Abstract
Herbicides are important tools for weed control in modern agriculture. In the search for potential herbicidal natural products from fungal species, harzianum A and B were identified from the biofertilizer fungus, Trichoderma brevicompactum. In the phytotoxicity assays on the dicot species Brassica chinensis, harzianum A and B reduced both shoot and root lengths at low concentrations and inhibited the seed germination at 2 μg mL−1. In addition, harzianum A and B also exhibited phytotoxicity against monocots, Oryza sativa L. cv. Nipponbare and Echinochloa crusgalli L. Beauv.. Compared with a common herbicide, 2,4-dichlorophenoxyacetic acid, harzianum A and B performed similar activity in a pot assay, and were more effective in post-emergence than pre-emergence conditions. Harzianum A and B have potential as efficient herbicide for controlling important dicotyledon and monocotyledon weeds at low concentrations. They can be sprayed in liquid form in both pre- and post-emergence conditions. Our results confirmed the importance of these molecules for the development of new herbicides.
Keywords: Trichoderma brevicompactum, Harzianum, Brassica chinensis, Echinochloa crusgalli L. Beauv., Phytotoxicity
Introduction
Weeds cause significant negative impact on crop productivity and consequent economic losses due to their competition with crops for soil, water and nutrients (Charudattan 2001; Rajcan and Swanton 2001; Tilman et al. 2002). Cultural, mechanical, chemical and biological methods are the most prominent approaches used for weed management. Among these, chemical herbicides remain the most effective method to date, because of their ease of application and greater accessibility for farmers; the dependence on these chemicals is increasing in recent decades, especially in emerging countries (Araniti et al. 2019). However, the intensive and indiscriminate application of these chemicals, especially those that are very persistent in agricultural end-products and not easily biodegradable, is one of the major causes of environmental pollution and human health threat. In addition, the rapid evolution of weed resistance calls for urgent need to develop new herbicides with high biological activity, as well as low or no toxicity, compared to the commercial herbicides (Araniti et al. 2019; Dayan and Duke 2014). Microbial natural products have long been playing important roles in medicine and agriculture; more than half of all small molecule drugs approved for use are derived from natural products, a large portion of which comes from microorganisms (Newman and Cragg 2020). However, until now, natural products have much less contribution in herbicide development compared to other pesticides (Copping and Duke 2007; Huter 2011), although many natural products and derivatives have been reported with herbicidal activity (Araniti et al. 2015, 2019; Duke et al. 2002; Mitchell et al. 2001; Zhou et al. 2019).
Fungi of the genus Trichoderma are commonly found in nature, including soil, water, household dust, foods, and decaying organic material, and are widely used as biological control agents for fungal phytopathogens and for the production of enzymes (Harman et al. 2004). Trichoderma spp. produce a number of natural products belonging to several classes of chemicals with different biological activities, such as antibiotic peptides and peptaibols, volatile pyrones and lactones, plant growth factors, siderophores, etc. (Benitez et al. 2004; Vinale et al. 2008). They also produce the mycotoxins, trichothecenes (Corley et al. 1994; Nielsen et al. 2005). Trichothecenes are a group of sesquiterpenoid-derived natural products with various patterns of oxygenations and esterifications of a core tricyclic structure with an epoxide function (Cardoza et al. 2011).
Trichothecenes are generally phytotoxic. They can cause necrosis, chlorosis and mortality to plants, enabling them to mediate a wide variety of plant diseases, including wilts, stalk rot, root rot and leaf rot in many important crop and ornamental plants, by binding to 60s ribosomes and interrupting protein synthesis in eukaryotic cells, which makes them potential herbicides (Bin-Umer et al. 2011; McCormick et al. 2011; Nishiuchi et al. 2006; Tijerino et al. 2011; Wang et al. 2006). However, this potential was hindered by their toxicity to humans and livestock e.g., vomiting, alimentary hemorrhaging, and dermatitis (McCormick et al. 2011). Nevertheless, this toxicity is highly dependent on the structure. Trichothecenes can be divided into four types according to functional groups. Type A has a functional group other than a keto group at C-8. This is the largest group and includes toxins like T-2 toxin. Type B trichothecenes have a keto group at C-8 and include the most widespread trichothecene deoxynivalenol. Type C has a second epoxide ring at C-7,8 or C-9,10 and toxins from Type D contain a macrocyclic ring between C-4 and C-15 with two ester-linkages. Simple trichothecenes including type A and type B are generally less toxic than macrocyclic trichothecenes (Abbas et al. 2013). In addition, peracetylation of type B trichothecenes and de-epoxidation of type A trichothecenes both substantially reduced mammalian toxicity with little effect on phytotoxicity (Abbas et al. 2013). Thus, type A and type B trichothecenes and their derivatives can be potential bioherbicide candidates as long as they possess high phytotoxicity and low mammalian toxicity. One of the type A trichothecenes, harzianum A (HA) consists of the core trichothecene structure (12,13-epoxytrichothec-9-ene, EPT) with a linear polyketide-derived substituent (octa-2,4,6-trienedioyl) esterified to an oxygen at carbon atom, and was first isolated from Trichoderma harzianum and then T. brevicompactum (Cardoza et al. 2019; Corley et al. 1994; Nielsen et al. 2005). Its cis–trans isomer, harzianum B (HB) was later identified from Hypocrea (teleomorph of Trichoderma) sp. F000527 (Jin et al. 2007). Both compounds were tested for cytotoxicity against HeLa, MCF-7, and HT1080 cell lines (Jin et al. 2007), but their phytoxicity was unknown.
In our search for potential herbicidal leading compounds from fungal strains, we identified HA and HB (1–2) from the biofertilizer fungus, T. brevicompactum (CGMCC19618). The herbicidal efficacy of HA and HB (1–2) was assayed on the dicot Brassica chinensis, monocot Oryza sativa L. cv. Nipponbare, and monocot weeds (Setaria viridis L. Beauv. and Echinochloa crusgalli L. Beauv.). The common commercial herbicide, 2,4-dichlorophenoxyacetic acid (2,4-D) was used as positive control. The results revealed the potential of HA and HB as herbicide.
Materials and methods
Screening of herbicidal compounds using B. chinensis
The dicot B. chinensis was selected for phytotoxicity testing to screen the fungal strains that produce herbicidal compounds. Briefly, 5 high-quality seeds were transferred to each well of the 96-well plate, with 50 μL water containing crude extract or purified compounds in the concentration range 0.2–2 mg mL−1 or 5–25 nM. Cultures were incubated at 25 °C under light/dark cycle of 16 h/8 h in a growth chamber. Each condition was triplicated and repeated at least three times. The germination rate was examined for visual signs of phytotoxicity. 2,4-Dichlorophenoxyacetic acid (2,4-D) was used as positive control.
Isolation and identification of harzianum A and B
The fungal strain used in this study was T. brevicompactum CGMCC19618. Preparation of fermentation extracts, analysis by liquid chromatography-mass spectrometry (LC–MS). HPLC-HRESIMS and MS–MS spectra were acquired on an Agilent 1290 Infinity II HPLC coupled with an Agilent QTOF 6530 instrument. 1H and 13C NMR spectra were obtained on an Agilent DD2 spectrometer at 600 MHz for 1H NMR and 150 MHz for 13C NMR. Two-liter fermentation broth was extracted using ethyl acetate. The extracts were first subjected to silica gel (25 g) column chromatography and eluted with a gradient of chloroform/methanol to yield five fractions (Fraction A, v/v 100:0, 250 mL; Fraction B, v/v 99:1, 250 mL; Fraction C, v/v 98:2, 250 mL; Fraction D, v/v 95:5, 250 mL; Fraction E, v/v 90:10, 250 mL). Each of these fractions was analyzed by HPLC–MS. Fractions containing the target compounds were subsequently purified by semi-preparative HPLC on an Agilent Eclipse XDB-C18 reversed-phase column (5 mm, 9.4 mm × 250 mm) using an Agilent 1260 Infinity II system.
Harzianum A (1): 1H NMR (400 MHz, methanol-d4) δ 7.90 (1H, dd, J = 15.1, 11.6 Hz, H-4′), 7.39 (1H, dd, J = 15.3, 11.3 Hz, H-6′), 6.78 (1H, t, J = 11.4 Hz, H-3′), 6.71 (1H, dd, J = 15.0, 11.3 Hz, H-5′), 6.07 (1H, d, J = 15.3 Hz, H-7′), 5.82 (1H, d, J = 11.4 Hz, H-2′), 5.70 (1H, dd, J = 7.9, 3.5 Hz, H-4), 5.38 (1H, d, J = 5.3 Hz, H-10), 3.78 (1H, d, J = 5.2 Hz, H-2), 3.70 (1H, d, J = 5.3 Hz, H-11), 3.11 (1H, d, J = 3.9 Hz, H-13α), 2.91 (1H, d, J = 3.9 Hz, H-11β), 2.57 (1H, dd, J = 15.4, 7.8 Hz, H-3α), 2.04 (1H, m, H-3β), 2.00 (2H, m, H-8), 1.94 (1H, m, H-7β), 1.71 (3H, s, H-16), 1.48 (1H, m, H-7α), 0.97 (3H, s, H-15), 0.73 (3H, s, H-14); 13C NMR (100 MHz, methanol-d4) δ 169.8 (C-8′), 167.1 (C-1′), 145.0 (C-6′), 144.3 (C-3′), 141.2 (C-9), 139.4 (C-5′), 136.9 (C-4′), 126.1 (C-7′), 121.5 (C-2′), 119.8 (C-10), 80.4 (C-2), 76.6 (C-4), 72.0 (C-11), 66.6 (C-12), 50.5 (C-5), 48.7 (C-13), 41.5 (C-6), 37.6 (C-3), 28.9 (C-8), 25.5 (C-7), 23.3 (C-16), 16.3 (C-15), 6.5 (C-14); (+)-HRESIMS m/z 401.2003 [M+H]+ (calcd. for C23H29O6, 401.1964).
Harzianum B (2): 1H NMR (400 MHz, methanol-d4) δ 7.40 (1H, dd, J = 15.4, 10.0 Hz, H-6′), 7.34 (1H, dd, J = 15.4, 10.0 Hz, H-3′), 6.79 (2H, d, J = 10.0 Hz, H-4′ and H-5′), 6.11 (1H, d, J = 15.4 Hz, H-7′), 6.07 (1H, d, J = 15.4 Hz, H-2′), 5.69 (1H, dd, J = 7.9, 3.5 Hz, H-4), 5.38 (1H, d, J = 5.3 Hz, H-10), 3.75 (1H, d, J = 5.2 Hz, H-2), 3.70 (1H, d, J = 5.3 Hz, H-11), 3.10 (1H, d, J = 3.9 Hz, H-13α), 2.90 (1H, d, J = 3.9 Hz, H-11β), 2.55 (1H, dd, J = 15.4, 7.8 Hz, H-3α), 2.04 (1H, m, H-3β), 2.00 (2H, m, H-8), 1.94 (1H, m, H-7β), 1.71 (3H, s, H-16), 1.48 (1H, m, H-7α), 0.96 (3H, s, H-15), 0.72 (3H, s, H-14); 13C NMR (100 MHz, methanol-d4) δ 169.8 (C-8′), 167.7 (C-1′), 144.9 (C-6′), 144.5 (C-3′), 141.2 (C-9), 138.8 (C-5′), 138.2 (C-4′), 126.2 (C-7′), 125.2 (C-2′), 119.8 (C-10), 80.5 (C-2), 76.8 (C-4), 72.0 (C-11), 66.6 (C-12), 50.5 (C-5), 48.6 (C-13), 41.6 (C-6), 37.4 (C-3), 28.9 (C-8), 25.5 (C-7), 23.3 (C-16), 16.3 (C-15), 6.3 (C-14); (+)-HRESIMS m/z 401.2003 [M+H]+ (calcd. for C23H29O6, 401.1964).
Phytotoxicity assays of harzianum A and B
The seeds of B. chinensis, O. sativa L. cv. Nipponbare, S. viridis L. Beauv. or E. crusgalli L. Beauv. were surface sterilized in 0.5% NaOCl (3 min), and then rinsed six times in distilled water. 1 μL of HA and HB (1–2) was applied to five seeds at doses from 1 ng to 100 μg per seed (Vinale et al. 2009). Equal volume of methanol was added to the control seeds. The treated seeds were sown in Petri dishes (100 × 150 mm) containing agar medium (0.8% w/v). Plates were placed vertically in the growth chamber (25 ± 2 °C temperature, 55% HR, and 16 h/8 h light/dark circle) to promote geotropic root growth. After treatment, seedlings were collected and separated into shoot and root. Growth inhibition was measured as reduced root or shoot length relative to the negative control (1 μL methanol). 2,4-D was used as positive control in all experiments. Each treatment was repeated two times.
Pot assays of harzianum A and B
The herbicidal activity of HA and HB (1–2) mixture was tested against the dicot B. chinensis in pot assays with 2,4-D as positive control. The test compounds were dissolved in 10 μL methanol and diluted with 5 mL water to the required concentrations, and applied to pot-grown plants in a greenhouse. Plastic pots with a diameter of 9.5 cm were filled with garden soil to a depth of 8 cm. Approximately 15 seeds of the tested weeds were sown in the soil at a depth of 1–2 cm and grown at a temperature of 25 °C in a greenhouse. The air relative humidity was 50%. For pre-emergence assays, 5 mL of the HA and HB solution was sprayed to the soil. For post-emergence assays, the B. chinensis was treated at the two-leaf stage with designated dosages with three replicates. The solvent (10 μL methanol in 5 mL water) was used as negative control. Herbicidal activity was evaluated visually 6 days post treatment.
Results
Identification of the herbicidal compounds, harzianum A and B
In the search for herbicidal compounds, crude extracts of fungal strains isolated from soil were tested using B. chinensis. One crude extract completely inhibited germination at a concentration of 0.2 mg mL−1. This extract was from a fungal strain that belongs to the species T. brevicompactum.
The herbicidal compounds were identified from the crude extract by bioactivity-oriented isolation. Filtered culture broth of T. harzianum was extracted exhaustively with ethyl acetate. The reddish-brown residue recovered was subjected to silica gel with gradient elution. The phytotoxic fraction was purified by semi-preparative HPLC to yield a mixture of two known compounds HA (1) and HB (2), which are geometrical isomers with an estimated ratio of 3:1 according to the integration of 1H NMR spectrum. 1 and 2 belong to type A trichothecene and consist of the core trichothecene structure (12,13-epoxytrichothec-9-ene, EPT) connected with a linear polyketide-derived substituent (octa-2,4,6-trienedioyl) via an ester bond at C-4. Their HRMS and NMR spectroscopic data are consistent with reported values (see “Experimental” section) (Jin et al. 2007).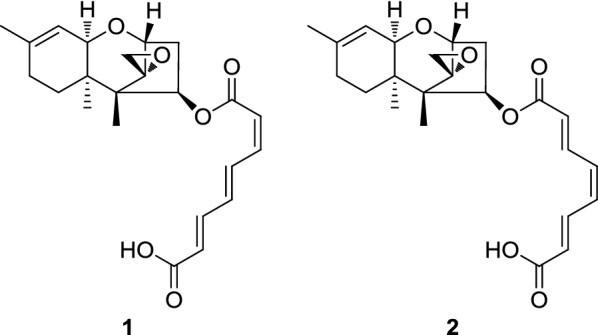
Phytotoxicity of harzianum A and B to B. chinensis
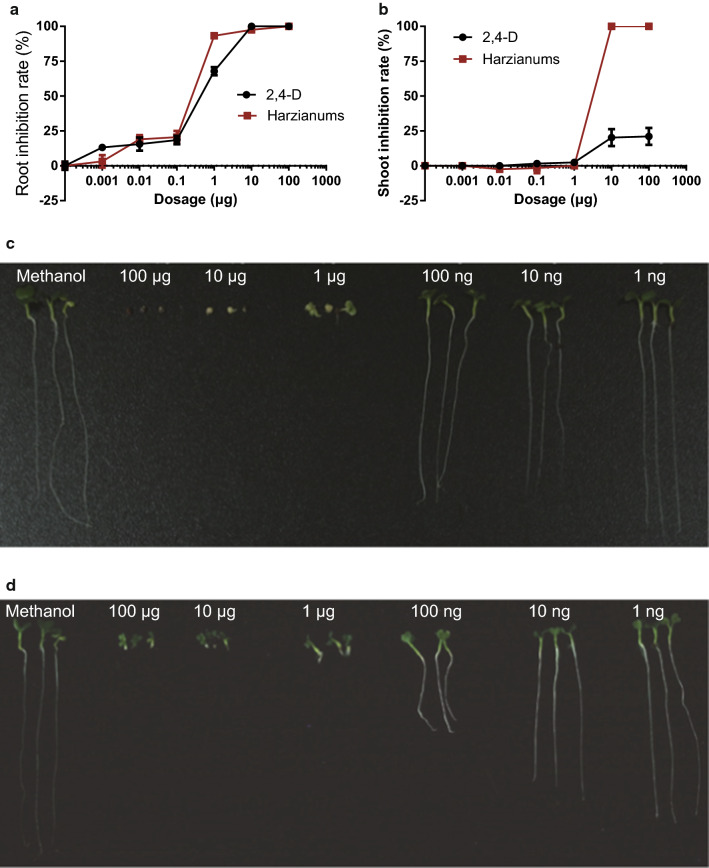
HA and HB (1–2) caused a strong inhibitory effect on the germination of B. chinensis seeds at a concentration of 5 μM. In general, the performances of HA and HB were comparable to the positive control, 2,4-D. When directly applied to the seeds at low concentrations (1–100 ng), the compounds caused significant root length reduction with inhibition values ranging from 3.2% (1 ng) to 20.6% (100 ng) at p values < 0.05 (Fig. 1a). However, the shoot length was not affected at these dosages. As the concentration increased (≥ 1 μg), the root growth inhibition by HA and HB (1–2) dramatically increased to 93.3%, which was higher than the value of 2,4-D (67.9%). When the concentration was increased to 10 μg, failure of development was observed on both shoot and root (Fig. 1a, b). In addition, the cotyledons appeared to be depigmented (Fig. 1c). In comparison, the positive control, 2,4-D, induced a significant shoot growth reduction at concentrations higher than 10 μg, with inhibition values ranging from 20.3 and 20.1%, respectively, and depigmentation was not observed (Fig. 1).
Fig. 1.
The phytotoxic effect of HA and HB (1–2) on B. chinensis compared to the positive control, 2,4-D. The inhibition rate of a root or b shoot length by HA and HB or 2,4-D (n = 5). The photographs of B. chinensis seedlings after treatment with c HA and HB or d 2,4-D
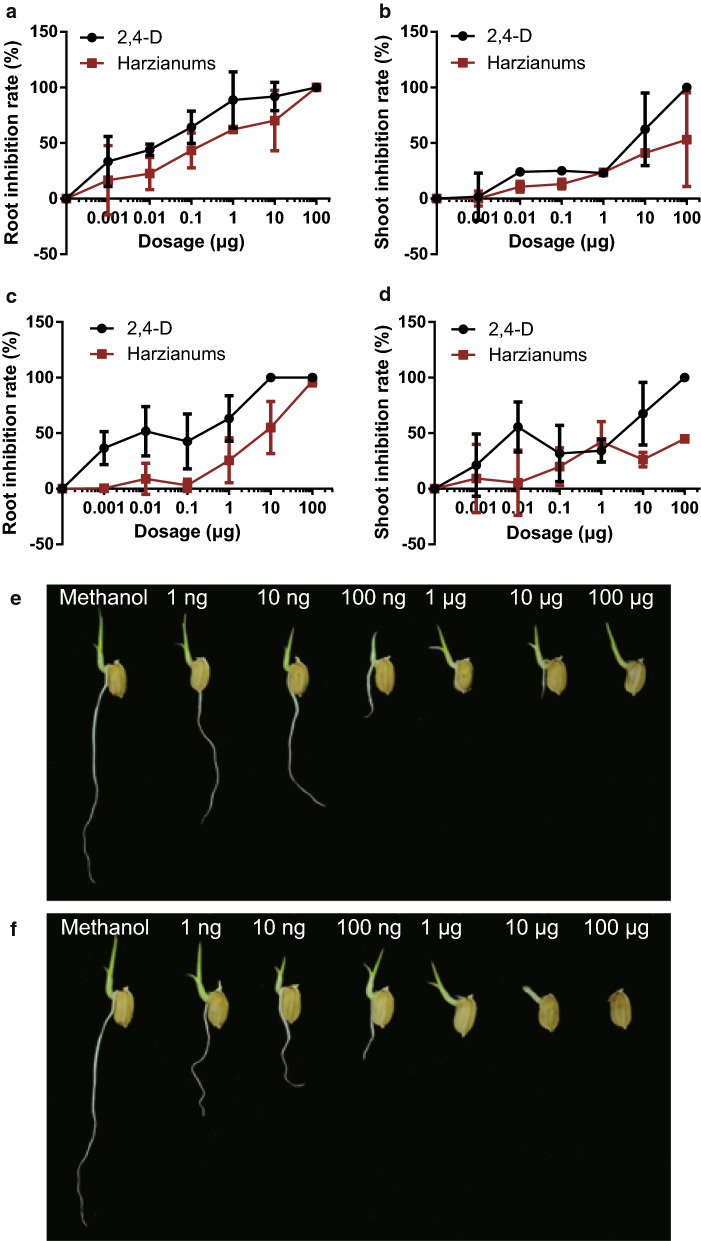
The herbicidal activities of HA and HB (1–2) to B. chinensis were further evaluated in a greenhouse with 2,4-D used as positive control. The compounds were tested at dosages of 0.2, 0.4, and 0.8 μM m−2 for pre-emergence and post-emergence herbicidal activity. When sprayed after germination, harzianums showed good herbicidal activity at all three dosages at 6 days, which was comparable to 2,4-D (Fig. 2a). The seedling survival rate of B. chinensis was 35–60% for the harzianums, much lower than that of 2,4-D. In contrast, when applied to the soil before germination, the performance of harzianums was not as good as 2,4-D (Fig. 2b), and the germination was not significantly affected.
Fig. 2.
The herbicidal activities of HA and HB (1–2) on B. chinensis in pot assays in a pre- and b post-emergence conditions
Phytotoxicity of harzianum A and B to monocots
To determine the crop/weed selectivity, the phytotoxicity assays of HA and HB (1–2) were carried out on the monocots, i.e., rice (O. sativa L. cv. Nipponbare) and two weeds (S. viridis L. Beauv. and E. crusgalli L. Beauv.). The herbicidal effectiveness was compared with that of 2,4-D based on germination and biomass production (shoot and root length), at dosage ranging from 1 ng to 100 μg. The most significant reduction by HA and HB was observed on rice (Fig. 3). Significant root length reduction (16.51–100%, p < 0.05) was observed at the concentration range from 1 ng to 100 μg, though it was lower, compared to that caused by commercial herbicide (33.4–100%). This inhibitory effect was also evident, at lower extent, on E. crusgalli L. Beauv., resulting in a reduction of 25.5, 55.1, and 96.9% at concentrations of 1, 10 and 100 μg, respectively. In general, the phytotoxicity of HA and HB was less effective than that of 2,4-D to the root development of rice and E. crusgalli L. Beauv. (Fig. 3a, c), with IC50 values of 42.9 ng and 63.0 ng for 2,4-D, and 186.2 ng and 9.16 μg for HA and HB. The shoot length was less affected by HA and HB with IC50 values larger than 100 μg, while 2,4-D confirmed greater phytotoxicity to shoot length development at concentrations of 10 and 100 μg for both rice and E. crusgalli L. Beauv. However, S. viridis L. Beauv. did not appear to be significantly affected by HA and HB or 2,4-D up to 10 μg dosage.
Fig. 3.
The phytotoxic effect of HA and HB (1–2) on rice or E. crusgalli L. Beauv. compared to the positive control, 2,4-D. The inhibition rate of a, c root or b, d shoot length by HA and HB or 2,4-D to a, b rice or c, dE. crusgalli L. Beauv., respectively (n = 5), and the photographs of rice seedlings after treatment with e HA and HB or f 2,4-D
Discussion
In the search for a potential natural product-based herbicide, harzianum A and B (HA and HB) were found to be produced by the biofertilizer fungus, T. brevicompactum. HA and HB can exert significant phytotoxic effects on both dicot and monocot, but varied in different species. The growth inhibition by HA and HB mainly occurred in the root system. When grown in soil, application of the compounds on soil before the seedling emergence was less effective than after. In summary, HA and HB exhibited potential to be herbicides.
HA was first isolated from T. harzianum and then T. brevicompactum (Cardoza et al. 2019; Corley et al. 1994; Nielsen et al. 2005), while HB was later identified from Hypocrea sp. F000527 (Jin et al. 2007). HB showed weak cytotoxicity against HeLa, MCF-7, and HT1080 cell lines with IC50 values of 74.18, 74.04 and 15.63 μg mL−1, while HA was more toxic with IC50 values of 5.07, 10.13 and 0.65 μg mL−1, respectively (Jin et al. 2007). It seemed that the cis–trans–trans stereochemistry of HA contributes to this cytotoxicity. Therefore, to further evaluate the herbicidal potential of HA and HB, it is necessary to test them individually.
Acknowledgements
We thank the Research Facility Center of Biotechnology Research Institute for providing HPLC-HRMSMS equipment. We thank the Beijing Center for Physical and Chemical Analysis for providing access to their NMR device.
Authors’ contributions
LZ, YX and BD conceived and designed research. MY, EF, CW, YZ and QY conducted experiments. BD contributed the fungal strain. LZ and CW analyzed data. LZ wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Key Research and Development Program of China Grant 2017YFD0201301-06 (to L.Z.), the National Key Research and Development Program of China (2018YFA0901800 to Y.X.), the National Natural Science Foundation of China (31570093 and 31870076 to Y. X and 21807110 to C. W.), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP to Y.X. and Y2020XK20 to L.Z.).
Availability of data and materials
The datasets on which the conclusions of the manuscript rely to were presented in the main paper.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors declare their consent for publication.
Competing interests
All authors declare that he/she has no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Miaomiao Yin and Opemipo Esther Fasoyin contributed equally to this work
Contributor Information
Yuquan Xu, Email: xuyuquan@caas.cn.
Liwen Zhang, Email: zhangliwen@caas.cn.
References
- Abbas HK, Yoshizawa T, Shier WT. Cytotoxicity and phytotoxicity of trichothecene mycotoxins produced by Fusarium spp. Toxicon. 2013;74:68–75. doi: 10.1016/j.toxicon.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Araniti F, Mancuso R, Lupini A, Giofre SV, Sunseri F, Gabriele B, Abenavoli MR. Phytotoxic potential and biological activity of three synthetic coumarin derivatives as new natural-like herbicides. Molecules. 2015;20(10):17883–17902. doi: 10.3390/molecules201017883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araniti F, Mancuso R, Lupini A, Sunseri F, Abenavoli MR, Gabriele B. Benzofuran-2-acetic esters as a new class of natural-like herbicides. Pest Manag Sci. 2019 doi: 10.1002/ps.5528. [DOI] [PubMed] [Google Scholar]
- Benitez T, Rincon AM, Limon MC, Codon AC. Biocontrol mechanisms of Trichoderma strains. Int Microbiol. 2004;7(4):249–260. [PubMed] [Google Scholar]
- Bin-Umer MA, McLaughlin JE, Basu D, McCormick S, Tumer NE. Trichothecene mycotoxins inhibit mitochondrial translation–implication for the mechanism of toxicity. Toxins. 2011;3(12):1484–1501. doi: 10.3390/toxins3121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoza RE, Malmierca MG, Hermosa MR, Alexander NJ, McCormick SP, Proctor RH, Tijerino AM, Rumbero A, Monte E, Gutierrez S. Identification of loci and functional characterization of trichothecene biosynthesis genes in filamentous fungi of the genus Trichoderma. Appl Environ Microbiol. 2011;77(14):4867–4877. doi: 10.1128/aem.00595-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoza RE, McCormick SP, Lindo L, Kim HS, Olivera ER, Nelson DR, Proctor RH, Gutierrez S. A cytochrome P450 monooxygenase gene required for biosynthesis of the trichothecene toxin harzianum A in Trichoderma. Appl Microbiol Biotechnol. 2019;103(19):8087–8103. doi: 10.1007/s00253-019-10047-2. [DOI] [PubMed] [Google Scholar]
- Charudattan R. Biological control of weeds by means of plant pathogens: significance for integrated weed management in modern agro-ecology. Biocontrol. 2001;46(2):229–260. doi: 10.1023/a:1011477531101. [DOI] [Google Scholar]
- Copping LG, Duke SO. Natural products that have been used commercially as crop protection agents. Pest Manag Sci. 2007;63(6):524–554. doi: 10.1002/ps.1378. [DOI] [PubMed] [Google Scholar]
- Corley DG, Miller-Wideman M, Durley RC. Isolation and structure of harzianum A: a new trichothecene from Trichoderma harzianum. J Nat Prod. 1994;57(3):422–425. doi: 10.1021/np50105a019. [DOI] [PubMed] [Google Scholar]
- Dayan FE, Duke SO. Natural compounds as next-generation herbicides. Plant Physiol. 2014;166(3):1090–1105. doi: 10.1104/pp.114.239061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Dayan FE, Rimando AM, Schrader KK, Aliotta G, Oliva A, Romagni JG. Chemicals from nature for weed management. Weed Sci. 2002;50(2):138–151. doi: 10.1614/0043-1745(2002)050[0138:IPCFNF]2.0.CO;2. [DOI] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species - opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2(1):43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- Huter OF. Use of natural products in the crop protection industry. Phytochem Rev. 2011;10(2):185–194. doi: 10.1007/s11101-010-9168-y. [DOI] [Google Scholar]
- Jin HZ, Lee JH, Zhang WD, Lee HB, Hong YS, Kim YH, Lee JJ. Harzianums A and B produced by a fungal strain, Hypocrea sp. F000527, and their cytotoxicity against tumor cell lines. J Asian Nat Prod Res. 2007;9(3–5):203–207. doi: 10.1080/10286020500531977. [DOI] [PubMed] [Google Scholar]
- McCormick SP, Stanley AM, Stover NA, Alexander NJ. Trichothecenes: from simple to complex mycotoxins. Toxins. 2011;3(7):802–814. doi: 10.3390/toxins3070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G, Bartlett DW, Fraser TE, Hawkes TR, Holt DC, Townson JK, Wichert RA. Mesotrione: a new selective herbicide for use in maize. Pest Manag Sci. 2001;57(2):120–128. doi: 10.1002/1526-4998(200102)57. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Nielsen KF, Grafenhan T, Zafari D, Thrane U. Trichothecene production by Trichoderma brevicompactum. J Agric Food Chem. 2005;53(21):8190–8196. doi: 10.1021/jf051279b. [DOI] [PubMed] [Google Scholar]
- Nishiuchi T, Masuda D, Nakashita H, Ichimura K, Shinozaki K, Yoshida S, Kimura M, Yamaguchi I, Yamaguchi K. Fusarium phytotoxin trichothecenes have an elicitor-like activity in Arabidopsis thaliana, but the activity differed significantly among their molecular species. Mol Plant-Microbe In. 2006;19(5):512–520. doi: 10.1094/mpmi-19-0512. [DOI] [PubMed] [Google Scholar]
- Rajcan I, Swanton CJ. Understanding maize-weed competition: resource competition, light quality and the whole plant. Field Crop Res. 2001;71(2):139–150. doi: 10.1016/s0378-4290(01)00159-9. [DOI] [Google Scholar]
- Tijerino A, Cardoza RE, Moraga J, Malmierca MG, Vicente F, Aleu J, Collado IG, Gutierrez S, Monte E, Hermosa R. Overexpression of the trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet Biol. 2011;48(3):285–296. doi: 10.1016/j.fgb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418(6898):671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. Trichoderma-plant-pathogen interactions. Soil Biol Biochem. 2008;40(1):1–10. doi: 10.1016/j.soilbio.2007.07.002. [DOI] [Google Scholar]
- Vinale F, Flematti G, Sivasithamparam K, Lorito M, Marra R, Skelton BW, Ghisalberti EL. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J Nat Prod. 2009;72(11):2032–2035. doi: 10.1021/np900548p. [DOI] [PubMed] [Google Scholar]
- Wang H, Hwang SF, Eudes F, Chang KF, Howard RJ, Turnbull GD. Trichothecenes and aggressiveness of Fusarium graminearum causing seedling blight and root rot in cereals. Plant Pathol. 2006;55(2):224–230. doi: 10.1111/j.1365-3059.2006.01339.x. [DOI] [Google Scholar]
- Zhou B, Wang H, Meng B, Wei R, Wang L, An C, Chen S, Yang C, Qiang S. An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest Manag Sci. 2019;75(9):2482–2489. doi: 10.1002/ps.5402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets on which the conclusions of the manuscript rely to were presented in the main paper.