Abstract
Intermittent administration of PTH type 1 receptor (PTH1R) agonists increases bone remodeling, with greater stimulation of bone formation relative to bone resorption causing net gains in bone mass. This pharmacodynamic feature underlies the bone-building effects of teriparatide and abaloparatide, the only PTH1R agonists approved to reduce osteoporotic fracture risk in postmenopausal women. This study in 8-week-old female mice compared bone resorption and formation responses to these agents delivered at the same 10 μg/kg dose, and a 40 μg/kg abaloparatide dose was also included to reflect its 4-fold higher approved clinical dose. Peptides or vehicle were administered by daily supra-calvarial subcutaneous injection for 12 days, and local (calvarial) and systemic (L5 vertebral and tibial) responses were evaluated by histomorphometry. Terminal bone histomorphometry data indicated that calvarial resorption cavities were similar in both abaloparatide groups versus vehicle controls, whereas the teriparatide group had more calvarial resorption cavities compared with the vehicle or abaloparatide 40 μg/kg groups. The bone resorption marker serum CTX was significantly lower in the abaloparatide 40 μg/kg group and similar in the other two active treatment groups compared with vehicle controls. Both peptides increased trabecular bone formation rate (BFR) in L5 and proximal tibia versus vehicle, and L5 BFR was higher with abaloparatide 40 μg/kg versus teriparatide. At the tibial diaphysis, periosteal BFR was higher with abaloparatide 40 μg/kg versus vehicle or teriparatide, and endocortical BFR was higher with teriparatide but not with abaloparatide 10 or 40 μg/kg versus vehicle. Few differences in structural or microarchitectural bone parameters were observed with this brief duration of treatment. In summary, calvarial bone resorption cavity counts were higher in the teriparatide group versus the vehicle and abaloparatide 40 μg/kg groups, and the abaloparatide 40 μg/kg group had lower serum CTX versus vehicle. L5 and tibial trabecular bone formation indices were higher in all three active treatment groups versus vehicle. The abaloparatide 40 μg/kg group had higher L5 trabecular BFR and tibial periosteal BFR versus teriparatide, whereas tibial endocortical BFR was higher with teriparatide but not abaloparatide. Together, these findings in female mice indicate that an improved balance of bone formation versus bone resorption is established shortly after initiating treatment with abaloparatide.
Keywords: PTH, PTHrP, Bone anabolic agent, Osteoporosis, Bone histomorphometry, Calvaria, Skull, Vertebrae, Tibia, Long bone
Highlights
-
•
PTH receptor (PTH-R) agonists increase bone density by stimulating bone formation.
-
•
PTH-R agonists differ in their propensity to increase bone resorption.
-
•
Female mice were treated for 12 d with PTH-R agonists abaloparatide or teriparatide.
-
•
The systemic resorption marker serum CTX was lower with abaloparatide vs vehicle.
-
•
Calvarial resorption cavities were higher with teriparatide but not abaloparatide.
1. Introduction
Bone strength and fracture resistance are largely related to bone mass, which reflects the net activities of bone-forming osteoblasts and bone-resorbing osteoclasts. The PTH type 1 receptor (PTH1R) agonists PTH(1–34) (teriparatide; TPTD) (Mosekilde et al., 1995; Sato et al., 2004; Neer et al., 2001) and abaloparatide (Bahar et al., 2016; Varela et al., 2017a; Doyle et al., 2018; Leder et al., 2015; Miller et al., 2016) increase bone mass and bone strength in animals and reduce fracture risk in women with postmenopausal osteoporosis (PMO) by increasing bone formation. Human data indicate that both agents also increase bone resorption as part of their remodeling-based mechanism of action, though increases in serum bone resorption markers are smaller with abaloparatide compared with teriparatide (Leder et al., 2015; Miller et al., 2016). These differential effects on bone resorption may contribute to greater gains in bone mineral density (BMD) at hip, femoral neck and lumbar spine with abaloparatide versus teriparatide (Miller et al., 2016).
Several differences between abaloparatide and teriparatide may influence their effects on bone resorption, bone formation, and bone mass, including their molecular derivations. Teriparatide is an amino-terminal fragment of PTH(1–84), an important calcium-mobilizing hormone that acts in part by stimulating bone resorption. This role is reflected in the phenotype of genetically modified mice that lack endogenous PTH, which have fewer osteoclasts, lower serum calcium, and higher bone mass (Miao et al., 2004a; Amizuka et al., 1996). Abaloparatide (previously known as BIM-44058 or BA058) is an analog of human PTH-related peptide (PTHrP), a paracrine factor that acts as a physiological regulator of bone formation (Martin, 2005). Endogenous PTHrP plays a key role in maintaining bone formation in the mature skeleton, and PTHrP-deficient mice exhibit fewer osteoblasts, lower mineral apposition rate, and lower trabecular bone volume (Miao et al., 2004b; Amizuka et al., 1996). Several amino acid substitutions (Gonnelli and Caffarelli, 2016) may explain why abaloparatide causes greater BMD gains in mice compared with a similar dose of native PTHrP(1–36) (Le Henaff et al., 2020).
Studies in estrogen-deficient ovariectomized (OVX) rats indicate significant increases in bone formation, bone mass, and bone strength when abaloparatide is administered for several weeks to several months at doses as low as 0.25–1.0 μg/kg/d (Varela et al., 2017b; Makino et al., 2018; Varela et al., 2017a). Studies in rats and non-human primate show no increases in biochemical or histomorphometric bone resorption parameters with abaloparatide at the highest doses tested, which ranged up to 25 μg/kg (Varela et al., 2017b; Doyle et al., 2018; Makino et al., 2018; Besschetnova et al., 2019; Chandler et al., 2019). Apart from one fracture healing study (Bernhardsson and Aspenberg, 2018), mouse studies have thus far only compared the effects of abaloparatide and teriparatide at 20 and/or 80 μg/kg, with both doses associated with increases in BMD and systemic biochemical markers of bone formation and bone resorption (Sahbani et al., 2019; Le Henaff et al., 2020). It is appropriate to compare these agents at similar doses to evaluate their relative potencies, as was done in several mouse studies (Ricarte et al., 2018; Sahbani et al., 2019; Le Henaff et al., 2020), but there is also clinical relevance in comparing their effects at the same multiple of their FDA-approved fixed dose, which is 80 μg/d for abaloparatide (Miller et al., 2016) and 20 μg/d for teriparatide (Neer et al., 2001). The current study provides the first direct comparisons of the effects of abaloparatide and teriparatide on static and dynamic bone histomorphometry parameters and bone turnover markers using the same dose, and at doses that reflect the 4-fold higher FDA-approved dose of abaloparatide versus teriparatide.
It can be challenging to quantify bone resorption by histomorphometry in rodents because their eroded surfaces are difficult to ascertain and their long bones rarely show increased cortical porosity in response to intermittent PTH1R agonist therapy. However, mouse calvarial bones exhibit natural porosity in the form of readily identifiable osteoclast-rich cavities that increase or decrease when bone resorption or bone formation are altered (Rhee et al., 2013; Eger et al., 2019). Furthermore, PTH1R agonists increase the ability of osteoblast-lineage cells from mouse calvaria to promote osteoclastogenesis (Shinoda et al., 2010), and recent data indicate that teriparatide therapy is associated with significant bone loss in the skull of women with PMO (Paggiosi et al., 2018). We therefore studied the effects of subcutaneous supra-calvarial injections of abaloparatide and teriparatide on histological parameters of bone resorption in the calvaria of female mice. A short 12-day course of therapy was chosen to remain within the window of a single remodeling period, which is approximately 14 days in mice (Jilka, 2013). Systemic responses to these agents were also evaluated by static and dynamic bone histomorphometry analyses of the lumbar vertebra and tibia, and by serum-based biochemical markers of bone turnover.
2. Materials and methods
2.1. Study design
All animal procedures were approved by the Radius Health IACUC and were performed in an AAALAC-accredited vivarium at Radius Health (HubLab/Vertex, Boston, MA, USA). Thirty-two 8-week-old ovary-intact female C57Bl/6 J mice were acquired from Jackson Labs (Bar Harbor, ME, USA) and acclimated for 3 days in polycarbonate cages with aspen wood shaving bedding (Lab Supply Inc.), with four mice per cage. Vivarium conditions included 22 °C ambient temperature and a 12-h light-dark cycle. Animals received water in pouches (Lab Products Hydropac machine) and rodent LabDiet 5053 ad libitum (PicoLab at Brentwood, MO, USA). Mice were weighed weekly and underwent daily cage-side observations for health and behavior.
After acclimation, mice were divided into 4 groups of 8 animals each based on similar average body weight. These groups received vehicle (sterile saline), TPTD at 10 μg/kg (Bachem; TPTD10 group), or abaloparatide at 10 or 40 μg/kg (ABL10 and ABL40 group, respectively; Radius Health Inc.). Specific peptide content was 85% for abaloparatide and 90.3% for teriparatide, and purity was approximately 99% for both agents. All treatments were administered for 12 consecutive days by subcutaneous injection above the right calvaria in a 100 μL injection volume. The final treatment injections were administered 2–4 h prior to a terminal blood draw and sacrifice by an overdose of 5% of isoflurane, followed by necropsy. Seven days and 2 days prior to necropsy, animals were intraperitoneally injected with the fluorochromes alizarin red complexone (30 mg/kg) and calcein green (20 mg/kg) (Sigma), respectively, both in 2% sodium bicarbonate. At necropsy the intact calvarial bones, 5th lumbar vertebra (L5), and right tibia were harvested and processed for bone histomorphometry.
2.2. Histomorphometry
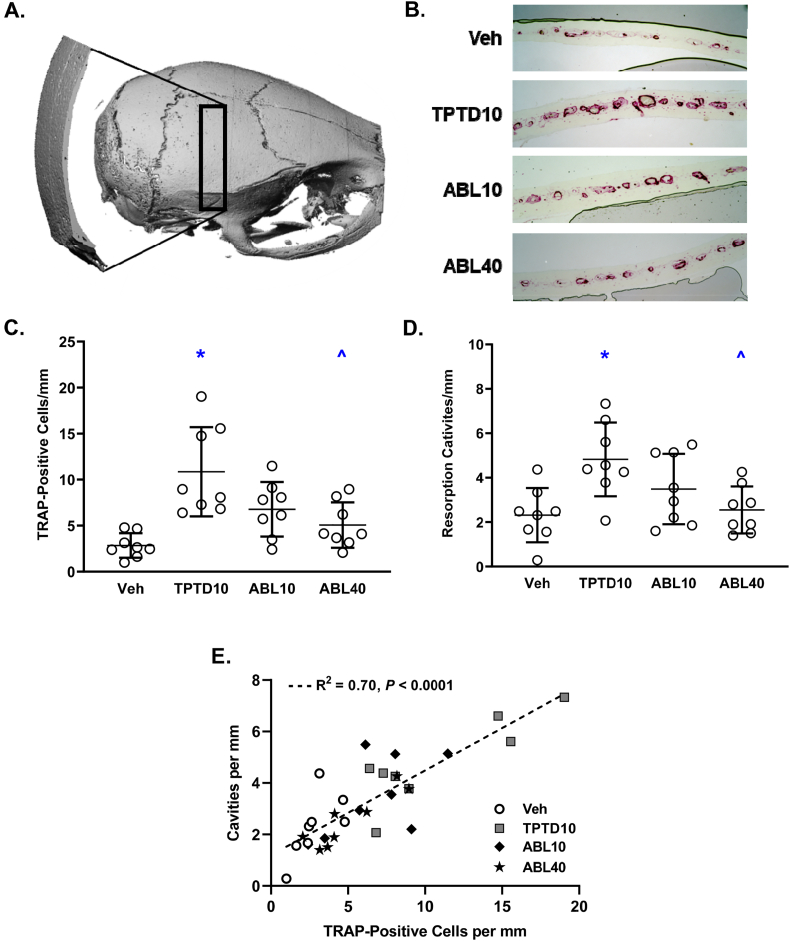
Calvarial bones were fixed for 24 h in 10% neutral buffered formalin (NBF; Universal Medical Inc.), decalcified in 0.5 M EDTA (pH 8.0; Millipore) for 7 days, dehydrated by graded ethanol-xylene, and embedded in paraffin. Twenty-four 4-μm thick coronal cross-sections were obtained from each bone with a microtome (Thermo Shandon Finesse ME microtome 10,609) and mounted on glass slides. Two slides 10 sections apart were deparaffinized with xylene and alcohol, washed in deionized water, incubated in acetate buffer pH 5.0, and stained for tartrate-resistant acid phosphatase (TRAP; Leukocyte TRAP kit, Sigma-Aldrich), followed by counterstaining with 0.02% Fast green. Photomicrographs of the stained calvarial sections were obtained with a digital microscope (VH-5000 series, Keyence, USA), and the number of TRAP-positive cells and resorption cavities were determined using ImageJ2 software (National Institutes of Health, Bethesda, MD, USA). Fig. 1A illustrates the region of histomorphometry analyses, which comprised the right hemicalvaria excluding the medial 0.75 mm region of bone adjacent to the sagittal suture.
Fig. 1.
Effects of daily supra-calvarial injections of vehicle (Veh, saline) teriparatide (10 μg/kg; TPTD10) or abaloparatide (10 or 40 μg/kg; ABL10 and ABL40, respectively) on the numbers of TRAP-positive cells and resorption cavities in calvarial bones. A: Micro-CT reconstruction of a mouse skull showing the region of histological sectioning (black box), above which the daily s.c. peptide injections were administered. B: Representative histologic cross-sections showing TRAP-stained cells (purple) occupying calvarial resorption cavities. C: Number of TRAP-positive cells per mm of calvarial length. D: Number of resorption cavities per mm of calvarial length. E: Linear regressions of TRAP-stained cells versus resorption cavities. Data represent means ± SD, n = 8/group; * P < 0.05 vs Veh control, ^P < 0.05 vs TPTD10 by ordinary one-way ANOVA and Tukey's post-test.
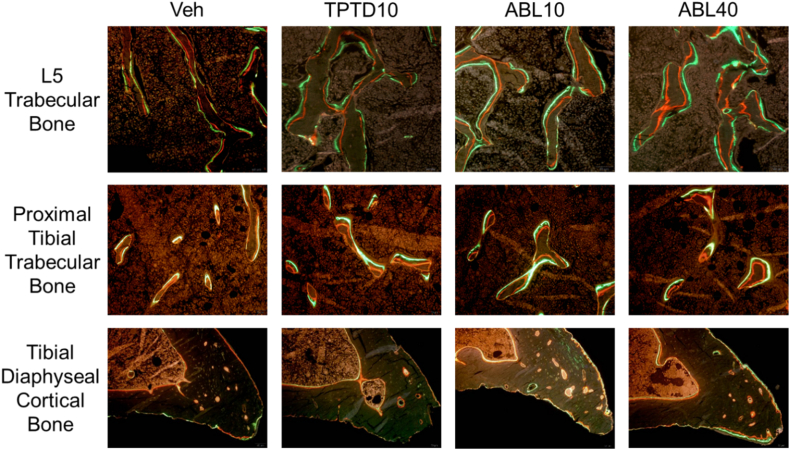
L5 and the right tibia were collected at necropsy, cleaned of soft tissue, fixed in 10% NBF for 3 days, and stored in 70% ethanol. A hand saw was used to bisect the tibial diaphysis midway between the proximal and distal tibiofibular junctions. The proximal portion of the tibia and L5 were then dehydrated in graded ethanol, cleared with xylene, embedded undecalcified in methylmethacrylate, and sectioned at 5-μm thickness with an RM2255 microtome (Leica, Germany). Consecutive coronal sections were cut through the middle of the proximal tibial metaphysis and the L5 vertebral body, and cross-sections of the tibial diaphysis were also collected. One section was left unstained for dynamic histomorphometry analyses under fluorescent light, another was stained with toluidine blue (pH 3.7), and another was stained for TRAP. For TRAP staining, sections were deplasticized in acetone, rehydrated, and incubated for 20 min in acetate buffer (0.2 M sodium acetate, 50 mM sodium tartrate pH 5.0). Sections were then placed in TRAP staining solution (0.5 mg/mL naphthol AS-MX phosphate and 1.1 mg/mL Fast Red TR salt in acetate buffer) at 37 °C until sufficient color developed, followed by rinsing in water. TRAP-stained sections were then counterstained in toluidine blue for 3–5 s, rinsed in water, air-dried, and mounted with Histomount (National Diagnostics).
L5 and tibial histomorphometry analyses were performed using a Nikon E800 microscope equipped with an Olympus DP71 digital camera to capture images via Olympus CellSens software. Data were obtained via Osteomeasure software (Osteometrics Inc., Decatur, GA, USA) using standardized bone histomorphometry analyses and nomenclature (Dempster et al., 2013). Data for structural parameters represent averages obtained from unstained, toluidine blue-stained, and TRAP-stained sections. Structural parameters included trabecular bone volume per total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), cortical tissue area (Ct.T.Ar), cortical bone area (Ct.B.Ar), cortical marrow area (Ct.Ma.Ar), cortical bone volume per total volume (Ct.BV/TV), cortical thickness (Ct.Th), periosteal perimeter (Ps.Pm), and endocortical perimeter (Ec.Pm). TB-stained sections were used to determine osteoblast surface per bone surface (Ob.S/BS), osteoid surface per bone surface (OS/BS) and osteoid thickness (O·Th). TRAP-stained sections were used to determine osteoclast surface per bone surface (Oc.S/BS). Dynamic parameters of bone formation were obtained from unstained sections under fluorescent light, including trabecular mineralizing surface per bone surface (Tb.MS/BS), trabecular mineral apposition rate (Tb.MAR), trabecular bone formation rate per bone surface (Tb.BFR/BS), trabecular BFR per bone volume (Tb.BFR/BV), periosteal and endocortical MS/BS (Ps.MS/BS and Ec.MS/BS, respectively), periosteal and endocortical MAR (Ps.MAR and Ec.MAR, respectively), periosteal and endocortical BFR/BS (Ps.BFR/BS and Ec.BFR/BS, respectively), and periosteal and endocortical BFR/BV (Ps.BFR/BV and Ec.BFR/BV, respectively).
2.3. RANKL, OPG, and biochemical markers of bone turnover
Blood (~0.5 mL) was collected in the morning from the submandibular vein of unfasted animals 2–4 h after the last injection (treatment day 12) and prepared as serum that was stored at −80 °C. Serum levels of the bone resorption marker serum C-terminal telopeptide of type 1 collagen (CTX) were determined using RatLaps EIA kits (IDS, Gaithersburg, MD, USA). To minimize potential biases related to diurnal CTX variation, including that which may be influenced by food consumption (Christgau, 2000), the order and schedule of bleeding was designed to balance the average time of day of blood draws across the 4 groups. Serum levels of the bone formation marker N-terminal propeptide of type 1 procollagen (P1NP) were measured using Rat/Mouse P1NP EIA kits from IDS. Serum levels of the pro-resorptive cytokine RANKL (receptor activator of nuclear factor kappa-B ligand) and the antiresorptive cytokine OPG (osteoprotegerin) were determined using mouse ELISA kits from Thermo Scientific (Waltham, MA, USA).
2.4. Statistical analysis
Bone histomorphometry and bone turnover marker data were analyzed by ordinary one-way ANOVA, followed by Tukey's post-test that compared all groups to each other. All between-group differences that achieved statistical significance are indicated in the Figures and Tables. A p value of <0.05 was used to indicate statistically significant differences. Data are expressed as group means ± standard deviation (SD). ANOVA tests and linear regression analyses were performed with Prism GraphPad V8.3.
3. Results
3.1. General health
All animals survived in good health until scheduled necropsy. Treatments were well tolerated and had no effect on food consumption or body weight, or on appearance or behavior by daily cage-side observations.
3.2. Effects of abaloparatide and teriparatide on calvarial bone
The numbers of TRAP-positive calvarial cells and calvarial resorption cavities were similar in the ABL10 and ABL40 groups versus vehicle controls, and both variables were higher in the TPTD10 group compared with the vehicle and ABL40 groups (Fig. 1C and D). Fig. 1B depicts the TRAP-positive cells and resorption cavities in representative calvarial histology sections for each group. Linear regression analysis across all four groups indicate a strong positive relationship between the number of TRAP-positive cells and the number of calvarial resorption cavities (r2 = 0.70, p < 0.0001; Fig. 1E).
3.3. Effects of abaloparatide and teriparatide on trabecular bone histomorphometry
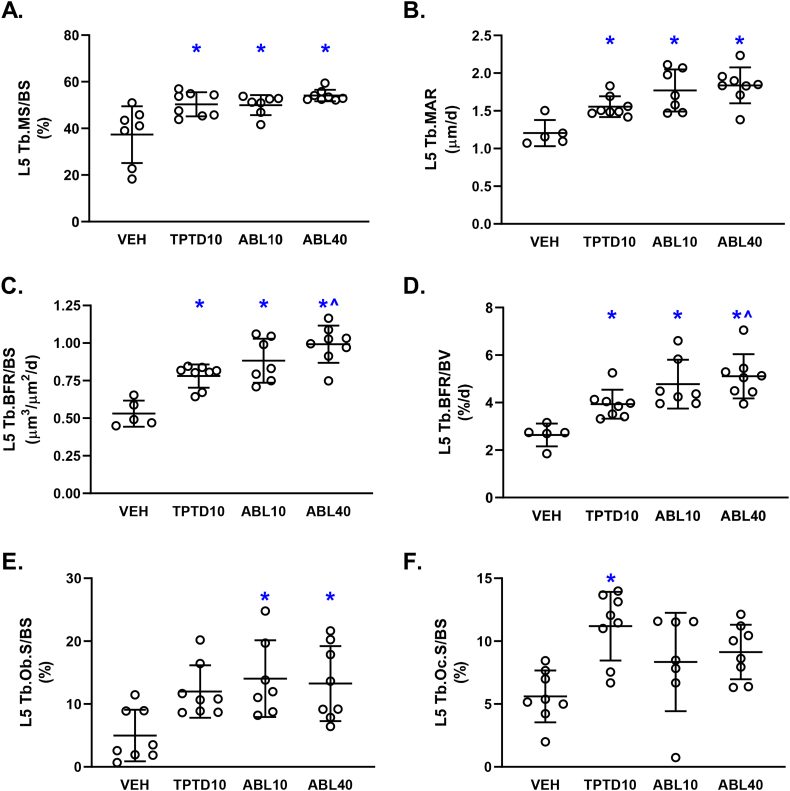
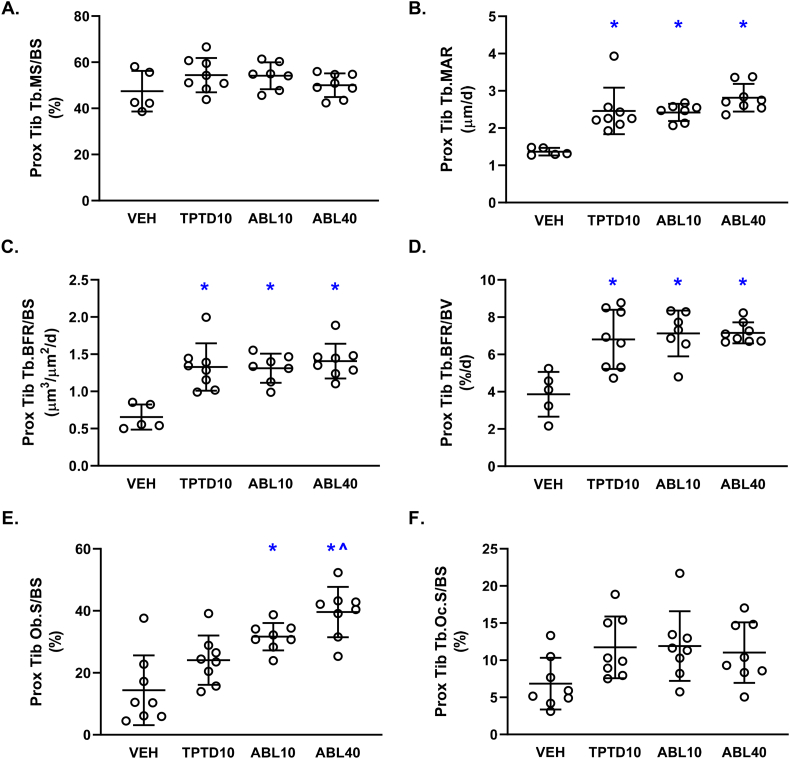
Representative fluorescent photomicrographs of trabecular bone in L5 and the proximal tibia are shown in Fig. 2. Dynamic histomorphometry analyses showed that the TPTD10, ABL10, and ABL40 groups had higher trabecular bone formation rates (Tb.BFR/BS and Tb.BFR/BV) in L5 and the proximal tibia compared with vehicle controls. (Figs. 3C-D and 4C-D). The ABL40 group had higher L5 Tb.BFR/BS and Tb.BFR/BV compared with the TPTD10 group (Fig. 3C-D). Higher trabecular bone formation rates with abaloparatide and teriparatide were due to higher MAR at both sites (Fig. 3B and 4B), and to higher MS/BS in L5 (Fig. 3A). The percentage of L5 and proximal tibial trabecular surfaces occupied by osteoblasts (Tb.Ob.S/BS) was higher in the ABL10 and ABL40 groups but not the TPTD10 group in comparison with vehicle controls (Fig. 3E and 4E). The ABL40 group also had higher proximal tibial Tb.Ob.S/BS compared with the TPTD10 group (Fig. 4E). The percentage of L5 trabecular surfaces occupied by osteoclasts (Tb.Oc.S/BS) was significantly higher in the TPTD10 group but not in the ABL10 or ABL40 groups versus vehicle controls (Fig. 3F). There were no significant between-group differences in Tb.Oc.S/BS for the proximal tibia (Fig. 4F).
Fig. 2.
Representative fluorochrome-labeled bone sections viewed by fluorescence microscopy. Red labels reflect alizarin red complexone administered 7 days prior to necropsy, and green labels reflect calcein green administered 2 days prior to necropsy. Veh, vehicle; TPTD10, teriparatide 10 μg/kg; ABL10, abaloparatide 10 μg/kg; ABL40, abaloparatide 40 μg/kg.
Fig. 3.
Trabecular bone histomorphometry data for the 5th lumbar vertebra (L5) after 12 days of daily supra-calvarial injections of vehicle (Veh, saline), teriparatide (10 μg/kg; TPTD10) or abaloparatide (10 or 40 μg/kg; ABL10 and ABL40, respectively). Data represent means ± SD, n = 8/group. * P < 0.05 vs Veh control, ^P < 0.05 vs TPTD10 by ordinary one-way ANOVA and Tukey's post-test.
Fig. 4.
Trabecular bone histomorphometry data for the proximal tibial metaphysis after 12 days of daily supra-calvarial injections of vehicle (Veh, saline) teriparatide (10 μg/kg; TPTD10) or abaloparatide (10 or 40 μg/kg; ABL10 and ABL40, respectively). Data represent means ± SD, n = 8/group. * P < 0.05 vs Veh control; ^P < 0.05 vs TPTD10 by ordinary one-way ANOVA and Tukey's post-test.
There were few treatment-related differences in trabecular microarchitectural parameters during this 12-day study. For L5, there were no between-group differences in trabecular BV/TV, Tb.Th, Tb.N, or Tb.Sp (Suppl. Table 1). There were also no differences in osteoid surface (OS/BS) or osteoid thickness (O·Th) (Suppl. Table 1). At the proximal tibia, the only statistically significant between-group differences in microarchitectural or osteoid variables were higher Tb.Th in the ABL40 group versus vehicle, and higher BV/TV in the TPTD10 group versus ABL10 (Suppl. Table 1).
3.4. Effects of abaloparatide and teriparatide on cortical bone
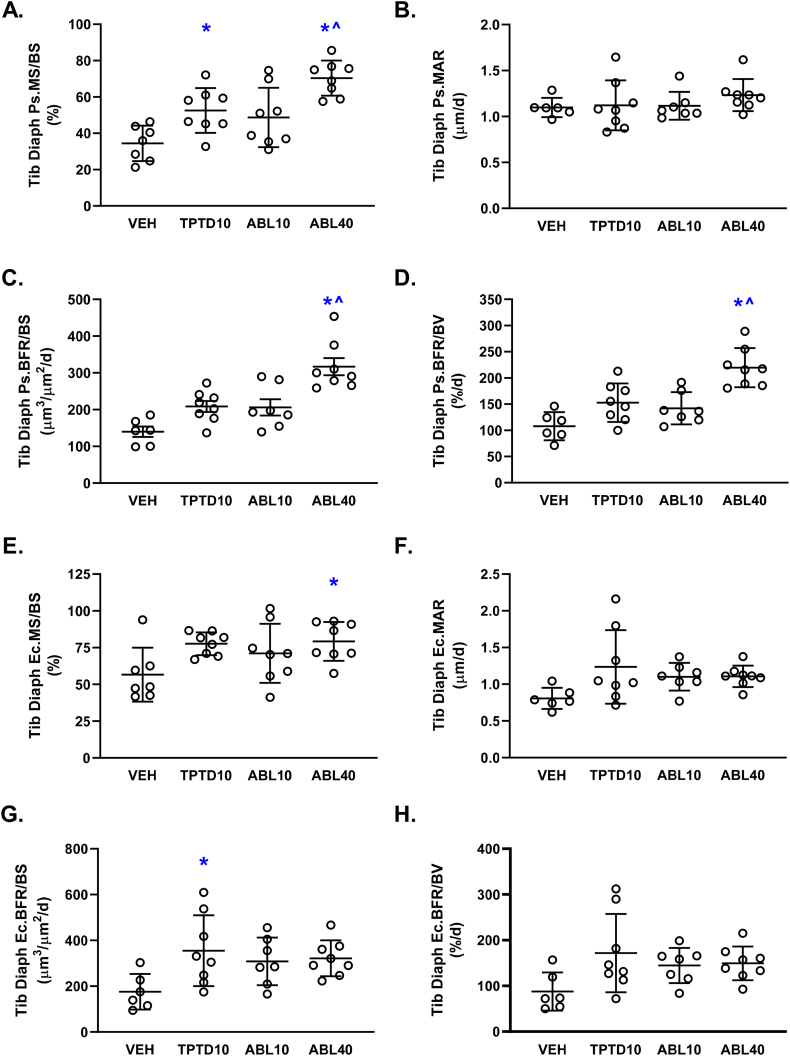
Representative fluorescent photomicrographs of tibial diaphyseal cross-sections are shown in Fig. 2. Dynamic histomorphometry analyses indicated significantly greater periosteal bone formation rates (Ps.BFR/BS and Ps.BFR/BV) in the ABL40 group in comparison with the vehicle and TPTD10 groups (Fig. 5C and D). These relative increases were related to significantly higher periosteal mineralizing surface (Ps.MS/BS) in the ABL40 group versus vehicle and TPTD10 (Fig. 5A), with no between-group differences observed for periosteal MAR (Fig. 5B). The TPTD10 group also had higher Ps.MS/BS versus vehicle (Fig. 5A). Few between-group differences were observed for endocortical bone formation parameters, which were limited to higher endocortical mineralizing surface (Ec.MS/BS) in the ABL40 group and higher surface-referent endocortical bone formation rate (Ec.BFR/BS) in the TPTD10 group compared with vehicle controls (Fig. 5E and G, respectively). Static histomorphometry analyses of tibial diaphyseal structural parameters indicated no between-group differences in Ct.T.Ar, Ct.Ma.Ar, Ct.BV/TV, Ct.Th, Ps.Pm, or Ec.Pm (Suppl. Table 2). Ct.B.Ar was higher in the TPTD10 group versus Veh controls (Suppl. Table 2).
Fig. 5.
Cortical bone histomorphometry data for the tibial diaphysis after 12 days of daily supra-calvarial injections of vehicle (Veh, saline), teriparatide (10 μg/kg; TPTD10) or abaloparatide (10 or 40 μg/kg; ABL10 and ABL40, respectively). Data represent means ± SD, n = 8/group. * P < 0.05 vs Veh control; ^ P < 0.05 vs TPTD10 by ordinary one-way ANOVA and Tukey's post-test.
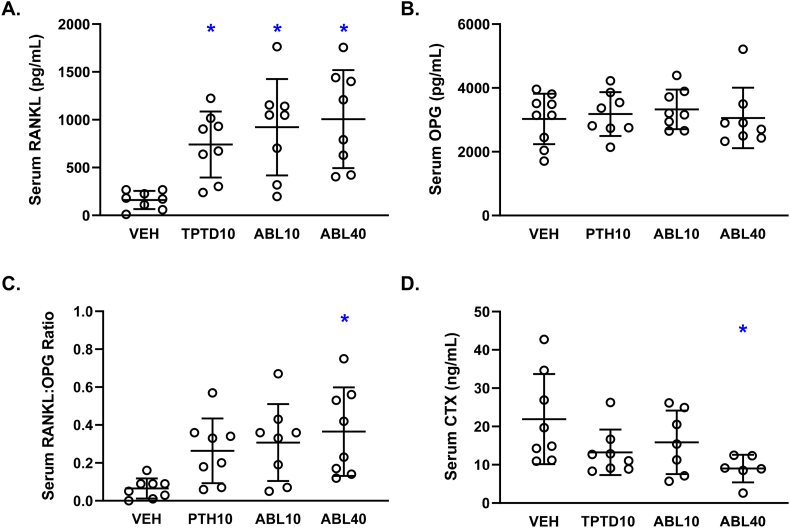
3.5. Effects of abaloparatide or teriparatide on RANKL, OPG, and biochemical markers of bone turnover
Mean serum concentrations of the pro-resorptive cytokine RANKL were similarly elevated in all three active treatment groups compared with Veh controls (Fig. 6A), whereas serum levels of the antiresorptive cytokine OPG were similar in all 4 groups (Fig. 6B). The serum RANKL:OPG ratio was also similar among the 3 active treatment groups, and was significantly higher in the ABL40 group versus vehicle controls (Fig. 6C). Serum levels of the bone resorption marker CTX were similar among the 3 active treatment groups and significantly lower in the ABL40 group versus controls (Fig. 6D). Linear regression analyses indicated an inverse correlation between serum RANKL and serum CTX (overall r = −0.56, p = 0.002), and between serum RANKL:OPG ratio versus CTX (r = −0.52, p = 0.004). Serum OPG showed no relationship with CTX (r = 0.10, p = 0.61). Serum RANKL, serum OPG, and serum RANKL:OPG ratio did not correlate with L5 or proximal tibial Oc.S/BS, or with calvarial resorption cavity number or calvarial osteoclast number (range of overall r values = 0.01–0.22, range of p values = 0.23–0.96). Mean serum concentrations of the bone formation marker P1NP were similar in all four groups (data not shown).
Fig. 6.
Biochemical markers and mediators of bone turnover after 12 days of daily supra-calvarial injections of vehicle (Veh, saline), teriparatide (10 μg/kg; TPTD10) or abaloparatide (10 or 40 μg/kg; ABL10 and ABL40, respectively). A. Serum concentrations of the pro-resorptive cytokine RANKL (receptor activator of nuclear factor κ-B ligand). B. Serum concentrations of the antiresorptive cytokine OPG (osteoprotegerin). C. Serum RANKL:OPG ratio. D. Serum concentrations of the bone resorption marker CTX (C-telopeptide of type 1 collagen). Data represent means ± SD, n = 7–8/group. * P < 0.05 vs Veh control by ordinary one-way ANOVA and Tukey's post-test.
4. Discussion
Abaloparatide was originally selected among a series of PTHrP analogs for its ability to increase bone formation and BMD with less calcium-mobilizing potential than teriparatide (Culler et al., 2001). This pharmacodynamic profile was targeted because PTH-induced calcium mobilization typically involves increased bone resorption, which can limit BMD gains (Tsai et al., 2013; Kostenuik et al., 2001) and contribute to dose-limiting hypercalcemia (Morony et al., 2005; Morony et al., 1999). The current experiment in female mice is the first preclinical study to directly compare the effects of abaloparatide versus teriparatide on histomorphometric and biochemical parameters of bone resorption and bone formation using the same dose as well as doses that reflect the 4-fold difference in their clinically approved doses. Both peptides were administered subcutaneously over the calvaria bones with the goal of potentiating calvarial bone resorption responses. The results indicate that calvarial resorption cavities and TRAP-positive calvarial osteoclasts were increased after 12 days of treatment with teriparatide, but not with the same dose or a 4-fold higher dose abaloparatide. The only previous comparative data on the effects of abaloparatide and teriparatide on structural parameters of bone resorption are from histomorphometric analyses of iliac crest bone biopsies of postmenopausal women with osteoporosis. Those results indicated similar effects of abaloparatide and teriparatide on cortical porosity, whereas trabecular eroded surfaces were lower with abaloparatide but not with teriparatide in comparison with subjects treated with placebo (Moreira et al., 2017).
The clinical relevance of the current animal model may be questioned because calvarial bones are not associated with osteoporotic fractures in humans. Furthermore, these mice were not ovariectomized or osteopenic, and new calvarial cavities that appeared in response to teriparatide may have undergone some degree of refilling by osteoblasts had the treatment duration been longer. Yet this calvarial response to teriparatide is not without clinical precedents. Radionuclide imaging indicates that the calvarial bones of women with PMO are highly metabolically responsive to teriparatide treatment (Moore et al., 2010), and teriparatide therapy is associated with substantial BMD loss in the skull of women with PMO, similar to reductions in BMD of the legs and peripheral skeleton in general (Paggiosi et al., 2018). While teriparatide treatment does not decrease and usually increases BMD in the legs and other post-cranial sites of mice and other small animals, the apparent similarities in calvarial bone responses to teriparatide in mice versus women suggests that this may represent a useful small-animal model for investigating mechanisms of osteoclastic responses to PTH1R agonists.
Histomorphometry of L5 vertebra indicated higher trabecular bone formation parameters in all three active treatment groups compared with vehicle controls, and surface- and volume-referent BFR was higher with ABL40 versus TPTD10. Direct comparisons between the latter two groups may have clinical relevance because abaloparatide is administered clinically at a 4-fold higher daily dose compared with teriparatide, due in part to abaloparatide's lower propensity to increase blood calcium (Miller et al., 2016; Leder et al., 2015). A previous study in mice with a femoral fracture also modeled this 4-fold difference in clinical dosing (Bernhardsson and Aspenberg, 2018), and another study in intact mice tested a 4-fold dose range of abaloparatide and teriparatide, but statistical comparisons were apparently limited to groups receiving the same dose of each peptide (Sahbani et al., 2019). L5 histomorphometry also showed higher trabecular osteoblast surface in both abaloparatide groups, and higher trabecular osteoclast surface in the teriparatide group, in comparison with vehicle controls. Higher osteoclast surface does not in itself prove an increase in bone resorption with teriparatide, and L5 trabecular microarchitectural parameters provided no indications of bone loss in the teriparatide group, perhaps due to increased trabecular bone formation.
Histomorphometry of the proximal tibia also showed higher trabecular BFR in all active treatment groups, and osteoblast surface was higher in both abaloparatide groups but not in the teriparatide group versus vehicle controls. Higher trabecular BFR and osteoblast surface without an increase in osteoclast surface has also been observed in abaloparatide-treated ovariectomized rats (Varela et al., 2017b). A recent study in male mice showed that 6 weeks of abaloparatide or teriparatide at 80 μg/kg led to greater trabecular BFR, osteoblast surface, BV/TV, and trabecular thickness in the distal femur, and trabecular thickness was greater in the abaloparatide versus teriparatide group (Le Henaff et al., 2020). The current study showed few significant between-group differences in trabecular architectural parameters, potentially due to the relatively short 12-day treatment period.
Cortical histomorphometry of the tibial diaphysis indicated higher endocortical BFR in the TPTD10 group versus vehicle controls, and higher periosteal BFR in the ABL40 group, compared with the vehicle and TPTD10 groups. These differences were not accompanied by differences in periosteal or endocortical perimeters, perhaps due to the short treatment period. Previous data showed that cortical thickness of the femoral diaphysis in male mice was significantly greater after 6 weeks of abaloparatide 80 μg/kg/d but not teriparatide 80 μg/kg/d compared with vehicle controls (Le Henaff et al., 2020). The current study's shorter treatment period may also explain the lack of effects of abaloparatide and teriparatide on the systemic bone formation marker serum P1NP (data not shown). Other animal and human studies with longer treatment durations show that abaloparatide increases serum P1NP (Doyle et al., 2018; Varela et al., 2017b; Sahbani et al., 2019; Le Henaff et al., 2020; Miller et al., 2016), as does teriparatide (Sahbani et al., 2019; Le Henaff et al., 2020; Miller et al., 2016).
The bone resorption marker serum CTX was similar in the TPTD10 and ABL10 groups and lower in the ABL40 group versus vehicle controls. Mice were not overnight-fasted for CTX measurements, but abaloparatide treatment does not influence food consumption (Varela et al., 2017a; Varela et al., 2017b; Doyle et al., 2018), and any effects of nocturnal feeding on CTX would likely represent a systematic bias. Furthermore, a study in overnight-fasted postmenopausal women showed a significant reduction in serum CTX during the first two weeks of teriparatide treatment (Glover et al., 2009), suggesting lower CTX in the ABL40 group may be a real albeit short-term response. Studies in overnight-fasted rats and non-human primates show that abaloparatide dosed at up to 25 μg/kg did not affect serum CTX at time points ranging from 8 weeks to 16 months, and was occasionally associated with reductions in other systemic bone resorption markers (Chandler et al., 2019; Doyle et al., 2018; Varela et al., 2017b; Makino et al., 2018). A previous study in female mice showed no increases in serum CTX after 1 month of abaloparatide administered at 20 or 80 μg/kg, whereas higher CTX was observed in male mice receiving abaloparatide or teriparatide at 80 μg/kg for 6 weeks (Le Henaff et al., 2020). Women with PMO show no change in serum CTX after 1 month of abaloparatide and modest increases thereafter that are smaller than increases observed with teriparatide (Miller et al., 2016).
Serum RANKL was similarly elevated in all three active treatment groups compared with vehicle controls, the potential influence of which remains unclear because serum RANKL showed no correlation with tibial, vertebral, or calvarial osteoclast variables, and serum RANKL was inversely related to serum CTX. RANKL exists in soluble and membrane-bound forms, the latter of which is particularly efficient at promoting osteoclastogenesis but is not measurable in serum (Nakashima et al., 2000). Other rodent studies showed that serum markers of bone resorption correlated positively with RANKL measured in bone marrow cells or bone marrow plasma, but did not correlate with RANKL levels in peripheral blood (Li et al., 2009). As such, the soluble circulating RANKL measured in the current study may not accurately reflect tissue-level bone resorption. Other studies assessing the effects of abaloparatide on serum RANKL or skeletal Rankl mRNA expression yielded mixed results. One study in female mice reported no effect of 30 days of abaloparatide or teriparatide on serum RANKL (Sahbani et al., 2019). A 6-week study in male mice showed no effect of abaloparatide (80 μg/kg) on Rankl mRNA expression in cortical bone extracts, whereas the same dose of teriparatide increased skeletal Rankl expression 3-fold (Ricarte et al., 2018). Lesser increases in Rankl mRNA expression were reported in osteoblast-like cell cultures treated with abaloparatide versus equivalent doses of teriparatide (Makino et al., 2018; Ricarte et al., 2018). Cortical bone extracts from a subset of three animals per group from the current study show no significant differences in Rankl mRNA expression among the three active treatment groups (data not shown). Together, these results suggest that differential regulation of RANKL may at best represent a partial explanation for differential effects of abaloparatide versus teriparatide on bone resorption, at least in rodents. The lack of changes in serum OPG levels is consistent with previous data from mice treated with abaloparatide at 20 or 80 μg/kg (Sahbani et al., 2019).
This study has several limitations, including its single time point. Earlier responses to abaloparatide and teriparatide were assessed in a mouse study of similar design, which showed higher numbers of calvarial resorption cavities and TRAP-positive calvarial cells after 5 days of supracalvarial injections of teriparatide 10 μg/kg but not abaloparatide 10 μg/kg (Radius Health, data on file). However, treatment durations longer than 12 days, or different doses, could have different effects on calvarial resorption and other variables. The volume of calvarial resorption cavities was not quantified due to occasional tissue artefacts related to the delicate nature of the thin calvarial sections, but this did not meaningfully impact the reliability of cavity counts or osteoclast counts. The extent to which the supracalvarial route of injection impacted the results is unknown, though the dynamic and cellular histomorphometry findings for L5 and the tibia were qualitatively similar to those observed in animals receiving abaloparatide via standard post-cranial subcutaneous injections (Varela et al., 2017b; Doyle et al., 2018; Chandler et al., 2019). The lack of bone micro-CT data is another limitation, as such analyses may have revealed structural or micro-architectural changes that were not apparent by histomorphometry. Finally, although some histomorphometric parameters of bone formation were higher in the ABL40 group versus the TPTD10 group after 12 days of treatment, bone histomorphometry data for women with PMO indicate that trabecular bone formation rates were similar after 12–18 months of treatment with abaloparatide 80 μg/d versus teriparatide 20 μg/d (Moreira et al., 2017).
In summary, 12 days of supra-calvarial injections of abaloparatide or teriparatide at a similar multiple of their respective clinical doses was associated with differential changes in several bone formation and resorption-related parameters in mice. Calvarial bone resorption parameters were increased with teriparatide relative to vehicle and abaloparatide, and bone formation rates for L5 trabecular bone and the tibial periosteum were greater with abaloparatide versus vehicle and teriparatide. In contrast to the teriparatide group, the abaloparatide 40 μg/kg group showed no increase in trabecular osteoclast surfaces and had significantly lower serum CTX compared with vehicle controls. Few microarchitectural changes were observed in this 12-day study, though Ct.B.Ar of the tibial diaphysis was higher with teriparatide and Tb.Th of the proximal tibia was higher with ABL40 versus vehicle. These results provide novel tissue-level evidence that abaloparatide treatment induces an early improvement in the balance of bone formation versus bone resorption.
Transparency document
Transparency document
CRediT authorship contribution statement
Heike Arlt: Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Tara Mullarkey: Formal analysis, Writing - review & editing. Dorothy Hu: Formal analysis, Writing - review & editing. Roland Baron: Data curation, Writing - review & editing. Michael S. Ominsky: Writing - review & editing. Bruce Mitlak: Writing - review & editing. Beate Lanske: Conceptualization, Data curation, Writing - original draft, Writing - review & editing.Tatiana Besschetnova:Conceptualization, Formal analysis, Data curation, Writing - original draft, Writing - review & editing.
Acknowledgments
Acknowledgements
The authors thank the histology core of the Center for Skeletal Research at MGH for processing and staining calvariae.
Medical writing support was provided by Paul Kostenuik, PhD, of Phylon Pharma Services, and funded by Radius Health.
Funding
This work was funded by Radius Health Inc.
Acknowledgments
Declaration of competing interest
Authors HA, TM, MO, BM, BL, and TB are employees of Radius Health and may own shares of Radius stock.
Footnotes
Some of these data were presented in abstract form at the annual meetings of the Endocrine Society (ENDO), March 23-26, 2019, in New Orleans, LA, USA, and the American Society for Bone and Mineral Research, Sept. 20-23, 2019, in Orlando, FL, USA.
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2020.100291.
Appendix A. Supplementary data
Supplementary tables
References
- Amizuka N., Karaplis A.C., Henderson J.E. Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev. Biol. 1996;175:166–176. doi: 10.1006/dbio.1996.0104. [DOI] [PubMed] [Google Scholar]
- Bahar H., Gallacher K., Downall J. Six weeks of daily abaloparatide treatment increased vertebral and femoral bone mineral density, microarchitecture and strength in ovariectomized osteopenic rats. Calcif. Tissue Int. 2016;99:489–499. doi: 10.1007/s00223-016-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardsson M., Aspenberg P. Abaloparatide versus teriparatide: a head to head comparison of effects on fracture healing in mouse models. Acta Orthop. 2018;89:674–677. doi: 10.1080/17453674.2018.1523771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova T., Brooks D.J., Hu D. Abaloparatide improves cortical geometry and trabecular microarchitecture and increases vertebral and femoral neck strength in a rat model of male osteoporosis. Bone. 2019;124:148–157. doi: 10.1016/j.bone.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Chandler H., Lanske B., Varela A. Abaloparatide, a novel osteoanabolic PTHrP analog, increases cortical and trabecular bone mass and architecture in orchiectomized rats by increasing bone formation without increasing bone resorption. Bone. 2019;120:148–155. doi: 10.1016/j.bone.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Christgau Stephan. Circadian variation in serum CrossLaps concentration is reduced in fasting individuals. Clin. Chem. 2000;46:431. [PubMed] [Google Scholar]
- Culler M.D., Dong J., Shen Y. BIM-44058, a novel analog of PTHrP with enhanced bone building activity, but decreased calcium-mobilization potential. J. Bone Miner. Res. 2001;16:S540. [Google Scholar]
- Dempster D.W., Compston J.E., Drezner M.K. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J. Bone Miner. Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle N., Varela A., Haile S. Abaloparatide, a novel PTH receptor agonist, increased bone mass and strength in ovariectomized cynomolgus monkeys by increasing bone formation without increasing bone resorption. Osteoporos. Int. 2018;29:685–697. doi: 10.1007/s00198-017-4323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger M., Bader M., Bree D. Bone anabolic response in the calvaria following mild traumatic brain injury is mediated by the cannabinoid-1 receptor. Sci. Rep. 2019;9:16196. doi: 10.1038/s41598-019-51720-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S.J., Eastell R., McCloskey E.V. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009;45:1053–1058. doi: 10.1016/j.bone.2009.07.091. [DOI] [PubMed] [Google Scholar]
- Gonnelli S., Caffarelli C. Abaloparatide. Clin Cases Miner Bone Metab. 2016;13:106–109. doi: 10.11138/ccmbm/2016.13.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka R.L. The relevance of mouse models for investigating age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1209–1217. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenuik P.J., Capparelli C., Morony S. OPG and PTH-(1-34) have additive effects on bone density and mechanical strength in osteopenic ovariectomized rats. Endocrinology. 2001;142:4295–4305. doi: 10.1210/endo.142.10.8437. [DOI] [PubMed] [Google Scholar]
- Le Henaff C., Ricarte F., Finnie B. Abaloparatide at the same dose has the same effects on bone as PTH (1-34) in mice. J Bone Miner Res. 2020;35(4):714–724. doi: 10.1002/jbmr.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder B.Z., O’Dea L.S., Zanchetta J.R. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 2015;100:697–706. doi: 10.1210/jc.2014-3718. [DOI] [PubMed] [Google Scholar]
- Li X., Ominsky M.S., Stolina M. Increased RANK ligand in bone marrow of orchiectomized rats and prevention of their bone loss by the RANK ligand inhibitor osteoprotegerin. Bone. 2009;45:669–676. doi: 10.1016/j.bone.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Makino A., Takagi H., Takahashi Y. Abaloparatide exerts bone anabolic effects with less stimulation of bone resorption-related factors: a comparison with teriparatide. Calcif. Tissue Int. 2018;103:289–297. doi: 10.1007/s00223-018-0422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T.J. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J. Clin. Invest. 2005;115:2322–2324. doi: 10.1172/JCI26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao D., He B., Lanske B. Skeletal abnormalities in PTH-null mice are influenced by dietary calcium. Endocrinology. 2004;145:2046–2053. doi: 10.1210/en.2003-1097. [DOI] [PubMed] [Google Scholar]
- Miao D., Li J., Xue Y. Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology. 2004;145:3554–3562. doi: 10.1210/en.2003-1695. [DOI] [PubMed] [Google Scholar]
- Miller P.D., Hattersley G., Riis B.J. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- Moore A.E., Blake G.M., Taylor K.A. Assessment of regional changes in skeletal metabolism following 3 and 18 months of teriparatide treatment. J. Bone Miner. Res. 2010;25:960–967. doi: 10.1359/jbmr.091108. [DOI] [PubMed] [Google Scholar]
- Moreira C.A., Fitzpatrick L.A., Wang Y. Effects of abaloparatide-SC (BA058) on bone histology and histomorphometry: the ACTIVE phase 3 trial. Bone. 2017;97:314–319. doi: 10.1016/j.bone.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Morony S., Capparelli C., Lee R. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1β, TNF-α, PTH, PTHrP, and 1,25(OH)2D3. J. Bone Miner. Res. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- Morony S., Warmington K., Adamu S. The inhibition of RANKL causes greater suppression of bone resorption and hypercalcemia compared with bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology. 2005;146:3235–3243. doi: 10.1210/en.2004-1583. [DOI] [PubMed] [Google Scholar]
- Mosekilde L., Danielsen C.C., Sogaard C.H. The anabolic effects of parathyroid hormone on cortical bone mass, dimensions and strength--assessed in a sexually mature, ovariectomized rat model. Bone. 1995;16:223–230. doi: 10.1016/8756-3282(94)00033-v. [DOI] [PubMed] [Google Scholar]
- Nakashima T., Kobayashi Y., Yamasaki S. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem. Biophys. Res. Commun. 2000;275:768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- Neer R.M., Arnaud C.D., Zanchetta J.R. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Paggiosi M.A., Yang L., Blackwell D. Teriparatide treatment exerts differential effects on the central and peripheral skeleton: results from the MOAT study. Osteoporos. Int. 2018;29:1367–1378. doi: 10.1007/s00198-018-4445-5. [DOI] [PubMed] [Google Scholar]
- Rhee Y., Lee E.Y., Lezcano V. Resorption controls bone anabolism driven by parathyroid hormone (PTH) receptor signaling in osteocytes. J. Biol. Chem. 2013;288:29809–29820. doi: 10.1074/jbc.M113.485938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricarte F.R., Le Henaff C., Kolupaeva V.G. Parathyroid hormone(1-34) and its analogs differentially modulate osteoblastic Rankl expression via PKA/SIK2/SIK3 and PP1/PP2A-CRTC3 signaling. J. Biol. Chem. 2018;293:20200–20213. doi: 10.1074/jbc.RA118.004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahbani K., Cardozo C.P., Bauman W.A. Abaloparatide exhibits greater osteoanabolic response and higher cAMP stimulation and Β-arrestin recruitment than teriparatide. Physiol Rep. 2019;7 doi: 10.14814/phy2.14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Westmore M., Ma Y.L. Teriparatide [PTH-(1-34)] strengthens the proximal femur of ovariectomized nonhuman primates despite increasing porosity. J. Bone Miner. Res. 2004;19:623–629. doi: 10.1359/JBMR.040112. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Kawaguchi H., Higashikawa A. Mechanisms underlying catabolic and anabolic functions of parathyroid hormone on bone by combination of culture systems of mouse cells. J. Cell. Biochem. 2010;109:755–763. doi: 10.1002/jcb.22454. [DOI] [PubMed] [Google Scholar]
- Tsai Joy N., Uihlein Alexander V., Lee Hang. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382:50–56. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela A., Chouinard L., Lesage E. One year of abaloparatide, a selective peptide activator of the PTH1 receptor, increased bone mass and strength in ovariectomized rats. Bone. 2017;95:143–150. doi: 10.1016/j.bone.2016.11.027. [DOI] [PubMed] [Google Scholar]
- Varela A., Chouinard L., Lesage E. One year of abaloparatide, a selective activator of the PTH1 receptor, increased bone formation and bone mass in osteopenic ovariectomized rats without increasing bone resorption. J. Bone Miner. Res. 2017;32:24–33. doi: 10.1002/jbmr.3003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Supplementary tables