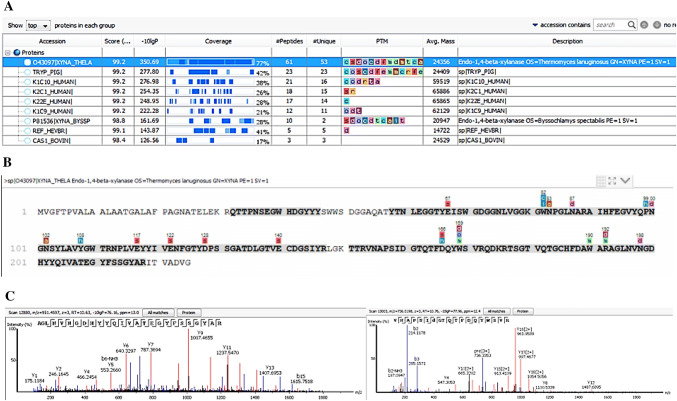
Peaks (Bioinformatics Solutions) database search on the produced peptides produced matches against a xylanase from the T. lanuginosus SS-8 strain. Amino acid sequence coverage showed that the xylanase, XynA, produced by the T. lanuginosus VAPS-24 strain compares well with the xylanase from strain T. lanuginosus SS-8 (UniProt ID O43097) over a query coverage of 77% (Fig. 2a, b). XynA showed significant matches with two peptides, AGLNVNGDHYYQIVATEGYFSSGYAR (2853.3115 MH+) and VNAPSIDGTQTFDQYWSVR (2184.035 MH+), identified as GH family 11 putative domains (Fig. 2c). The two peptides had ion score values of 76.16 and 77.96, respectively (Fig. 2c). The ion score is defined as − 10 log (P), where P is the probability that an observed match is a random event and individual ions scores of > 34 indicate identity or extensive homology (P < 0.05) [11]. However, seventeen post-translational modifications (PTMs) were identified on some amino acids in the XynA sequence which were not present on the SS-8 homolog, with three of these modifications occurring in the two GH family 11 putative domains and one of these PTMs (sulfonation) occurring on one of the two glutamic acid residues (residue E-117, Fig. 2B) of xylanases reported to be involved in enzymatic activity as noted by Wakarchuk and co-workers [12]. Upon purification from the growth media by ultra-filtration, the molecular mass of the T. lanuginosus VAPS-24 xylanase, XynA, was determined to be 20 kDa using SDS-PAGE (Fig. 1). The molecular weight of the xylanase was similar to that of XynSS8 from a T. lanuginosus SS-8 strain obtained by nanoLC ESI MS (21.3 kDa) [13]. The amino acid sequence of XynA was then determined using tryptic mapping. Tryptic mapping illustrated that XynA is highly similar to XynSS8 from a T. lanuginosus SS-8 strain, however, numerous PTMs were present on XynA which seemed to be absent on XynSS8 (Fig. 2). Similar to our work, Silva and co-workers reported differences in MS/MS fragmentation of their Emericella nidulans xylanase compared to that reported for other Emericella nidulans isolates—this was thought to be as a result of different isoforms of the enzyme from this fungal species, as the enzyme was produced from the same fungus, but grown under different conditions to those reported previously [14]. As a result, there were differences in PTMs and/or amino acid sequences of these isoforms. From this data, we confirmed that XynA from strain VAPS24 is a xylanase belonging to GH family 11 and an isoform of the xylanase produced by other strains of Thermomyces lanuginosus species, and as a result, the enzyme can be used as a representative model of Thermomyces species derived xylanases. Having this model representative protein structure can aid in numerous studies, such as docking and molecular dynamics, to better understand enzyme characteristics (i.e. catalytic mechanism or inhibition) and in facilitating protein engineering approaches to design variants with desirable properties (i.e. improved catalytic efficiency or thermos-stability).
Fig. 2.
Amino acid sequene identification of T. lanuginosus VAPS-24 XynA by tryptic mapping and MS/MS. a Protein match hit for T. lanuginosus VAPS-24 XynA against SS-8 xylanase in Peaks database showing 77% identity, b protein coverage view of T. lanuginosus VAPS-24 XynA against T. lanuginosus SS-8 xylanase sequence with matched peptides shown in bold (shaded grey), while PTMs are shown above amino acid residues as coloured letters and c MS/MS spectra for the two putative GH11 family domains found in T. lanuginosus VAPS-24 XynA
Acknowledgements
We would like to thank Dr. Stoyan Stoychev (CSIR, South Africa) for identifying the xylanase used in this study using tryptic mapping/MS. The authors are also grateful for the financial support received for this study from the National Research Foundation (NRF) of South Africa and Rhodes University (Sandisa Imbewu). Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s), and therefore the NRF does not accept liability in regard thereto. VK is thankful to UGC New Delhi, India, for awarding him the Junior Research Fellowship [F.17-63/2008 (SA-I)]. PS acknowledges the support from the Department of Biotechnology, Government of India (Grant No. BT/PR27437/BCE/8/1433/2018), SERB, Department of Science and Technology, Government of India (DST Fast Track Grant No. SR/FT/LS-31/2012) and the infrastructural support from Department of Science and Technology, Government of India through a FIST Grant (Grant No. 1196 SR/FST/LS-I/2017/4).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pratyoosh Shukla, Email: pratyoosh.shukla@gmail.com.
Brett I. Pletschke, Email: b.pletschke@ru.ac.za
References
- 1.de Oliveira Gorgulho Silva C, Filho EXF. A review of holocellulase production using pretreated lignocellulosic substrates. BioEnergy Res. 2017;10:592–602. doi: 10.1007/s12155-017-9815-x. [DOI] [Google Scholar]
- 2.Patel SK, Gupta RK, Kumar V, Mardina P, Lestari R, Kalia VC, Choi MS, Lee JK. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Madlala AM, Prior BA. Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol Rev. 2003;27:3–16. doi: 10.1016/S0168-6445(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 4.Meng DD, Ying Y, Chen XH, Lu M, Ning K, Wang LS, Li FL. Distinct roles for carbohydrate-binding modules of glycoside hydrolase 10 (GH10) and GH11 xylanases from Caldicellulosiruptor sp. strain F32 in thermostability and catalytic efficiency. Appl Environ Microbiol. 2015;81:2006–2014. doi: 10.1128/AEM.03677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khucharoenphaisan K, Tokuyama S, Kitpreechavanich V. Purification and characterization of a high-thermostable β-xylanase from newly isolated Thermomyces lanuginosus THKU-49. Mycoscience. 2010;51:405–410. doi: 10.1007/S10267-010-0054-7. [DOI] [Google Scholar]
- 6.Singh KR, Lee JK, Selvaraj C, Singh R, Li J, Kim SY, Kalia VC. Protein engineering approaches in the post-genomic era. Curr Protein Pep Sci. 2018;19:5–15. doi: 10.2174/1389203718666161117114243. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Chhabra D, Shukla P. Xylanase production from Thermomyces lanuginosus VAPS-24 using low cost agro-industrial residues via hybrid optimization tools and its potential use for saccharification. Bioresour Technol. 2017;243:1009–1019. doi: 10.1016/j.biortech.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli UK. Cleavage of structura l proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Xin L, Shan B, Chen W, Xie M, Yuen D, Zhang W, Zhang Z, Lajoie GA, Ma B. PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteomics. 2012;11:M111.010587. doi: 10.1074/mcp.M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vafiadi C, Christakopoulos P, Topakas E. Purification, characterization and mass spectrometric identification of two thermophilic xylanases from Sporotrichum thermophile. Process Biochem. 2010;45:419–424. doi: 10.1016/j.procbio.2009.10.009. [DOI] [Google Scholar]
- 12.Wakarchuk WW, Campbell RL, Sung WL, Davoodi J, Yaguchi M. Mutational and crystallographic analyses of the active site residues of the Bacillus circulans xylanase. Protein Sci. 1994;3:467–475. doi: 10.1002/pro.5560030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrivastava S, Shukla P, Deepalakshmi PD, Mukhopadhyay K. Characterization, cloning and functional expression of novel xylanase from Thermomyces lanuginosus SS-8 isolated from self-heating plant wreckage material. World J Microbiol Biotechnol. 2013;29:2407–2415. doi: 10.1007/s11274-013-1409-y. [DOI] [PubMed] [Google Scholar]
- 14.Silva CDOG, Aquino EN, Ricart CAO, Midorikawa GEO, Miller RNG. GH11 xylanase from Emericella nidulans with low sensitivity to inhibition by ethanol and lignocellulose-derived phenolic compounds. FEMS Microbiol Lett. 2015;362:1–8. doi: 10.1093/femsle/fnv094. [DOI] [PubMed] [Google Scholar]